SUMMARY
While progenitor-restricted factors broadly specify area identities in developing neocortex, the downstream regulatory elements involved in acquisition of those identities in post-mitotic neurons are largely unknown. Here, we identify Bhlhb5, a transcription factor expressed in layers II–V, as a post-mitotic regulator of area identity. Bhlhb5 is initially expressed in a high caudomedial to low rostrolateral gradient that transforms into a sharp border between sensory and rostral motor cortices. Bhlhb5-null mice exhibit aberrant expression of area-specific genes and structural organization in the somatosensory and caudal motor cortices. In somatosensory cortex, Bhlhb5-null mice display post-synaptic disorganization of vibrissal barrels. In caudal motor cortex, Bhlhb5-null mice exhibit anomalous differentiation of corticospinal motor neurons, accompanied by failure of corticospinal tract formation. Together, these results demonstrate Bhlhb5’s function as an area-specific transcription factor that regulates the post-mitotic acquisition of area identities and elucidate the genetic hierarchy between progenitors and post-mitotic neurons driving neocortical arealization.
INTRODUCTION
The tangential plane of the six layered mammalian neocortex is comprised of areas that process cognitive, motor, and sensory information. Neocortical areas are characterized by unique molecular profiles, architecture, and connectivity, and are delineated by sharp boundaries (O'Leary and Nakagawa, 2002). Areal identities are specified in neocortical progenitors by complex interactions between signaling molecules and transcription factors (TFs). For example, the signaling molecule FGF8, secreted by the anterior neural ridge, represses the homeodomain TF Emx2 and the orphan nuclear receptor COUP-TF1 anteriorly, and probably generates their high caudal to low rostral gradients in neocortical progenitors (Fukuchi-Shimogori and Grove, 2001, 2003; Garel et al., 2003).
Targeted deletion of the caudomedially enriched Emx2 results in expansion of rostrolateral areas and contraction of caudomedial areas. A converse phenotype is observed in mice over-expressing Emx2 or null for Pax6, a paired-box TF expressed in a high rostrolateral to low caudomedial gradient (Bishop et al., 2000; Bishop et al., 2002; Hamasaki et al., 2004). Cortex-specific deletion of the caudolaterally enriched COUP-TF1 results in dramatic expansion of rostral areas in the caudal sensory cortex (Armentano et al., 2007). Thus, gradients of TFs along the rostrocaudal and mediolateral axes specify area/positional information in progenitors that is then inherited by post-mitotic progeny as they migrate radially out of the ventricular zone (VZ). This positional information is manifested as area-specific molecular, anatomical, and functional features in post-mitotic neurons (Grove and Fukuchi-Shimogori, 2003; O'Leary and Nakagawa, 2002).
This deep and important work on areal specification of progenitors motivates a fundamental question: What are the TFs in post-mitotic neurons that regulate the acquisition, manifestation, or execution of areal identities specified in progenitors by TFs such as Emx2 (Mallamaci and Stoykova, 2006; O'Leary et al., 2007; O'Leary and Nakagawa, 2002)? In other words, what are the post-mitotic transcriptional regulators that control precise differentiation of diverse neuron populations within distinct neocortical areas? While Emx2 and Pax6 have progenitor-restricted expression (Bishop et al., 2000; Gulisano et al., 1996; Simeone et al., 1992), COUP-TF1 is expressed in both caudolateral progenitors and post-mitotic neurons (Liu et al., 2000). However, cortex specific deletion of COUP-TF1, and gain-of-function studies, suggest that COUP-TF1 has a predominant role in specification of areal identities at the progenitor level (Armentano et al., 2007; Faedo et al., 2007). Several candidates, such as Emx1 and members of the Ten_m family, exhibit differential areal expression in the cortical plate, but either do not regulate, or have undemonstrated roles, in arealization (Bishop et al., 2002; Li et al., 2006; Muzio and Mallamaci, 2003). Here, we identify Bhlhb5 as a post-mitotically expressed TF that functions centrally in regulation and acquisition of areal identity in developing neurons of layers II–V in somatosensory and caudal motor cortices.
Bhlhb5 is a member of the Olig subfamily of the spatiotemporally restricted Class B basic-helix-loop-helix (bHLH) TFs (Bertrand et al., 2002). It is postulated to be a transrepressor, and is implicated in retinogenesis (Feng et al., 2006; Peyton et al., 1996; Xu et al., 2002). Bhlhb5 has not been previously identified as having a function in corticogenesis. Here, we demonstrate the post-mitotic expression of Bhlhb5 selectively in glutamatergic projection neurons of neocortical layers II–V. Midway through cortical neurogenesis, Bhlhb5 expression in the murine cortical plate parallels the high caudomedial to low rostrolateral gradient of Emx2 in progenitors. By birth, Bhlhb5 is expressed in the entire sensory cortex, thereby defining a sharp border between sensory and rostral motor cortices. We thus hypothesized that Bhlhb5 might either suppress rostral areal identities or preferentially impart sensory identities within the sensory domain in layers II–V.
To test these hypotheses, we analyzed area-specific markers and input-output connectivity in Bhlhb5-null mice. We observe a highly stereotypical disruption of gene expression patterns, with ectopic, absent or reduced expression of multiple area-specific genes in the somatosensory and caudal motor cortices. There is also significant disruption of the cytoarchitectonic somatosensory cortical barrels, and aberrant differentiation of caudal corticospinal motor neurons (CSMN) accompanied by failure of corticospinal tract formation, additionally notable in light of human BHLHB5’s mapping to a locus associated with hereditary spastic paraplegia (Hentati et al., 1994; Xu et al., 2002). Taken together, these data indicate that Bhlhb5 is required for acquisition of area-specific properties by post-mitotic neurons of the somatosensory and caudal motor cortices.
