Abstract
Successful RAIT strategies depend upon selecting radioisotopes with physical properties complementary to the biological properties of the targeting vehicle. Small, engineered anti-tumor antibody fragments are capable of rapid, highly specific tumor targeting in immunodeficient mouse models. We hypothesized that their rapid systemic elimination would make them ideal radioisotope carriers for the radioimmunotherapy (RAIT) of established tumors. The C6.5 diabody, a non-covalent anti-HER2 single-chain Fv dimer, has a T1/2 α (equilibration phase) of 0.7 hrs, a T1/2 β (elimination phase) of 6 hrs, and a T1/2 in tumor of approximately 30 hrs that favors the use of short-lived radioisotopes. In particular, the α-particle emitting radioisotope 211At (T1/2 = 7.2 hrs) was hypothesized to be very promising for diabody-directed RAIT. This hypothesis was tested in immunodeficient nude mice bearing established HER2/neu positive MDA-MB-361/DYT2 tumors treated with 211At-SAPS C6.5 diabody (N-succinimidyl N-(4-[211At]astatophenethyl)succinamate-C6.5 diabody) at or below the maximum tolerated dose. A single i.v. injection of 211At-SAPS C6.5 diabody lead to a 30 day delay in tumor growth when a 20 µCi dose was administered and a 57 day delay in tumor growth (60% tumor free after one year) when a 45 µCi dose was employed. Treatment of mice bearing the same tumors with 211At-SAPS T84.66 diabody targeting the carcinoembryonic antigen (CEA) at the same doses led to a delay in tumor growth, but no complete responses, likely due to substantially lower expression of this antigen on the MDA-MB-361/DYT2 tumors. A dose of 20 µCi of 211At-SAPS on an a diabody specific for the Müllerian Inhibiting Substance Type II Receptor which is minimally expressed on this tumor cell line did not impact tumor growth rate, demonstrating specificity. These findings indicate that diabody molecules can be effective agents for targeted radioimmunotherapy of solid tumors using powerful, short-lived α-emitting radioisotopes.
Introduction
Radioimmunotherapy (RAIT) exploits the highly specific antigen-specificity of anti-tumor antibody molecules to selectively target and retain therapeutic radioisotopes in tumors. RAIT has been associated with significant clinical therapeutic outcomes in the treatment of hematologic (diffuse) malignant diseases, which has led to the approval of two anti-CD20 MAbs for RAIT applications (1). However, similar clinical successes have yet to be achieved in the treatment of solid, established malignancies. This is thought to be due to a number of factors related to the size of the antibodies and the properties of commonly employed therapeutic radioisotopes (2, 3). These include limited tumor penetration and prolonged circulation of intact monoclonal antibodies (MAbs), which together impair the ability to treat a tumor and increases the bone marrow toxicity; the requirement for thousands of traversals of β-particles through a single tumor cell to mediate its death; and the disparate biological half-lives of intact MAbs and physical half-lives of the commonly employed β-emitting radioisotopes such as Iodine-131 (131I) and Yttrium-90 (90Y). To address these issues, we have focused on developing smaller antibody-based molecules that are capable of greater tumor targeting specificity and pairing them based upon their biological half-lives with high-energy therapeutic radioisotopes that have complementary physical half-lives.
Noncovalent single chain Fv (scFv) dimers, known as diabodies, can be formed by producing single-chain Fv (scFv) molecules with short (5 AA) linkers between their variable light (VL) and variable heavy (VH) chains (4). This prevents the VH and VL chains from a single molecule from associating with each other to form a functional scFv. Consequently, the VH from one molecule associates with the VL from a second molecule, and vice versa, to form a divalent protein capable of binding to two antigen molecules. We, and others, have previously reported that these Mr 55,000 diabody molecules exhibit a unique combination of highly specific, durable tumor localization and relatively rapid elimination from normal tissues (5–7).
We have produced the human C6.5 diabody, which is specific for the extracellular domain (ECD) of HER2/neu and exhibits a 40-fold increase in affinity over that observed with C6.5 scFv (5). The C6.5 diabody displays an exceptional combination of quantitative and selective tumor targeting in scid mice. At 24 hr post-injection, 6 % injected dose per gram (%ID/g) of radio-iodinated C6.5 diabody was retained in SK-OV-3 tumor xenografts in mice and tumor:blood ratios of 10:1 were observed. Diabodies thus represent an improved strategy for selective tumor targeting as compared with scFv, Fab or IgG molecules. Furthermore, as decreasing the size of the molecule increases both its diffusion rate into tumor (8) and its rate of elimination from circulation, the degree of penetration and the specificity of retention in the tumor are enhanced.
We have previously reported the observation that effective RAIT of established subcutaneous (s.c.) human tumor xenografts growing in immunodeficient mice can be accomplished using a radioimmunoconjugate of 90Y and the C6.5 diabody (9). However, in that study the relatively low linear energy transfer (LET) associated with the β-emission of 90Y’s necessitated doses equal to the LD10 to achieve significant tumor growth delays and doses equivalent to the LD20 before two of the 8 treated mice (25%) 6 exhibited durable complete responses. RAIT with 131I conjugated C6.5 diabody was also sub-optimal in the same mouse model (unpublished data). The β–emissions from 90Y and 131I, the other commonly employed RAIT radioisotope, have an LET of 0.2 keV/µm (10). An attractive alternative approach is to incorporate α–emitting radioisotopes with significantly higher LET emissions (reviewed in (10, 11)) into diabody-based RAIT strategies. We initially assessed the therapeutic potential of the α-emitting radioisotope Bismuth-213 (213Bi) conjugated to the C6.5 diabody and found that the radioisotope’s physical half-life of 45.6 minutes was too short to allow systemically-administered diabody to specifically localize in an established solid tumor (12). In the current study we evaluate the utility of pairing Astatine-211 (211At), an α-emitting radioisotope (T1/2 = 7.2 Hrs, LET= 97–99 keV/µm) with the C6.5 diabody. The longer physical half-life of 211At is complimentary to the relatively rapid tumor targeting and systemic clearance of the C6.5 diabody.
Methods
Antibodies and cell lines
The anti-HER2 C6.5 diabody and the GM17 diabody that is specific for the human Mullerian Inhibiting Substance Type II Receptor (MISIIR) were each expressed from E. coli and purified as previously described (5, 13). The anti-CEA T84.66 diabody was a kind gift of Dr. Anna Wu (Beckman Research Institute, City of Hope and University of California, Los Angeles) (14). The MDA-MB361/DYT2 cell line was a kind gift of Dr. Dajun Yang of Georgetown University. Cells were expanded in culture as described (9). In vitro expression of HER2, CEA and MISIIR were determined by flow cytometry as previously described (9) using the anti-HER2 MAb 520C9 (a kind gift of Chiron Corp, Emeryville, CA), the anti-CEA MAb CB30 (Cell Signaling Technology, Danvers MA) and the anti-MISIIR MAb 12G4 (a kind gift of Dr. Isabelle Teulon of the Centre de Recherche en Cancérologie de Montpellier, France) as primary antibodies and a fluorochrome-conjugated goat anti-mouse MAb (ICN Immunobiologicals, Costa Mesa, CA) as a secondary antibody. The degree of fluorescence was determined using a FACScan flow cytometer (Becton-Dickinson, San Jose, CA) and was analyzed using the CELLQuest software (Becton-Dickinson) (data not shown). In these studies the MDA-MB361/DYT2 cells were observed to express large quantities of HER2 (+++) and low levels of CEA (+). The flow cytometry assays also suggested that MDA-MB361/DYT2 cells express low levels of MISIIR (data not shown). Low level MISIIR expression on the MDA-MB361/DYT2 cells was confirmed by RT-PCR assays.