RESULTS
Bhlhb5 is Expressed in Post-Mitotic Glutamatergic Projection Neurons of the Neocortex
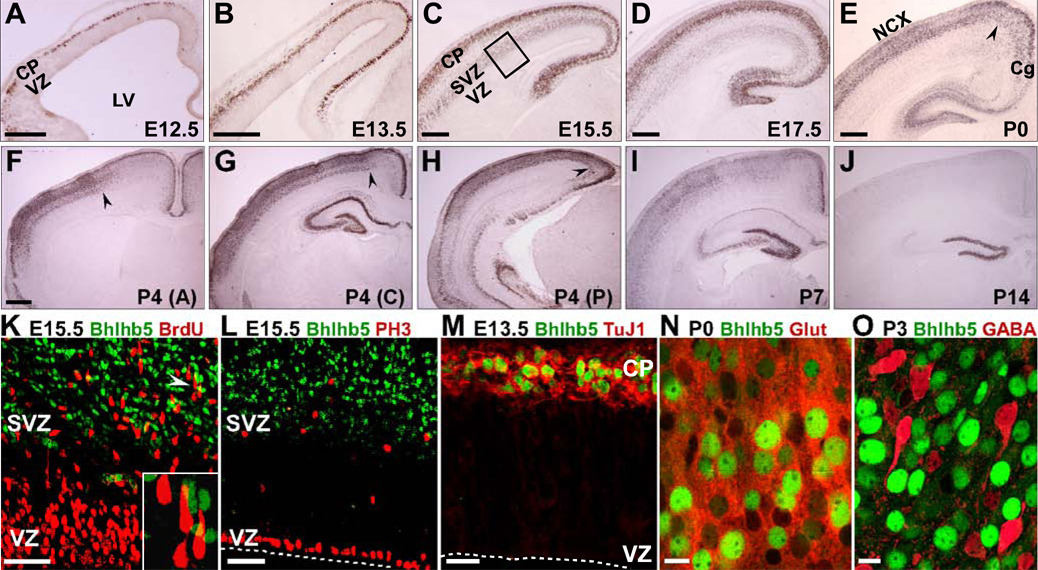
Immunocytochemistry reveals onset of Bhlhb5 expression in the nascent cortical plate of the dorsal telencephalon at embryonic day 12.5 (E12.5) (Figure 1A). During peak production of deep layer neurons between E12.5 and E13.5, Bhlhb5 is restricted to post-migrational neurons, with no expression in the proliferative ventricular zone (VZ) or in migrating neurons (Figures 1A and 1B). Between E15.5 and E17.5, when superficial layer neurons are generated, Bhlhb5 is expressed strongly in the cortical plate and weakly in presumptive migrating neurons and in the subventricular zone (SVZ; Figures 1C, 1D and S1A). Bhlhb5 does not colocalize with proliferating progenitors in S or M phase in either the VZ or the SVZ (Figures 1K, 1L and S1B) and is restricted to post-mitotic glutamatergic projection neurons during neurogenesis (Figure 1M, Figures S1C–S1I and 1N). Bhlhb5 is not expressed in cortical GABAergic interneurons (Figure 1O, S1J and S1K) or their extracortical sites of genesis, the medial and caudal ganglionic eminences (Figures S1L–S1N). Postnatally, Bhlhb5 begins to be downregulated at the junction of the cingulate cortex and neocortex (Figures 1E, 1G and 1H); by postnatal day 4 (P4), downregulation well into the neocortex is observed anteriorly (Figure 1F) with the exception of the most superficial layer. Downregulation continues from medial to lateral, exhibiting a markedly reduced expression by P14 (Figures 1I and 1J). These results are in agreement with previously published regional Bhlhb5 expression analysis at the transcript level (Kim et al., 2002).
Figure 1. Bhlhb5 is Expressed in Post-Mitotic Excitatory Neurons of Developing Neocortex.
(A–J) HRP-immunocytochemistry for Bhlhb5 on coronal brain sections.
(A and B) At E12.5 (A) and E13.5 (B), Bhlhb5 is expressed in the cortical plate (CP) but not the ventricular zone (VZ).
(C and D) At E15.5 (C) and E17.5 (D), Bhlhb5 is strongly expressed in the CP, weakly expressed in the subventricular zone (SVZ), but not in the VZ.
(E–J) Bhlhb5 starts downregulating at the border of neocortex (NCX) and cingulate cortex (Cg) at P0 (E). At P4, downregulation proceeds into NCX anteriorly (F), and becomes more pronounced at the NCX-Cg border posteriorly (G,H). Bhlhb5 starts downregulating throughout the NCX by P7 (I) with markedly reduced expression by P14 (J). Black arrowheads in (E–H) mark extent of downregulation.
(K–M) Bhlhb5 does not colocalize with the S-phase marker BrdU (K; 30 min pulse), or the M-phase marker PH3 (L) in the VZ or SVZ (boxed region in C), but colocalizes with the post-mitotic neuronal marker, Tuj1 (M). Inset in (K) is magnified view of area adjacent to white arrowhead; shows lack of Bhlhb5 and BrdU colocalization in SVZ.
(N) Bhlhb5 is expressed by a subset of glutamatergic neurons.
(O) Bhlhb5 is not expressed by GABAergic interneurons.
LV, lateral ventricle. Scale bars: 250 µm (A–E), 500 µm (F–J), 50 µm (K, L, N and O), 25 µm (M). Inset in (K) not to scale.
Patterned Expression of Bhllhb5 in Neocortical Layers II–V
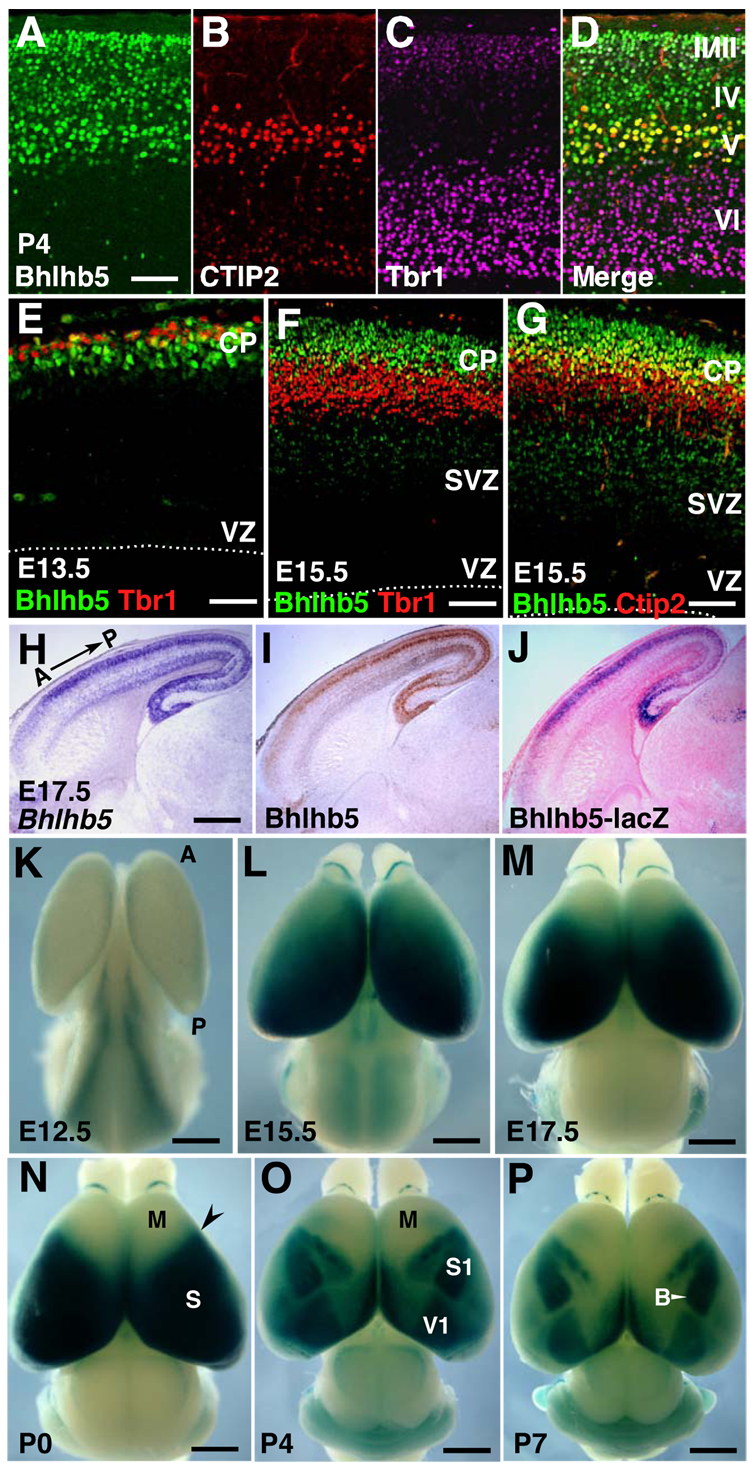
We next evaluated the more precise laminar and cell type-specific expression of Bhlhb5 at P4 via triple immunolabelling with CTIP2, a TF that specifically labels all subcerebral projection neurons (SCPN) including CSMN in layer V (Arlotta et al., 2005), and Tbr1, a TF enriched in layers II–III and in layer VI corticothalamic neurons (Hevner et al., 2001). Using Tbr1 and CTIP2 to delineate both laminar borders and SCPN identity, we find that Bhlhb5 is expressed in layers II, III, IV, and V, and clearly colocalizes with CTIP2+ SCPN of layer V (Figures 2A–2D). Within layer VI, Bhlhb5 is expressed in only very rare scattered Tbr1 negative neurons (Figure 2D). We next examined whether Bhlhb5 is expressed in the same populations earlier in development. During neurogenesis, Tbr1 is restricted to layer VI and subplate (Hevner et al., 2001), and Bhlhb5 does not colocalize with the overwhelming majority of Tbr1+ neurons at E13.5 and E15.5 (Figures 2E and 2F). These data indicate that there is essentially no Bhlhb5 expression in layer VI corticothalamic and subplate neurons. In contrast, many of the presumptive CSMN and other SCPN in developing layer V, identified by high level CTIP2 expression in the cortical plate (Arlotta et al., 2005; Lai et al., 2008; Molyneaux et al., 2005), express Bhlhb5 at E15.5. Bhlhb5 is also expressed in layers superficial to the CTIP2-expressing layer during development (Figure 2G).
Figure 2. Bhlhb5 is Expressed with Both Lamina and Area Specificity.
(A–D) Triple immunolabeling of Bhlhb5 (A) with CTIP2 (layer V) (B), and Tbr1 (layers II, III, VI and subplate) (C), reveals Bhlhb5 expression in layers II, III, IV, and V at P4.
(E–F) Bhlhb5 is not expressed by a majority of Tbr1+ neurons (layer VI and subplate) at E13.5 (E) and E15.5 (F).
(G) Bhlhb5 is expressed in a subset of CTIP2+ neurons (layer V; more superficial) and layers superficial to the CTIP2-expressing layer at E15.5.
(H–J) In situ hybridization (H), immunocytochemistry (I), and X-Gal staining (counterstained with eosin) (J) on adjacent sagittal sections from a Bhlhb5lacZ/+ heterozygote brain, reveals faithful recapitulation of Bhlhb5 mRNA and protein expression by Bhlhb5-lacZ reporter.