Radiolabelings
125I-SIPS and 211At-SAPS preparation
Iodine-125 was purchased from Perkin Elmer (Boston, MA, cat# NEZ033H). Astatine-211 was produced as described (15). Succinimidyl N-{4-[125I]iodophenethyl}succinamate (SIPS) and succinimidyl N-{4-[211At]astatophenethyl}succinamate (SAPS) were prepared as previously described (16).
In brief, SAPS was prepared by mixing 211At (5 – 10 mCi in 70 µL MeOH) with its tributylstannyl precursor as previously reported (16). Labeling with 211At was performed by mixing 5 µL of 2 mg/mL N-chlorosuccinimide (NCS) in MeOH with 20 µL of 25 mg/mL of the tributylstannyl precursor in MeOH containing 1% acetic acid. After 30 min incubation at room temperature, the reaction mixture was air dried and the residue was dissolved in 80 µL of EtOAc/MeOH 50/50 v/v. The 211At-labeled active ester was isolated by normal phase HPLC using the following gradient: 90% hexane (10% ethyl acetate) for 1 min and followed by a 9 min linear gradient to 100% EtOAc; a retention time of 8.4 for SAPS was routine. The flow rate for all elutions was 3 mL/min. The HPLC fractions containing the product were combined, dried in an air-stream, and used in the next step. Typical labeling efficiencies were 45±5%. SIPS was also prepared as previously reported (16). In brief, 20 µL of 25 mg/mL of tributylstannyl precursor in MeOH, 100 µL of CH3COOH/CH3OH (9/91 v/v), and 5 µL of 2 mg/mL NCS in MeOH were added to 125I (500 µCi). After 30 min at room temperature, the reaction mixture was dried under an air stream, re-dissolved in 80 µL of EtOAc/MeOH 50/50 v/v and purified by normal phase HPLC, using the same elution protocol described above for purification of SAPS; Rt for SIPS was 8.40 min routinely. Reaction radiochemical yields were 65±5%,
Conjugation to diabodies
125I-SIPS was conjugated to the C6.5 and T84.66 diabodies and 211At-SAPS was conjugated to the C6.5, T84.66 and GM17 anti-MISIIR diabodies using methods similar to those previously reported (17).
Briefly, 2.0 mL of absolute MeOH was added to the 125I-SIPS (0.26 mCi in 1.0 mL methylene chloride) or 211At-SAPS (1.4 – 2.0 mCi in 1.0 mL methylene chloride) and the mixture was dried completely under a gentle stream of nitrogen. The diabody was concentrated and diluted in 0.5 M borate buffer to a final concentration of approximately 1 mg/mL for the C6.5 and GM17 diabodies and 5.0 mg/mL for the T84.66 diabody, and added directly to the tube containing the dried 125I-SIPS or 211At-SAPS. Each diabody was added at a ratio of 5 mg diabody to 1 mCi of 211At. After a 15 min incubation at room temperature, 2.0 mL of PBS, pH 7.2, was added to the reaction vial and the reaction was chromatographed over a PD10 column (BioRad, Richmond, CA) that was equilibrated with PBS, pH 7.2. The column was eluted with an additional 5.0 mL of PBS and 0.5 mL fractions were collected. Each fraction was assayed in a dose calibrator for radioactivity and the fractions containing the radiolabeled diabody were pooled for Q.C.
Radiolabeled diabodies were assayed for radiochemical purity and function in ITLC assays and live cell binding studies as previously described (9). In the ITLC assay, one µL from each the reaction mixture and the final product were applied to silica ITLC strips (Biodex Medical Systems, Shirley, NY) and allowed to migrate using normal saline as a mobile phase. The strips were cut at the midpoint and the two halves were counted in a gamma well counter (Cobra Quantum, Packard Instruments, Meriden, CT). Each radiolabeled diabody exhibited a radiochemical purity of greater than or equal to 94%. The immunoreactivity of the radiopharmaceutical was determined in a live cell binding assay (18). Briefly, 10 ng of labeled diabody in 100 µL of media was added in triplicate into 15 mL polypropylene centrifuge tubes containing 3 × 106 SK-OV-3 cells. The cells were allowed to incubate for 30 min at r.t. One mL of PBS, 4°C, was added to each tube and they were centrifuged for 5 min at 500 × g at 4°C. Supernatants were separated from the cell pellets, both were transferred to 12 × 75 mm counting tubes and the percentage of radioactivity associated with the cell pellet was determined by counting in a gamma-counter. Live cell-binding assays routinely revealed that the 125I-SIPS C6.5 diabody and 211At-SAPS C6.5 diabody each exhibited an immunoreactivity greater than 40% on MDA-MB361/DYT2 tumor cells.
Biodistribution studies
Four to six-week-old inbred C.B17/Icr- scid mice were obtained from the Fox Chase Cancer Center Laboratory Animal Facility. 3 × 106 SK-OV-3 cells or 5 × 106 MDA-361/DYT2 cells in 0.1 mL of PBS were injected s.c. into the abdomen of each mouse. Approximately two months after the implantation of the SK-OV-3 cells and three weeks after the implantation of the MDA-361/DYT2 cells, the tumors had achieved a size of about 100 mm3 and the distribution studies were initiated.
Twenty micrograms of 125I-SIPS C6.5 diabody or 125I-SIPS T84.66 diabody (0.2 µCi/µg) were administered to each mouse by tail vein injection. Total injected doses were determined by counting the mice on a Series 30 multichannel analyzer/probe system (probe model #2007, Canberra, Meridian, CT). Blood sample collection and whole body counts of the mice were performed immediately after injection and just prior to euthanization. Groups of five or six mice were euthanized at 4 and 48 hr after injection; tumor, organ and blood retentions were determined as previously described (5, 18). The mean and standard error of the mean (SEM) for each group of data were calculated, and tumor to organ ratios (T:O) were determined.
Radioimmunotherapy studies
Male Balb/c nude mice were obtained at 8–12 weeks of age from Taconic Labs (Germantown, NY). Human tumor xenografts were established by implanting 5 × 106 MDA-MB361/DYT2 tumor cells s.c. on the abdomen as previously described (18). Tumor volumes were determined using the using the ellipsoidal formula: length (mm) × width (mm) × height (mm) × 0.52 (derived from π/6) (19). After approximately three weeks, the tumors were well established and the therapy studies were initiated. Cohorts of 5 – 7 mice bearing established s.c. tumors were treated with 20 µCi, 30 µCi or 45 µCi of 211At-SAPS C6.5 diabody or 211At-SAPS T84.66 diabody or 20 µCi of 211At-SAPS anti-MISIIR GM17 diabody at a dose of approximately 1 mCi/mg diabody or left untreated. The mean tumor sizes in the 211At-SAPS C6.5 diabody study at the time of treatment were 344 ± 8 mm3, 400 ± 78 mm3, 221 ± 22 mm3 and 423 ± 50 mm3, respectively, for the 20 µCi, 30 µCi, 45 µCi and control treatment groups. The mean tumor sizes in the 211At-SAPS T84.66 diabody study at the time of treatment were 526 ± 100 mm3, 427 ± 60 mm3, 358 ± 57 mm3 and 504 ± 66 mm3, respectively, for the 20 µCi, 30 µCi, 45 µCi and control treatment groups. The mean tumor sizes in the anti MISIIR GM17 diabody study were 534 ± 122 mm3 and 467 ± 52 mm3, respectively, for the treated and control groups. Following treatment, the mice were observed and weighed and their tumors were measured with calipers every three days. All studies described in this manuscript were performed under approved protocols following Fox Chase Cancer Center’s Institutional Animal Care and Use Committee guidelines. Mice were euthanized when tumor volumes exceeded 10% of the animal's body weight.