(K–P) Dorsal views of X-Gal histochemistry on whole mount brains. (K) At E12.5, Bhlhb5 is expressed highest medially in cingulate cortex and weakly in neocortex. (L) At E15.5, Bhlhb5 is expressed in a high caudomedial to low rostrolateral gradient in neocortex. (M,N) The Bhlhb5 gradient transforms into a sharp border (N; arrowhead) between the rostral motor (M) and sensory (S) cortical domains by P0. (O and P) Bhlhb5 expression is further restricted to primary sensory areas at P4 (O) and P7 (P) such as the primary somatosensory cortex (S1) and primary visual cortex (V1).
VZ, ventricular zone; SVZ, subventricular zone; CP, cortical plate; A, anterior; P, posterior; B, vibrissal barrel field. Scale bars: 50 µm (A–G), 500 µm (H–J), 1 mm (K–P).
Bhlhb5 expression varies strikingly along the A-P axis; there is a precipitous decrease in expression in the rostral cortical plate (Figure 2I). To determine the dynamics of Bhlhb5 expression across all cortical areas, we performed β-galactosidase enzymatic reactions on whole mount brains obtained from Bhlhb5lacZ/+ mice (Figure S2A; Feng et al., 2006). Analysis of adjacent Bhlhb5lacZ/+ brain sections demonstrated that Bhlhb5-lacZ faithfully recapitulates endogenous Bhlhb5 expression at the mRNA and protein levels (Figures 2H–2J).
X-Gal histochemistry at E12.5 reveals that Bhlhb5 expression is highest medially in the cingulate cortex with weak expression in the rest of the cortex (Figure 2K). At E15.5, Bhlhb5 is expressed in a high caudomedial to a low rostrolateral gradient that is strikingly similar to the Emx2 gradient in progenitors (Figure 2L). Between E15.5 and P0, Bhlhb5 expression increases laterally and the gradient gradually transforms into a sharp border between the presumptive rostral motor and sensory domains (Figures 2L–2N). During the first postnatal week, there is a further transformation from homogeneous expression across sensory cortex into discrete areas of high Bhlhb5 expression coincident with the primary sensory areas. At P4 and P7, Bhlhb5 is expressed in primary visual cortex (V1), primary auditory cortex (A1), and distinct primary somatosensory (S1) representations, including the vibrissal barrel field (Figures 2O and 2P). Thus, Bhlhb5 is unique as a TF in distinctly delineating the cortical map at the level of layer IV neurons.
Cell Survival and Cortical Lamination in Bhlhb5-null Mice are Largely Normal
We employed an in vivo loss-of-function approach to investigate Bhlhb5’s role in corticogenesis by crossing Bhlhb5lacZ/+ mice (henceforth referred to as heterozygotes) to obtain Bhlhb5lacZ/lacZ homozygous mutants (henceforth referred to as Bhlhb5-nulls) (Figure S2B). Due to deletion of the entire ORF, we did not expect or detect any Bhlhb5 protein via immunocytochemistry in Bhlhb5-nulls (Figure S2C). Heterozygotes are indistinguishable from wild types, and Bhlhb5-nulls are born at Mendelian frequencies and survive into adulthood.
At birth, Bhlhb5-nulls are similar to wild type mice in size (wt n=9; nulls n=7; p=0.57) but exhibit a 14% and a 40% reduction in bodyweight by P7 (wt n=11; null n=12; p<0.01) and one month (wt n=6; null n=6; p<0.01), respectively (Figure S2D and data not shown). While a 15% reduction in brain weight, and 37% increase in brain weight to body weight ratio is observed at one month in Bhlhb5-nulls, these indices are no different from wild type at P0 and P7. Nonetheless, Bhlhb5-null brains are slightly smaller in volume in the first postnatal week (Figure S5A–S5C’). We performed TUNEL staining on E17.5 and P5 wild type and Bhlhb5-null brain sections to assess apoptotic cell death; this analysis showed no change in the number of TUNEL+ cells in Bhlhb5-nulls versus wild types (data not shown). To evaluate the survival of Bhlhb5-expressing neurons, we performed X-Gal reactions on P5 heterozygous and Bhlhb5-null brain sections at comparative locations along the A-P axis. Bhlhb5-lacZ staining is highly similar in both groups (Figure S3A), with the exception of more intense staining in the nulls with two copies of lacZ, suggesting that the overwhelming majority of Bhlhb5-expressing neurons survive and migrate normally into the cortex in Bhlhb5-null mice. We also assessed cortical lamination by evaluating several lamina specific markers (Figures S3B–S3H). We observed a morphologically normal cortex with six distinct layers in Bhlhb5-nulls. However, there is a subtle and reproducible laminar abnormality in layer V of sensorimotor cortex, with compaction of its sub-layers (Figure S3F–S3H). Thus, overall, Bhlhb5 does not appear to function substantially in cell survival and cortical lamination.
Disruption of Somatosensory and Caudal Motor Chemoarchitecture in Bhlhb5-nulls
The caudomedial high to rostrolateral low gradient of Bhlhb5 at E15.5 that transforms into a sharp border between the sensory and rostral motor cortices by P0 (Figures 2L–2N), leads to consideration of two hypothetical roles during neocortical arealization: 1) Bhlhb5 might suppress rostral motor identity in layers II–V of the sensory cortex; or 2) Bhlhb5 might regulate the acquisition of sensory and caudal motor identities in layers II–V of these cortices. We first evaluated wild type and Bhlhb5-null mice for a broad set of genes (such as TFs, cell adhesion and axon guidance molecules) whose expression demarcates cortical areas in specific layers. We analyzed these between P1 and P5, when cortical layers can be delineated. For simplicity, we denote areas along the A–P axis medially as rostral motor, caudal motor, and occipital cortex, and laterally as rostral motor, somatosensory (parietal), and occipital cortex (see Figure 5A).