Renal Toxicity and histological studies
The diabody is primarily eliminated through the kidneys; therefore a possible outcome of diabody-based RAIT could be renal damage. As this type of damage would appear after a significant delay following therapy the long-term survivors were euthanized at one year after the treatment date and their kidneys were fixed in formalin and sections were obtained for histopathological examination. Sections were stained with hematoxylin and eosin and were then examined by the Fox Chase Cancer Center Histopathology Facility for abnormalities.
Statistics
Treatment cohorts were analyzed by one-way ANOVA using the GraphPad InStat 3 software package (GraphPad Software, San Diego, CA)
Results
Biodistribution Studies
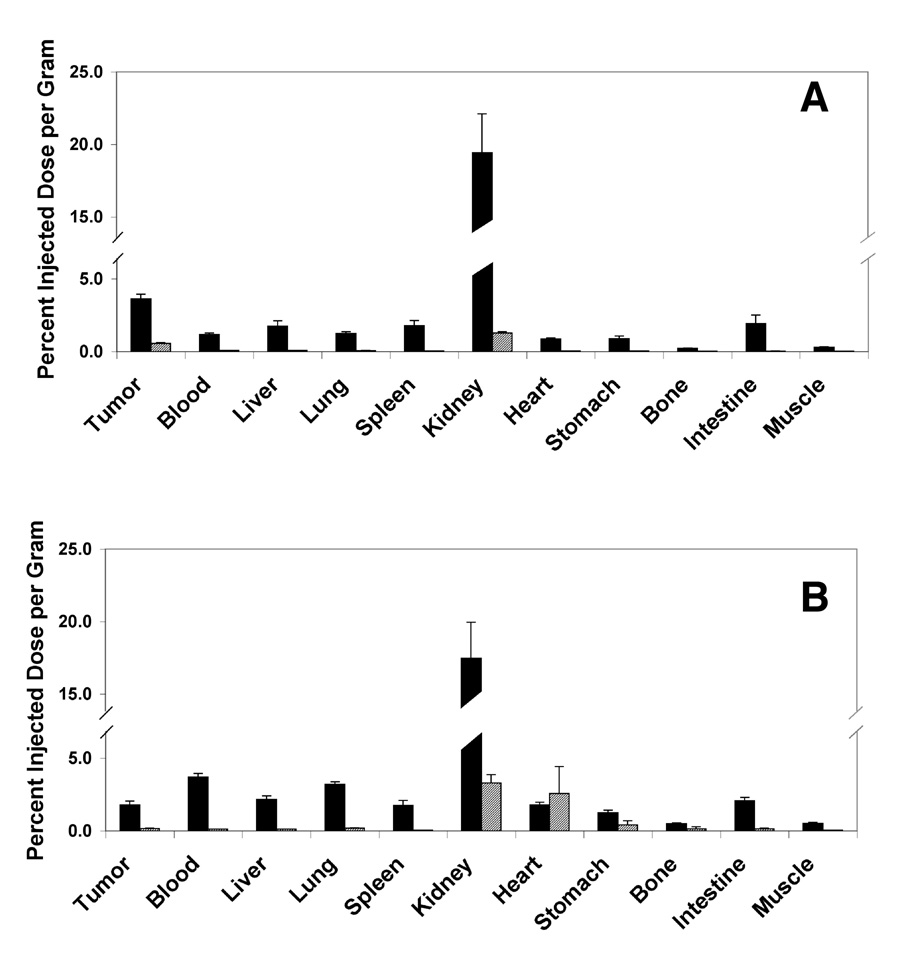
As the distribution of 211At conjugated agents is difficult to follow in vivo, biodistribution studies were performed using 125I-SIPS conjugated diabodies as surrogates for the 211At conjugated diabodies. The 4 hr and 48 hr biodistributions of the 125I-SIPS C6.5 diabody and 125I-SIPS T84.66 diabody were determined in scid mice bearing s.c. MDA-361/DYT2 tumor xenografts. At 4 hr post-injection, the tumor retention of the 125I-SIPS C6.5 diabody was 3.6% ID/g, two-fold or more greater than that retained in every major organ except the kidneys (19.4% ID/g) which are the site of elimination for this molecule (Figure 1). The rapid blood clearance was also reflected by the approximately 3:1 tumor:blood ratio at four hours post-injection. By 48 hours after administration, the quantity retained in the tumor had dropped to about 0.6 %ID/g, 6-fold or more greater than that retained in blood and all major organs except the kidneys (1.3 %ID/g).
Figure 1.
Biodistibutions of 125I-SIPS Diabodies. The biodistributions of C6.5 diabody (A) and T84.66 diabody (B) radioiodinated with 125I-SIPS were evaluated at 4 hours (solid black bars) and 48 hours (hatched bars) in cohorts of five scid mice bearing s.c. MDA-MB361/DYT2 tumors. Average tumor and organ uptake are presented as percentage of the injected dose localized per gram of tissue or ml of blood. Standard errors of the mean are indicated.
Radioimmunotherapy Studies
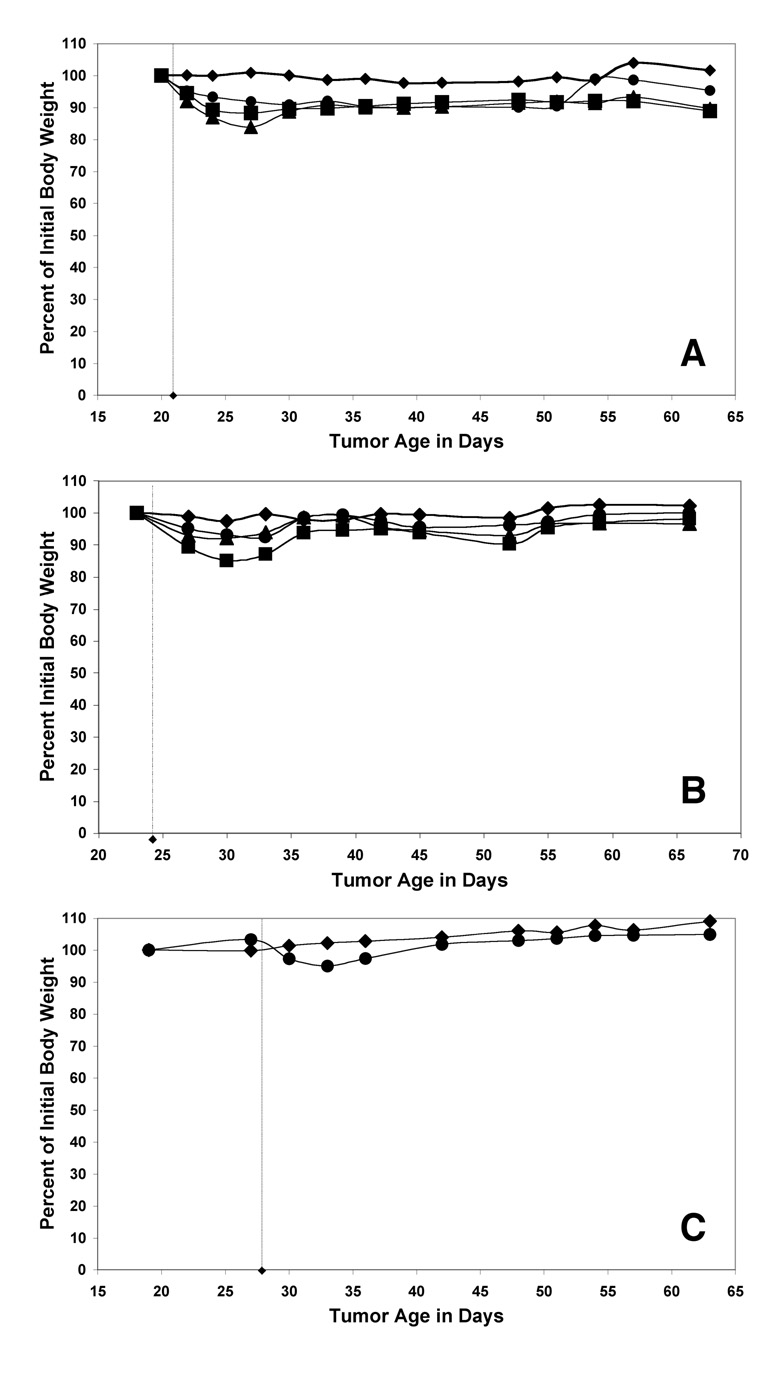
The efficacy and specificity of a single dose of 211At conjugated diabody was assessed in nude mice bearing established MDA-MB361/DYT2 tumors. The 211At-SAPS conjugated diabodies were well tolerated at all doses studied. In each group, maximum weight losses occurred at approximately one week following treatment and were typically less than or equal to 15% of the body weight for the 45 µCi groups (Figure 2). No fatalities occurred as a result of the treatment, but the mean body weights in the groups that received 45µCi were very slow to recover to their initial weights suggesting that the peak doses employed were close to the acute maximum tolerated dose in this model system.
Figure 2.
Weight loss is an indication of acute toxicity following radioimmunotherapy. The mean weights of the groups of nude mice treated with 211At-SAPS-C6.5 diabody (A), 211At-SAPS-T84.66 diabody (B) and 211At-SAPS-Anti-MISIIR diabody (C) are shown for the untreated control groups (diamonds), 20 µCi (circles), 30 µCi (triangles) and 45 µCi (squares) treatment groups
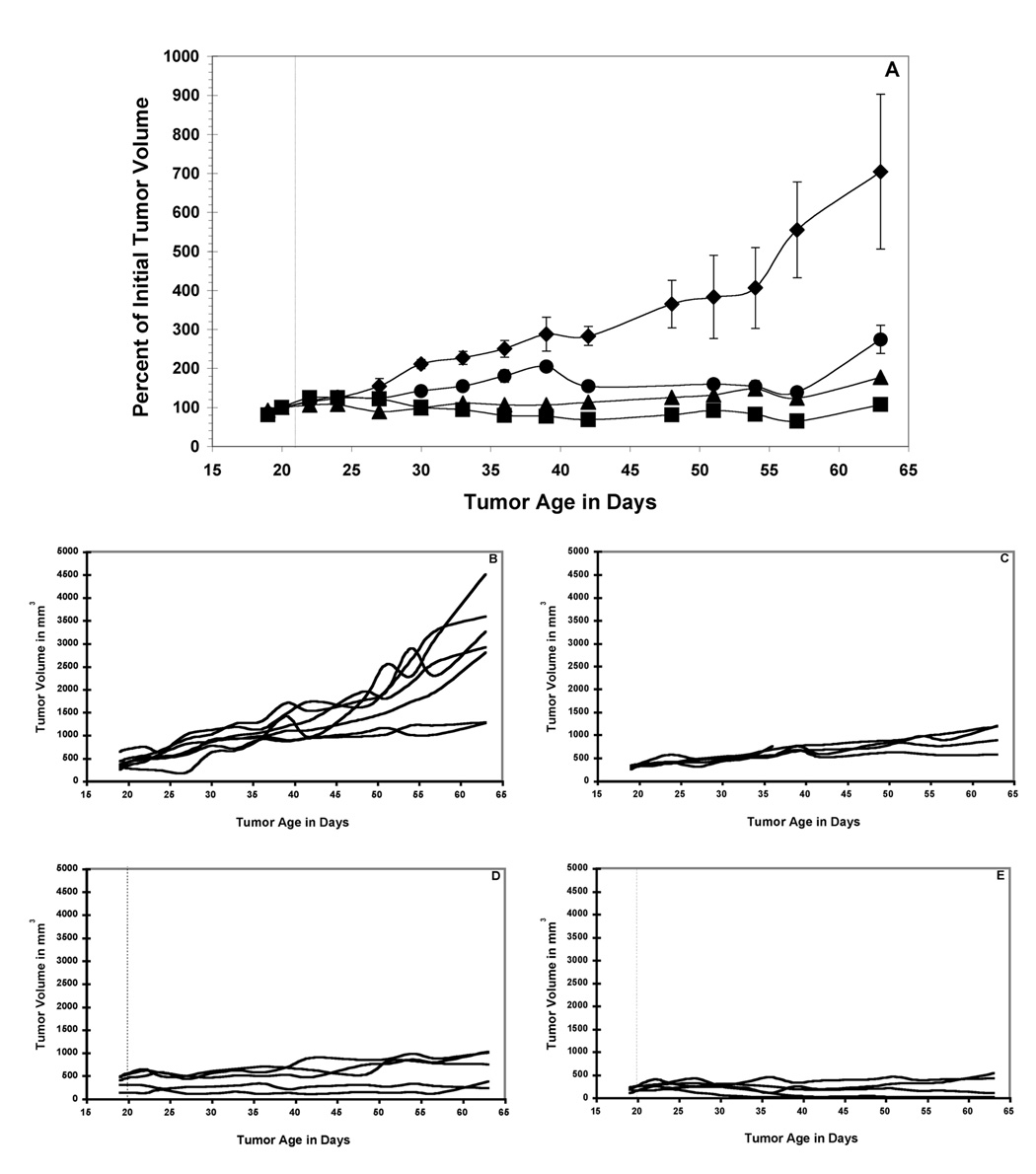
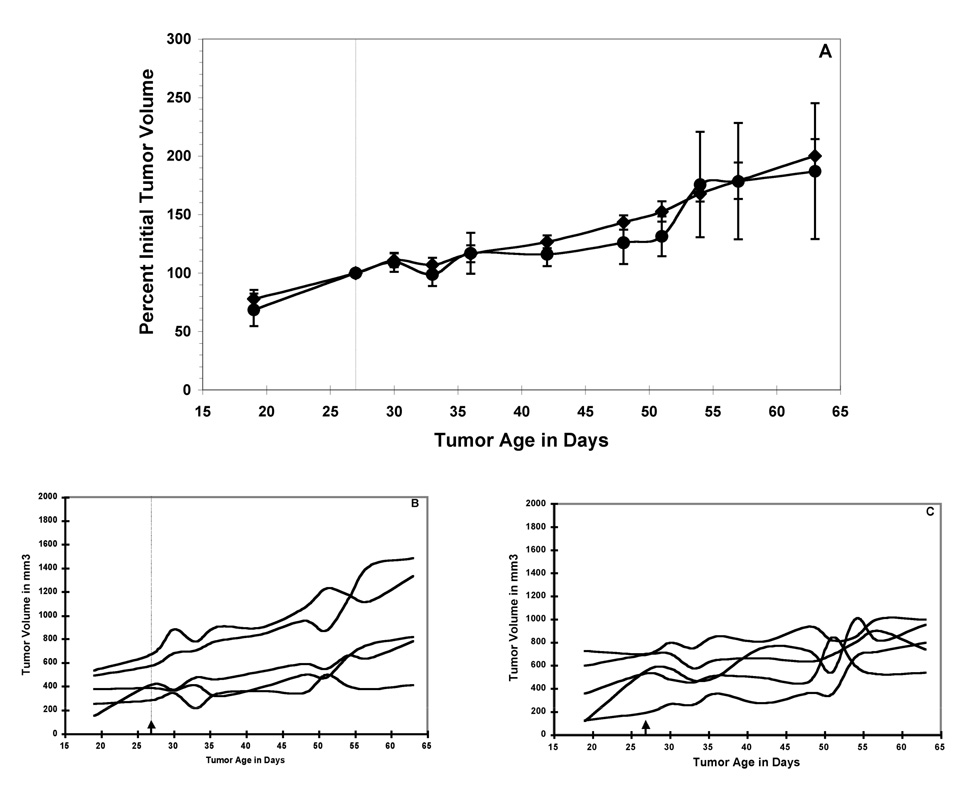
A single dose of 211At-SAPS C6.5 diabody administered to mice bearing established MDA-MB361/DYT2 tumors resulted in a dose-dependent anti-tumor response (Figure 3A). The tumors in the untreated mice grew rapidly throughout the study (Figure 3A and 3B). A single treatment with 20 µCi of 211At-SAPS C6.5 diabody resulted in a 10-day delay, as compared to the untreated control group, in the time it took for the mean tumor volumes to double (Figure 3A and 3C). Doses of 30 µCi and 45 µCi of 211At-SAPS C6.5 diabody delayed tumor growth significantly as compared to the untreated controls, such that the mean tumor volume did not double during the course of the study (Figure 3A, 3D, 3E) (significance of at least p < 0.05 from day 30 to the end of the study for both for both the 30 µCi and 45 µCi dose groups groups). At the highest treatment dose (45 µCi of 211At-SAPS C6.5 diabody), three of the five treated mice exhibited a full remission (Figure 3E). These animals were tumor free for one year following treatment, at which time they were euthanized and histopathological examination revealed no signs of tumors (data not shown).