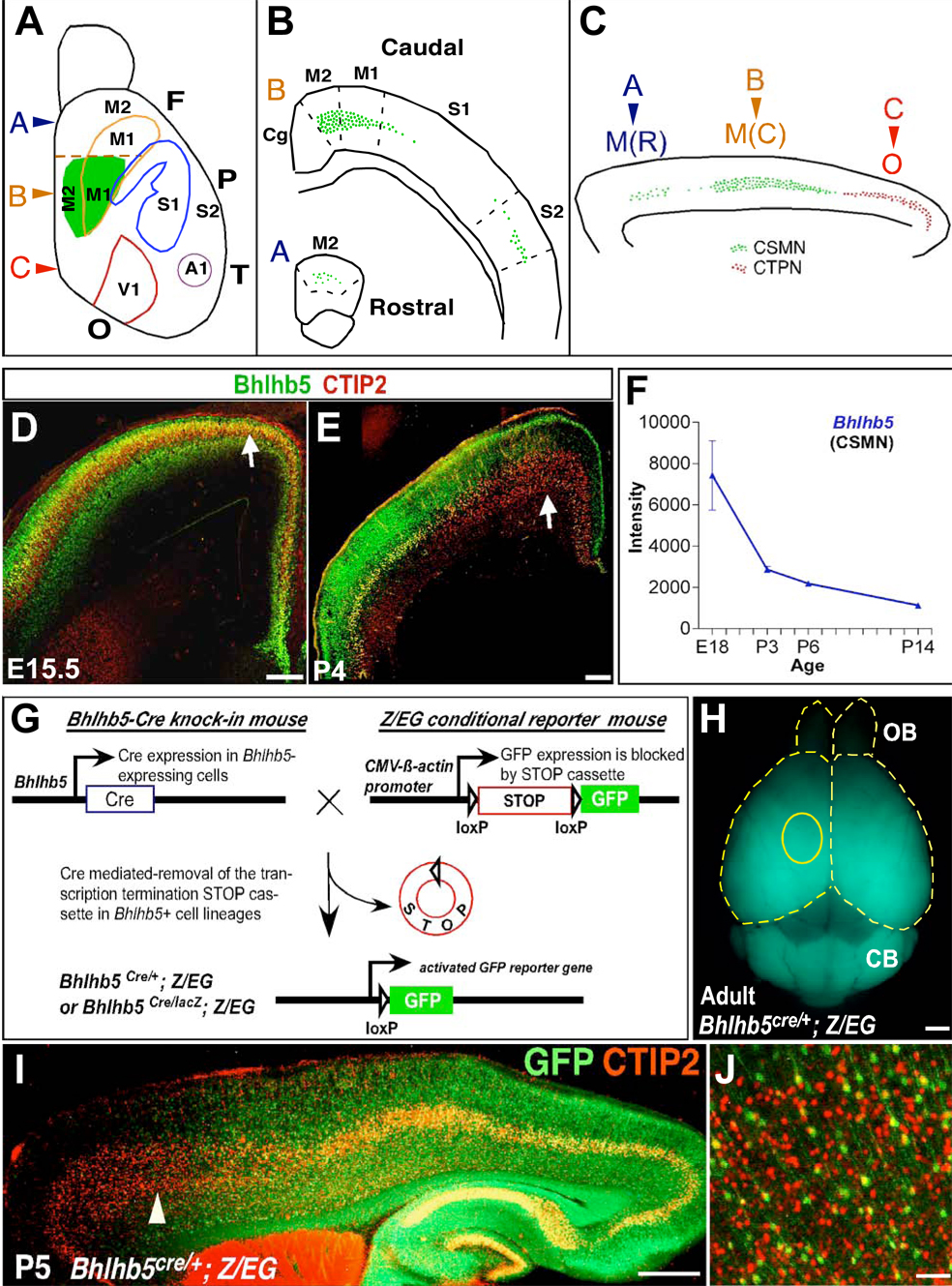
Figure 5. Bhlhb5 Genetic Lineage Tracing Demonstrates Differential Bhlhb5 Expression in Subcerebral Projection Neuron Subtypes along the Rostro-Caudal Axis.
(A–C) Schematic of the areal distribution of CSMN and CTPN. Medially, along the rostrocaudal axis, fewer CSMN (green) are located in rostral motor cortex (M(R)), more CSMN are located in caudal motor cortex (M(C)), and CTPN (red) are located in occipital cortex (O).
(D and E) Bhlhb5 colocalizes with SCPN marker, CTIP2, at E15.5 but not P4 in caudal motor cortex (arrows).
(F) Microarray expression profiling of Bhlhb5 in purified CSMN shows steep decline in expression between E18 and P14.
(G) Schematic of the genetic lineage tracing strategy to permanently mark cells of Bhlhb5+ lineage via continued GFP reporter production.
(H) Dorsal view of whole mount Bhlhb5cre/+; Z/EG/+ heterozygote adult brain reveals cumulative spatiotemporal expression of Bhlhb5 with high GFP fluorescence caudally and low fluorescence rostrally in the neocortex. Yellow circle delineates approximate location of caudal motor cortex.
(I and J) Double immunolabeling of GFP (Bhlhb5) and CTIP2 on P5 sagittal section of Bhlhb5cre/+; Z/EG/+ heterozygote brain. Bhlhb5 is expressed by a majority of presumptive CTPN in occipital cortex, CSMN in caudal motor cortex but not CSMN in rostral motor cortex. (J) Magnified view (arrowhead in I) shows very few GFP+ CTIP2+ neurons in rostral motor cortex.