Figure 3.
211At-SAPS-C6.5 diabody RAIT of nude mice bearing established MDA-MB361/DYT2 human breast tumors. Mean values +/− SEM of cohorts of 5 mice treated with a single dose of 20 µCi (circles), 30 µCi (triangles) and 45 µCi (squares) of 211At-SAPS-C6.5 diabody and a cohort of 7 untreated mice (diamonds) are presented (A). Also shown are tumor volumes from individual mice from the control group (B), 20 µCi treatment group (C), 30 µCi (D) and 45 µCi (E). Significance between treatment and control groups are indicated as follows * = p < 0.05; ** = p < 0.01.
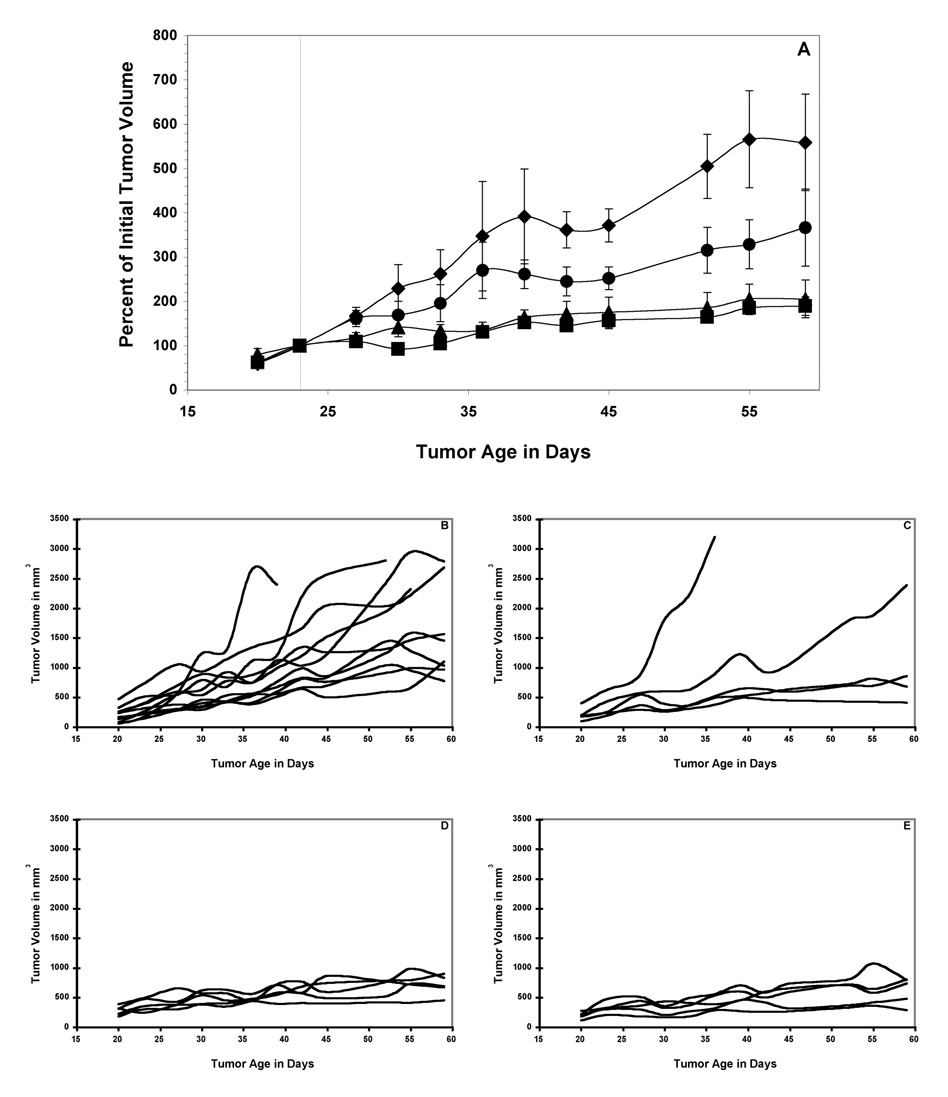
As with the C6.5 diabody above, treatment of mice bearing established MDA-MB361/DYT2 tumors with a single dose of 211At-SAPS T84.66 diabody resulted in a dose-dependent anti-tumor response (Figure 4A). 20 µCi of 211At-SAPS T84.66 diabody resulted in a 6-day delay in the mean tumor volume doubling time as compared to the control group (Figure 4A and 4C), while 30 µCi and 45 µCi doses of 211At-SAPS 16 T84.66 diabody further increased the delay in tumor growth as compared to the untreated controls (Figure 4A, 4D, 4E) (significance of at least p < 0.05 from day 42 to the end of the study for both the 30 µCi and 45 µCi dose groups). However, all of the tumors in the mice treated with 211At-SAPS T84.66 diabody eventually grew to 10% of the animal’s total body weight, requiring euthanization as per institutional guidelines.
Figure 4.
211At-SAPS-T84.66 diabody RAIT of nude mice bearing established MDA-MB361/DYT2 human breast tumors. Mean values +/− SEM of cohorts of 5 mice treated with a single dose of 20 µCi (circles), 30 µCi (triangles) and 45 µCi (squares) of 211At-SAPS-T84.66 diabody and a cohort of 11 untreated mice (diamonds) are presented (A). Also shown are tumor volumes from individual mice from the control group (B), 20 µCi treatment group (C), 30 µCi (D) and 45 µCi (E). Significance between treatment and control groups are indicated as follows * = p < 0.05; ** = p < 0.01.
In contrast to the results seen above with 20 µCi of 211At-SAPS conjugated C6.5 and T84.66 diabodies, treatment of mice bearing established MDA-MB361/DYT2 tumors with 20 µCi of 211At-SAPS MISIIR diabody, resulted in no delay in tumor growth over that observed in mice left untreated (Figures 5 A, B, C), likely a result of a combination of both the low expression levels of this antigen and the low affinity of GM17 diabody for MISIIR.
Figure 5.
211At-SAPS-Anti-MISIIR GM17 diabody RAIT of nude mice bearing established MDA-MB361/DYT2 human breast tumors. Mean values +/− SEM of a cohort of 5 mice treated with a single dose of 20 µCi (circles) of 211At-SAPS-Anti-MISIIR diabody or 5 untreated mice (diamonds) are presented (A). Also shown are tumor volumes from individual mice from the control group (B) and the 20 µCi treatment group (C). The differences between the values of the treatment and control groups were not significant.
Long-term toxicity
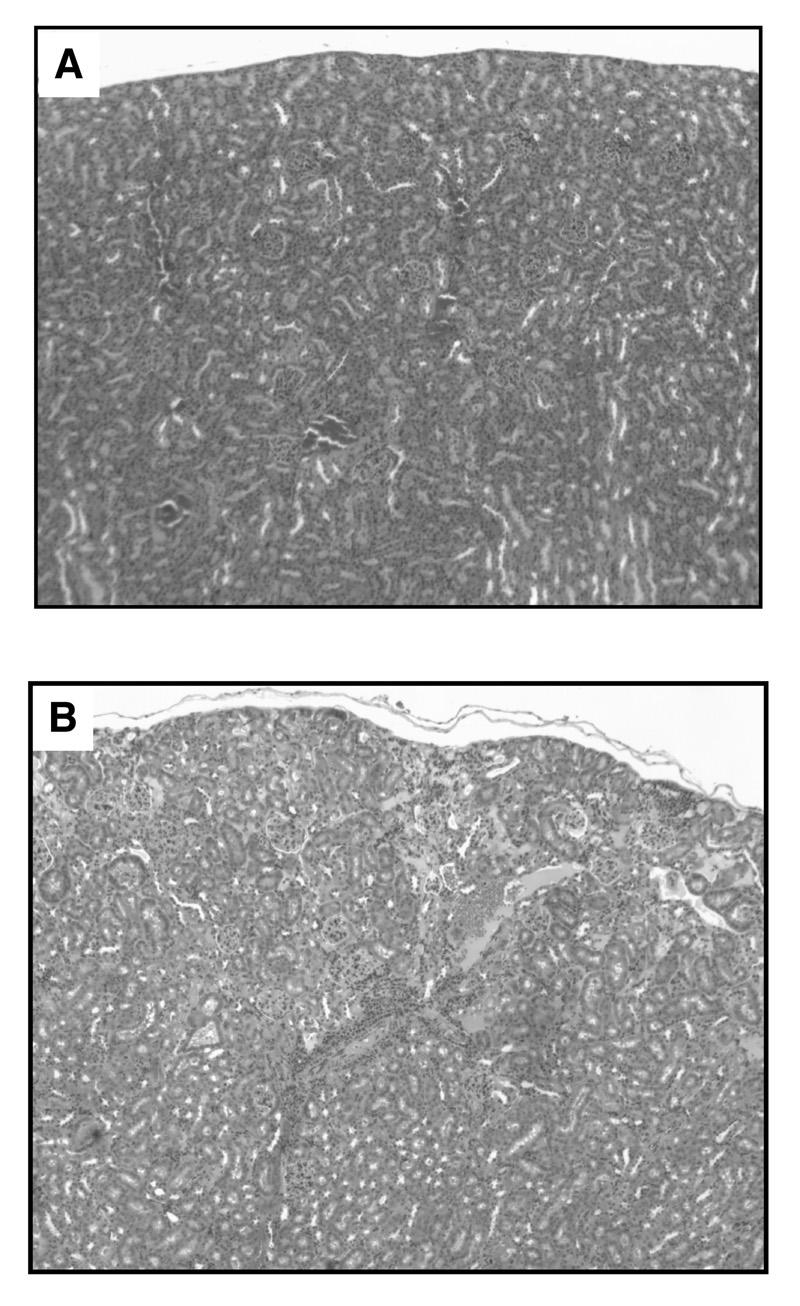
In order to assess long-term renal toxicity associated with 211At-SAPS diabody therapy, we compared the kidneys of mice one year following treatment with those of untreated age-matched control mice. The three mice that exhibited complete responses to 211At-SAPS C6.5 diabody therapy above and three control mice were euthanized and their kidneys were excised and processed for histopathological examination. While the kidneys of the untreated control animals and one of the treated animals appeared normal (Figure 6A), two of the three treated mice exhibited signs of renal damage. In these animals, both normal regions of their kidneys and regions with varying degrees of damage including edema and mild fibrosis in one mouse and focal cortical fibrosis and atrophy in the second mouse were present (Figure 6B).
Figure 6.
Renal toxicity. The kidneys of mice treated with 45 µCi of 211At-SAPS C6.5 diabody and untreated age-matched control mice were examined one year following therapy. Untreated age matched control mice (A) and one of the three treated mice exhibited no signs of renal damage, while two of the treated mice presented with renal damage. (B) a section revealing both normal kidney regions and fibrosis seen in one of the treated mice.
Discussion
RAIT-based strategies for the treatment of diffuse malignancies have exhibited significant successes in the clinical setting (20) and significant advances have been made in the preclinical treatment of solid malignancies (1). Typically these strategies are based upon the delivery of β-emitting radioisotopes to tumors via intact monoclonal antibodies. The two most frequently employed β-emitters are 90Y and 131I. As a result of their decay, both emitt β– particles with relatively long track lengths ranging up to several millimeters associated with a relatively low LET. These long track lengths extend the range of β-particle based RAIT well beyond the penetration range of the antibody carrier. This also allows for a “crossfire effect” in which tumor cells that lack the target antigen can still be effectively treated. However, the low LET associated with β-emitting radioisotopes necessitates up to 106 cell-surface decays or “hits” on a given tumor cell before a cytotoxic event occurs (21).
In contrast with β–particles, α–particles have relatively short track lengths and high LET (a few, 3–7, can kill a cell) (19). The radioisotope we employed in the present study, 211At, has an LET of 97–99 keV/µm, ~200 times greater than that associated with 90Y (10). While the short track length of α emissions significantly reduces the ability to kill tumor cells located distant from the decay event and limits the “crossfire effect” described above, their high potency can overcome these limitations if sufficient tumor penetration is achieved through conjugation to smaller antibody fragments, such as diabodies, that are capable of penetrating deeper into solid tumors. Localization of these α-particle emitting radioimmunoconjugates to perivascular tumor calls could also mediate an anti-vascular effect (22).
The relatively short physical half-lives of the commonly available, medically relevant, α–emitting radionuclides limit the ability of α–emitter based radioimmunoconjugates to be efficiently and safely administered by systemic routes for the treatment of solid tumors. As a result, the greatest successes using α-emitting radioisotopes conjugated to intact antibodies have resulted from i.p. delivery for the treatment of tumors that are localized in the peritoneal cavity (23, 24) and intrathecal delivery for the treatment of brain tumors (25).
An alternate strategy employed to address the limitations that short physical half-lives impose on RAIT with α-emitters such as 213Bi is the targeted delivery of one of 213Bi’s longer-lived parent radioisotopes, Actinium-225 (225Ac), which has a T1/2 of 10 days (26). This approach allows accumulation of 225Ac conjugated MAbs in the tumor over a longer period of time and then depends upon limited diffusion of 225Ac’s chain of α-emitting daughter radioisotopes during their subsequent combined 50-minute half-life. As five different decay events occur over this period, releasing a total of four α particles, RAIT with targeted 225Ac is reported to be associated with impressive therapeutic efficacy (27). However, renal toxicity associated with the release and systemic trafficking of daughter radioisotopes in the 225Ac/213Bi decay chain must be addressed before this methodology can achieve its full potential (28, 29).