F, Frontal cortex; P, Parietal cortex, T, Temporal cortex; O, Occipital cortex; M1, primary motor area; M2, secondary motor area; M(R), rostral motor cortex; M(C), caudal motor cortex; S1, primary somatosensory area; S2, secondary somatosensory area; A1, primary auditory area; V1; primary visual area; OB, olfactory bulb; CB, cerebellum. Scale bars: 200 µm (D,E), 1 mm (H), 500 µm (I), 50 µm (J).
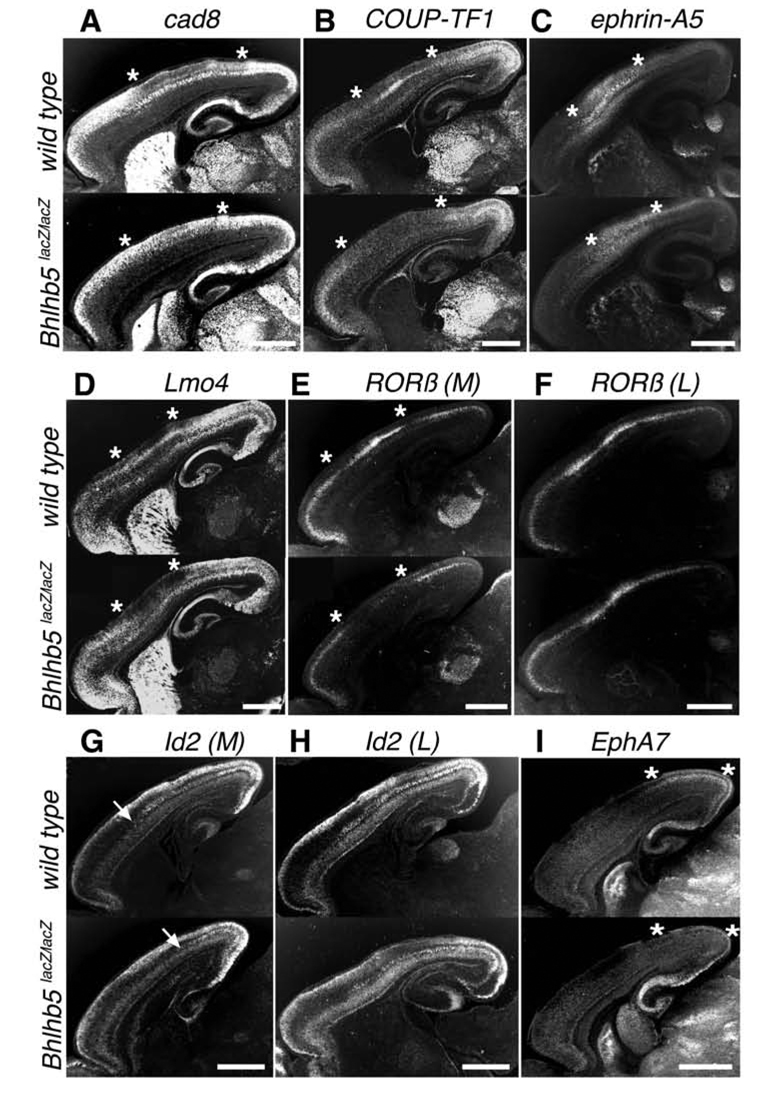
A directional shift of gene expression patterns is often indicative of re-specification of area identity, whereas a disruption is often indicative of an anomaly in acquisition of appropriate area identity (see Figure 9). In Bhlhb5-nulls, there is highly consistent disruption of normal patterns of area-specific gene expression that is most severe in somatosensory and caudal motor cortices. For example, in wild type neocortex, Cadherin 8 is expressed in layers II–III of the rostral motor and occipital, but not somatosensory cortex (Bishop et al., 2002; Suzuki et al., 1997). In sharp contrast, there is ectopic expression of Cadherin 8 in layers II–III of somatosensory cortex in Bhlhb5-nulls, resulting in a nearly homogeneous expression across all areas of the cortex (Figure 3A). The orphan nuclear receptor COUP-TF1 exhibits an abrupt decline in expression at the rostral motor-somatosensory and somatosensory-occipital transitions in layer IV of wild type mice (Liu et al., 2000; Zhou et al., 2001). While Bhlhb5-nulls exhibit largely normal COUP-TF1 expression in rostral motor and occipital cortices, there is a striking lack of expression in somatosensory cortex (Figure 3B). Ephrin-A5, a GPI-linked cell surface axon guidance molecule normally enriched in multiple layers of the somatosensory cortex (Bishop et al., 2002; Gao et al., 1998) exhibits quite decreased expression in layers IV–V in Bhlhb5-nulls (Figure 3C). Bhlhb5-nulls also exhibit a striking loss of the somatosensory expression of Lmo4 (Figure 3D), a transcriptional regulator normally uniformly expressed in layer IV (Bulchand et al., 2003).