In contrast with the work of others, we elected to utilize smaller antibody-based molecules with biological half-lives that are more complimentary to the short physical half-lives of the medically relevant α–emitting radionuclides. In selecting a tumor-targeting agent, diabody molecules represent a particularly attractive class of antibody-based molecules. Diabodies are noncovalent dimers of single-chain Fv molecules that are held together by the affinity of the variable heavy and variable light chains for each other (4). With a molecular weight of approximately 55 kDa, diabodies are expected to more efficiently penetrate into tumors than intact IgG molecules that are three times larger. Due to their divalent nature they also exhibit prolonged retention in tumors as compared to monovalent scFv molecules (5).
We have previously reported on the isolation of the C6.5 scFv from a human non-immune phage display library (30) and the development of the C6.5 diabody from this molecule (5). As the C6.5 diabody is nearly twice the size of the C6.5 scFv molecule, it exhibits a prolonged systemic retention in the mouse model. The C6.5 diabody also exhibits a higher functional affinity for HER2 than the C6.5 scFv (4 × 10−10 M vs. 1.6 × 10−8 M, respectively). Taken together, these factors confer the C6.5 diabody with a selective targeting advantage over the C6.5 scFv (5).
We have recently demonstrated that the C6.5 diabody is an effective vehicle for 90Y for the RAIT of established solid tumors in the preclinical setting (9). In that study, 20% of the animals treated with the highest dose of 90Y CHX-A” conjugated C6.5 diabody exhibited durable complete responses. However, the same dose was also associated with a 20% fatality rate due to treatment-related acute toxicity. In contrast, RAIT of solid tumor xenografts growing in nude mice using the extremely short-lived α-emitting radioisotope 213Bi (T1/2 = 46 min) conjugated to the C6.5 diabody revealed no delay in tumor growth, likely due to the inability of the diabody to localize in the tumor prior to the majority of the radioactive decay events (12). Based upon these results, we postulated that the most effective RAIT could be achieved through rational pairing of the biological half-life of the delivery agent and the physical half-life of the radioisotope. As the C6.5 diabody exhibits an elimination half-life (T1/2 β) of 6.4 hours in the circulation and a biological half-life of about 30 hours in tumor xenografts in the mouse model (5), we predicted that it would exhibit excellent anti-tumor efficacy when paired with the potent α-particle emitting radioisotope 211At (T1/2 = 7 hours).
A major drawback with employing a short-lived, novel radioisotope such as 211At is the difficulty associated with its acquisition, purification, conjugation and transport. In the current study, over two physical half-lives passed during these steps, severely limiting the doses we were able to administer to the animals. It is likely that the acute MTD was not achieved in our treatment studies. However, the slow recovery of weight loss by the cohorts of mice that received the highest treatment dose suggests that that it was close to the MTD. Unfortunately, the difficulty in production and transport of the 211At limited the dose employed in the study with the anti-MISIIR diabody to a single dose group that received 20 µCi.
In the studies described here, we observed that 211At can be an extremely effective radioisotope for the RAIT of solid tumors when it is conjugated to divalent 52–55 kDa diabody molecules. A single i.v. treatment with 211At conjugated to both the anti-HER2 C6.5 diabody and anti-CEA T84.66 diabody resulted in dose-dependent delays in tumor growth of MDA-MB361/DYT2 tumor xenografts in nude mice. At the highest dose administered, three of the five mice treated with 211At SAPS-C6.5 diabody exhibited durable complete responses and the remaining mice exhibited prolonged delays in tumor growth. In contrast, treatment of mice with the same dose of 211At SAPS-T84.66 diabody resulted in significant delays in tumor growth, but no complete responses. This likely reflected the significantly greater expression of HER2 on the MDA-MB361/DYT2 tumor cells as compared to CEA. However, the ultimate fate of the target antigen could also play a role in the efficacy of α-emitter RAIT. HER2 internalizes into tumor cells, bringing the short track-length 211At α emissions into closer proximity to the nucleus than would be the case with the conjugates targeting the relatively non-internalizing CEA antigen.
As diabodies fall below the threshold for first pass renal elimination, the kidneys often exhibit the greatest degree of retention of radioactivity of any normal organ (Figure 1), leading to the potential for significant renal toxicity. A recent publication by Jaggi et al reports that radiation damage to the kidneys following exposure to free α-emitting radioisotopes is progressive over a 10 to 40 week interval following exposure (29). In a prior study examining therapeutic efficacy of the β-emitting radioisotope 90Y conjugated to the C6.5 diabody in the same mouse model employed in the current study, we observed significant renal damage at one year following therapy (9). Accordingly, we decided to perform a preliminary evaluation of renal toxicity one year after treatment in the three mice whose tumors were cured with the 45 µCi dose of 211At-SAPS C6.5 diabody. Histopathological examination of the kidneys revealed that two of the three mice exhibited both regions of fibrosis and healthy regions in their kidneys as compared to the untreated, age-matched controls and the third treated mouse. While the cohort of animals studied is too small to draw significant conclusions, the degree of renal toxicity observed here appeared to be mild in contrast to the significant renal toxicity seen in some of the mice treated with 90Y conjugated C6.5 diabody. If this observation were repeated when larger cohorts of animals are studied, it would be consistent with the overall lower renal retention seen with halogen-based radioisotopes as compared to residualizing radiometals (9).
Conclusion
High LET, short-range α-emitters offer a promising alternative to lower LET β-emitters. However, to fully fulfill their potential for the systemic treatment of solid tumors and residual disease in an adjuvant setting, it is necessary to deliver these radioisotopes in a rapid and specific manner. Our report here represents the first successful use of small, 52 kDa, diabody molecules as vehicles for α-emitter RAIT of established tumors.