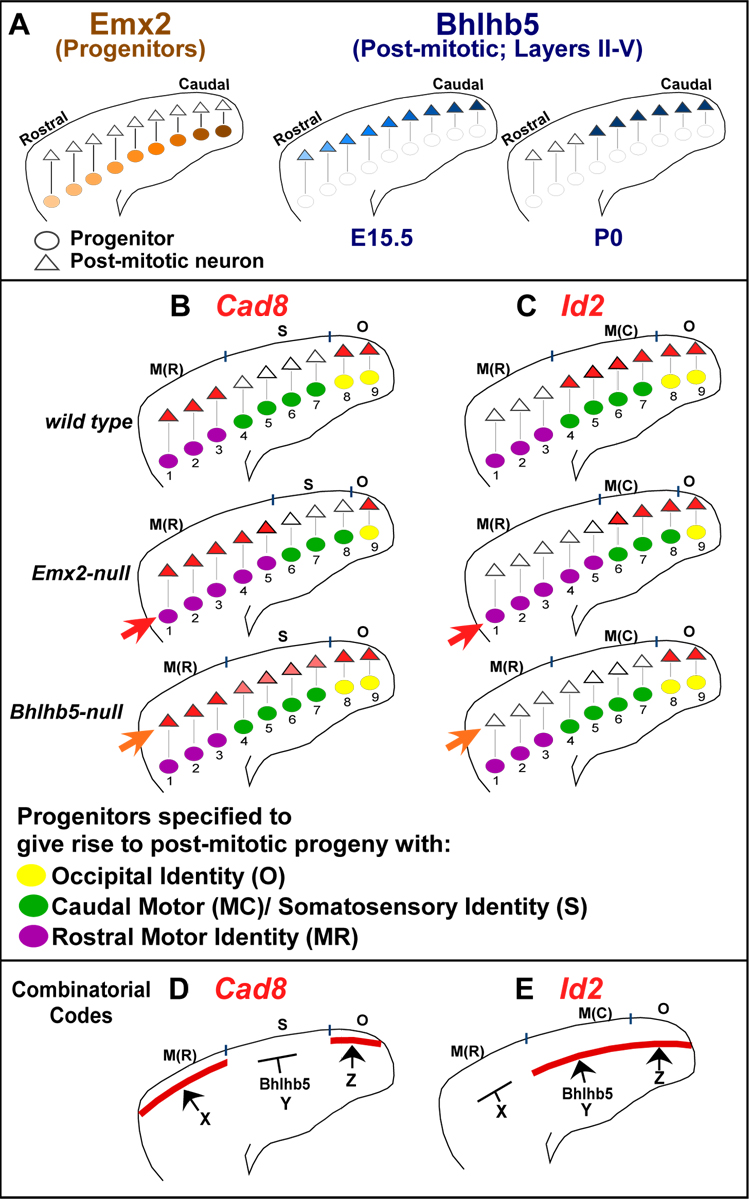
Figure 9. Distinct Progenitor and Post-Mitotic Effects on Specification and Acquisition of Area Identities.
(A) Emx2 is expressed in a high caudal to low rostral gradient in progenitors. Bhlhb5’s post-mitotic high caudal to low rostral gradient at E15.5 resolves into a sharp border between rostral motor and caudal sensory cortices by P0. Post-mitotic neurons inherit areal information specified in progenitors due to radial migration.
(B–C) The same molecular-genetic markers can indicate roles of a regulatory gene in specification or acquisition of area identities.
(B) Cad8 is normally expressed in layers II/III of rostral motor and occipital cortices, but not somatosensory cortex. In Emx2-nulls, a caudal shift of Cad8 expression pattern in the cortical plate (CP) suggests respecificiation of areal identity in a caudal direction, in progenitors (red arrow). In Bhlhb5-nulls, specification of areal identity is unaltered in progenitors (see Figure S5). In Bhlhb5-nulls, disruption of Cad8 pattern in CP (orange arrow) due to ectopic expression in somatosensory cortex (pink) suggests failure in acquisition of one somatosensory property in layers II/III.
(C) Id2 is expressed in layer V of the occipital and caudal motor cortices, but not rostral motor cortex. In Emx2-nulls, a caudal shift of Id2 expression pattern in CP suggests re-specification of areal identity in a caudal direction, in progenitors (red arrow). In CP (orange arrow) of Bhlhb5-nulls, disruption of Id2 expression in caudal motor cortex (but not occipital cortex) suggests a failure in acquisition of one caudal motor property in layer V.
(D–E) Combinatorial codes of regulatory molecules for generating abrupt areal borders of patterned genes in neocortex.
(D) Somatosensory suppression by Bhlhb5 generates Cad8 rostral motor-somatosensory and somatosensory-occipital transitions. Regulators of Cad8 expression in rostral motor and occipital cortex are currently unknown.
(E) Bhlhb5 generates the rostral motor-caudal motor Id2 transition, by controlling Id2 expression in caudal motor cortex. Regulators of Id2 expression in occipital cortex and putative repressors in rostral motor cortex are currently unknown.
M(R), rostral motor cortex; M(C), caudal motor cortex; S, somatosensory cortex; O, occipital cortex; X, Y, Z are undiscovered transcriptional regulators.
Figure 3. Loss of Bhlhb5 Function Disrupts Chemoarchitecture in Somatosensory and Caudal Motor Cortices.
In situ hybridization for various area-specific genes on sagittal wild type and Bhlhb5lacZ/lacZ (null) brain sections. Asterisks delineate area of gene expression change. In Bhlhb5-nulls, there is:
(A) Ectopic expression of Cadherin 8, in layers II/III of somatosensory cortex.
(B) Lack of COUP-TF1 expression in layer IV of somatosensory cortex.
(C) Decreased expression of ephrin-A5 in layers IV/V of somatosensory cortex.
(D) Lack of Lmo4 expression in layer IV of somatosensory cortex.
(E,F) Lack of RORβ expression in layer IV of caudal motor cortex (E), medially (M), but not laterally (L) in somatosensory cortex (F).
(G,H) Lack of Id2 expression in layer IV of caudal motor cortex (G), medially (G), but not laterally in somatosensory cortex (H).
(I) Lack of EphA7 expression in layer IV of occipital cortex.
Ages: P3 (A, C, E, F, G, H, I); P5 (B,D). For each gene, wt n=3; Bhlhb5-nulls n=3. Scale bars: 1 mm.