Acknowledgements
The authors would like to thank Dr. Louis Weiner, Heidi Simmons and Eva Horak of the Department of Medical Oncology for helpful discussions, Dr. Andres Klein-Szanto and Ms. Cass Renner of the Fox Chase Cancer Center Histopathology Facility and the members of the Fox Chase Cancer Center Laboratory Animal Resources Group for their expert assistance. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Supported by DOE Grant # DE-FG02-01ER63190 (GPA) and by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- 1.Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23:1137–1146. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 2.Robinson MK, Weiner LM, Adams GP. Improving Monoclonal Antibodies For Cancer Therapy. Drug Development Research. 2004;16:172–187. [Google Scholar]
- 3.Russeva MG, Adams GP. Radioimmunotherapy with engineered antibodies. Expert Opin Biol Ther. 2004;4:217–231. doi: 10.1517/14712598.4.2.217. [DOI] [PubMed] [Google Scholar]
- 4.Holliger P, Prospero T, Winter G. "Diabodies": small bivalent and bispecific antibody fragments. Proc Natl Acad Sci U S A. 1993;90:6444–6448. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams GP, Schier R, McCall AM, Crawford RS, Wolf EJ, Weiner LM, Marks JD. Prolonged in vivo tumour retention of a human diabody targeting the extracellular domain of human HER2/neu. Br J Cancer. 1998;77:1405–1412. doi: 10.1038/bjc.1998.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu AM, Chen W, Raubitschek A, Williams LE, Neumaier M, Fischer R, Hu SZ, Odom-Maryon T, Wong JY, Shively JE. Tumor localization of anti-CEA single-chain Fvs: improved targeting by non-covalent dimers. Immunotechnology. 1996;2:21–36. doi: 10.1016/1380-2933(95)00027-5. [DOI] [PubMed] [Google Scholar]
- 7.Power BE, Caine JM, Burns JE, Shapira DR, Hattarki MK, Tahtis K, Lee FT, Smyth FE, Scott AM, Kortt AA, Hudson PJ. Construction, expression and characterisation of a single-chain diabody derived from a humanised anti-Lewis Y cancer targeting antibody using a heat-inducible bacterial secretion vector. Cancer Immunol Immunother. 2001;50:241–250. doi: 10.1007/s002620100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain RK. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res (Suppl.) 1990;50:814s–819s. [PubMed] [Google Scholar]
- 9.Adams GP, Shaller CC, Dadachova E, Simmons HH, Horak EM, Tesfaye A, Klein-Szanto AJ, Marks JD, Brechbiel MW, Weiner LM. A single treatment of yttrium-90-labeled CHX-A"-C6.5 diabody inhibits the growth of established human tumor xenografts in immunodeficient mice. Cancer Res. 2004;64:6200–6206. doi: 10.1158/0008-5472.CAN-03-2382. [DOI] [PubMed] [Google Scholar]
- 10.Mulford DA, Scheinberg DA, Jurcic JG. The promise of targeted {alpha}-particle therapy. J Nucl Med. 2005;46 Suppl 1:199S–204S. [PubMed] [Google Scholar]
- 11.Zalutsky MR, Pozzi OR. Radioimmunotherapy with alpha-particle emitting radionuclides. Q J Nucl Med Mol Imaging. 2004;48:289–296. [PubMed] [Google Scholar]
- 12.Adams GP, Shaller CC, Chappell LL, Wu C, Horak EM, Simmons HH, Litwin S, Marks JD, Weiner LM, Brechbiel MW. Delivery of the alpha-emitting radioisotope bismuth-213 to solid tumors via single-chain Fv and diabody molecules. Nucl Med Biol. 2000;27:339–346. doi: 10.1016/s0969-8051(00)00103-7. [DOI] [PubMed] [Google Scholar]
- 13.Yuan Q-A, et al. MISIIR Paper. MCT; 2006. In Press. [Google Scholar]
- 14.Yazaki PJ, Shively L, Clark C, Cheung CW, Le W, Szpikowska B, Shively JE, Raubitschek AA, Wu AM. Mammalian expression and hollow fiber bioreactor production of recombinant anti-CEA diabody and minibody for clinical applications. J Immunol Methods. 2001;253:195–208. doi: 10.1016/s0022-1759(01)00388-x. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz UP, Plascjak P, Beitzel MP, Gansow OA, Eckelman WC, Waldmann TA. Preparation of 211At-labeled humanized anti-Tac using 211At produced in disposable internal and external bismuth targets. Nucl Med Biol. 1998;25:89–93. doi: 10.1016/s0969-8051(97)00165-0. [DOI] [PubMed] [Google Scholar]
- 16.Talanov VS, Yordanov AT, Garmestani K, Milenic DE, Arora HC, Plascjak PS, Eckelman WC, Waldmann TA, Brechbiel MW. Preparation and in vivo evaluation of novel linkers for 211At labeling of proteins. Nucl Med Biol. 2004;31:1061–1071. doi: 10.1016/j.nucmedbio.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Yordanov AT, Garmestani K, Zhang M, Zhang Z, Yao Z, Phillips KE, Herring B, Horak E, Beitzel MP, Schwarz UP, Gansow OA, Plascjak PS, Eckelman WC, Waldmann TA, Brechbiel MW. Preparation and in vivo evaluation of linkers for 211At labeling of humanized anti-Tac. Nucl Med Biol. 2001;28:845–856. doi: 10.1016/s0969-8051(01)00257-8. [DOI] [PubMed] [Google Scholar]
- 18.Adams GP, McCartney JE, Tai MS, Oppermann H, Huston JS, Stafford WF, 3rd, Bookman MA, Fand I, Houston LL, Weiner LM. Highly specific in vivo tumor targeting by monovalent and divalent forms of 741F8 anti-c-erbB-2 single-chain Fv. Cancer Res. 1993;53:4026–4034. [PubMed] [Google Scholar]
- 19.Hann HW, Stahlhut MW, Rubin R, Maddrey WC. Antitumor effect of deferoxamine on human hepatocellular carcinoma growing in athymic nude mice. Cancer. 1992;70:2051–2056. doi: 10.1002/1097-0142(19921015)70:8<2051::aid-cncr2820700806>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 21.Humm JL. A microdosimetric model of astatine-211 labeled antibodies for radioimmunotherapy. Int J Radiat Oncol Biol Phys. 1987;13:1767–1773. doi: 10.1016/0360-3016(87)90176-3. [DOI] [PubMed] [Google Scholar]
- 22.Kennel SJ, Mirzadeh S, Eckelman WC, Waldmann TA, Garmestani K, Yordanov AT, Stabin MG, Brechbiel MW. Vascular-targeted radioimmunotherapy with the alpha-particle emitter 211At. Radiation Research. 2002;157:633–641. doi: 10.1667/0033-7587(2002)157[0633:vtrwta]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Milenic DE, Garmestani K, Brady ED, Albert PS, Ma D, Abdulla A, Brechbiel MW. Alpha-particle radioimmunotherapy of disseminated peritoneal disease using a (212)Pb-labeled radioimmunoconjugate targeting HER2. Cancer Biother Radiopharm. 2005;20:557–568. doi: 10.1089/cbr.2005.20.557. [DOI] [PubMed] [Google Scholar]
- 24.Elgqvist J, Andersson H, Back T, Hultborn R, Jensen H, Karlsson B, Lindegren S, Palm S, Warnhammar E, Jacobsson L. Therapeutic efficacy and tumor dose estimations in radioimmunotherapy of intraperitoneally growing OVCAR-3 cells in nude mice with (211)At-labeled monoclonal antibody MX35. J Nucl Med. 2005;46:1907–1915. [PubMed] [Google Scholar]
- 25.Zalutsky MR, McLendon RE, Garg PK, Archer GE, Schuster JM, Bigner DD. Radioimmunotherapy of neoplastic meningitis in rats using an alpha-particle-emitting immunoconjugate. Cancer Res. 1994;54:4719–4725. [PubMed] [Google Scholar]
- 26.McDevitt MR, Ma D, Lai LT, Simon J, Borchardt P, Frank RK, Wu K, Pellegrini V, Curcio MJ, Miederer M, Bander NH, Scheinberg DA. Tumor therapy with targeted atomic nanogenerators. Science. 2001;294:1537–1540. doi: 10.1126/science.1064126. [DOI] [PubMed] [Google Scholar]
- 27.Borchardt PE, Yuan RR, Miederer M, McDevitt MR, Scheinberg DA. Targeted actinium-225 in vivo generators for therapy of ovarian cancer. Cancer Res. 2003;63:5084–5090. [PubMed] [Google Scholar]
- 28.Jaggi JS, Kappel BJ, McDevitt MR, Sgouros G, Flombaum CD, Cabassa C, Scheinberg DA. Efforts to control the errant products of a targeted in vivo generator. Cancer Res. 2005;65:4888–4895. doi: 10.1158/0008-5472.CAN-04-3096. [DOI] [PubMed] [Google Scholar]
- 29.Jaggi JS, Seshan SV, McDevitt MR, LaPerle K, Sgouros G, Scheinberg DA. Renal tubulointerstitial changes after internal irradiation with alpha-particle-emitting actinium daughters. J Am Soc Nephrol. 2005;16:2677–2689. doi: 10.1681/ASN.2004110945. [DOI] [PubMed] [Google Scholar]
- 30.Schier R, Marks JD, Wolf E, Apell G, Wong C, McCartney J, Bookmna M, Huston J, Weiner L, Adams G. In vitro and in vivo characterization of a human anti-c-erB2 single chain Fv isolated from a filamentous pahge antibody library. Immunotechnology. 1995;1:73–81. doi: 10.1016/1380-2933(95)00007-0. [DOI] [PubMed] [Google Scholar]