Intriguingly, Bhlhb5 regulates the expression of some area-specific markers selectively along the mediolateral axis. For example, RORβ, a layer IV enriched orphan nuclear receptor is expressed discontiguously across areas (Bishop et al., 2002; Park et al., 1997). While, RORβ’s expression is unchanged in the rostral motor and occipital cortex of Bhlhb5-nulls, there is a lack of expression medially in the caudal motor cortex, but not laterally in the somatosensory cortex in Bhlhb5-nulls (Figures 3E and 3F). Id2, an HLH TF, marks the presumptive border between the sensory and rostral motor cortex in layer V (Bulfone et al., 1995; Rubenstein et al., 1999). While Id2 expression persists in the occipital cortex of Bhlhb5-nulls, we observe a lack of expression medially in the caudal motor cortex, but not laterally in the somatosensory cortex in Bhlhb5-nulls (Figures 3G and 3H). EphA7 receptor tyrosine kinase is expressed in layer IV of the occipital cortex (Bishop et al., 2002; Mori et al., 1995). In Bhlhb5-nulls, there is striking absence of EphA7 in layer IV of the occipital cortex (Figure 3I). The neurotrophin receptor p75, expressed in layer VI and subplate caudally (Mackarehtschian et al., 1999), and the transcription factor Etv5, normally expressed in multiple layers of the rostral motor cortex and delineating the presumptive transition between the rostral motor and somatosensory cortex (Rash and Grove, 2006), were evaluated to investigate the specificity of Bhlhb5 effects. As expected, there were no changes in the expression patterns of p75 or Etv5 in Bhlhb5-nulls (Figure S4A and S4B), consistent with the lack of normal coincident Bhlhb5 expression with these two markers. Taken together, these data strongly indicate that loss of Bhlhb5 results in a stereotypical disruption of molecular identity in the caudal motor and somatosensory cortices.
Bhlhb5 Does Not Suppress Rostral Motor Identity in the Sensory Domain
The consistent disruption, but not caudal shift, of gene expression patterns in layers II–V observed in Bhlhb5-nulls suggested a novel role for Bhlhb5 in the acquisition of area-specific properties, rather than suppression of rostral motor identity in the caudal sensory domain. To investigate this hypothesis further, we evaluated the cortical area map in Bhlhb5-nulls by two methods: 1) Taking advantage of postnatal Bhlhb5-lacZ expression in primary areas, X-Gal reactions were performed on P6 control (Bhlhb5lacZ/+; n=6) and Bhlhb5-null (Bhlhb5lacZ/lacZ; n=5) whole mount brains (Figures S5A and S5A’); 2) HRP-immunohistochemistry for serotonin transporter (SERT) on P7 wild type (n=7) and Bhlhb5-null (Bhlhb5lacZ/lacZ; n=7) tangential cortical sections (Figures S5B and S5B’). The oblique line of primary somatosensory (S1) representations of fore limbs, trunk, etc. demarcates the rostral motor-sensory border with the rostral motor and sensory domains, rostral and caudal to it, respectively. X-Gal (p=0.96) and SERT (p=0.16) revealed no change in the ratio of the rostral motor to sensory domain surface area in Bhlhb5-nulls (Figure S5D). Thus, the rostral motor domain does not expand at the expense of the caudal sensory domain in Bhlhb5-nulls, supporting this hypothesis that Bhlhb5 functions in acquisition of area-specific properties, not in specifying area/positional identities.
This observation is further supported by the expansion (not reduction, as would be predicted by the alternate hypothesis of an areal specifying role) in the proportional sizes of some primary sensory areas, supporting a role in acquisition and refinement of sub-areal properties and later-stage differentiation. For example, a 20% (p<0.0001) and a 14% (p=0.04) increase in the ratio of primary visual area (V1) to total cortical surface area is observed in Bhlhb5-nulls via X-Gal and SERT, respectively (Figure S5E). To investigate S1, we quantified the area of the posteromedial barrel subfield (PMBSF), which represents the large facial vibrissae. SERT revealed a 26% increase (p<0.0001) in the ratio of PMBSF to total cortical surface area in Bhlhb5-nulls (Figure S5F). To corroborate this barrel field expansion via an independent marker, tangential sections through P7 wild type (n=7) and Bhlhb5-null (n=6) cortices were analyzed with cytochrome oxidase histochemistry (Wong-Riley and Welt, 1980) (Figures S5C and S5C’). Bhlhb5-nulls exhibited a similar 23% increase (p’0.0001) in the ratio of PMBSF to total cortical surface area (Figure S5F). Since the motor to sensory ratio is unchanged in Bhlhb5-nulls, the V1 and PMBSF expansions in Bhlhb5-nulls appear to occur within the sensory domain at the expense of other primary, secondary and/or association areas (irregularly shaped, and thus not accurately directly quantifiable). Additionally, the expansions of V1 and PMBSF may partially reflect an error in TCA pathfinding in layer IV (perhaps due to disruption of expression of genes including Lmo4 (Kashani et al., 2006)) rather than a re-specification of progenitor areal identity. Consistent with this interpretation, no changes are seen in the gradients of TFs that specify area identities – Emx2, Pax6 and COUP-TF1 – in E13.5 Bhlhb5-nulls (Figures S5G–S5I).
Post-Synaptic Disorganization of Vibrissal Barrels in Bhlhb5-nulls
While some known molecular markers and cortical maps are excellent assays to examine initial specification of areal identities, indicators to evaluate acquisition of and control over areal differentiation are largely unknown. Because there is severe disruption of areal gene expression laterally in somatosensory cortex and medially in caudal motor cortex in Bhlhb5-nulls, we further investigated the identities of these areas at an anatomical level. The vibrissal barrels are a highly reproducible anatomical marker for S1 in layer IV. Each barrel has a cell-dense barrel wall comprised of layer IV neurons and a cell-sparse barrel ‘hollow’ occupied by TCA’s from the ventroposteromedial (VPM) nucleus of the dorsal thalamus. The layer IV neurons direct their dendrites centrally into the barrel hollows where they synapse with VPM axon terminals. Barrel walls are separated from each other by septa (see Figures 4E–4G) (Woolsey et al., 1975; Woolsey and Van der Loos, 1970).
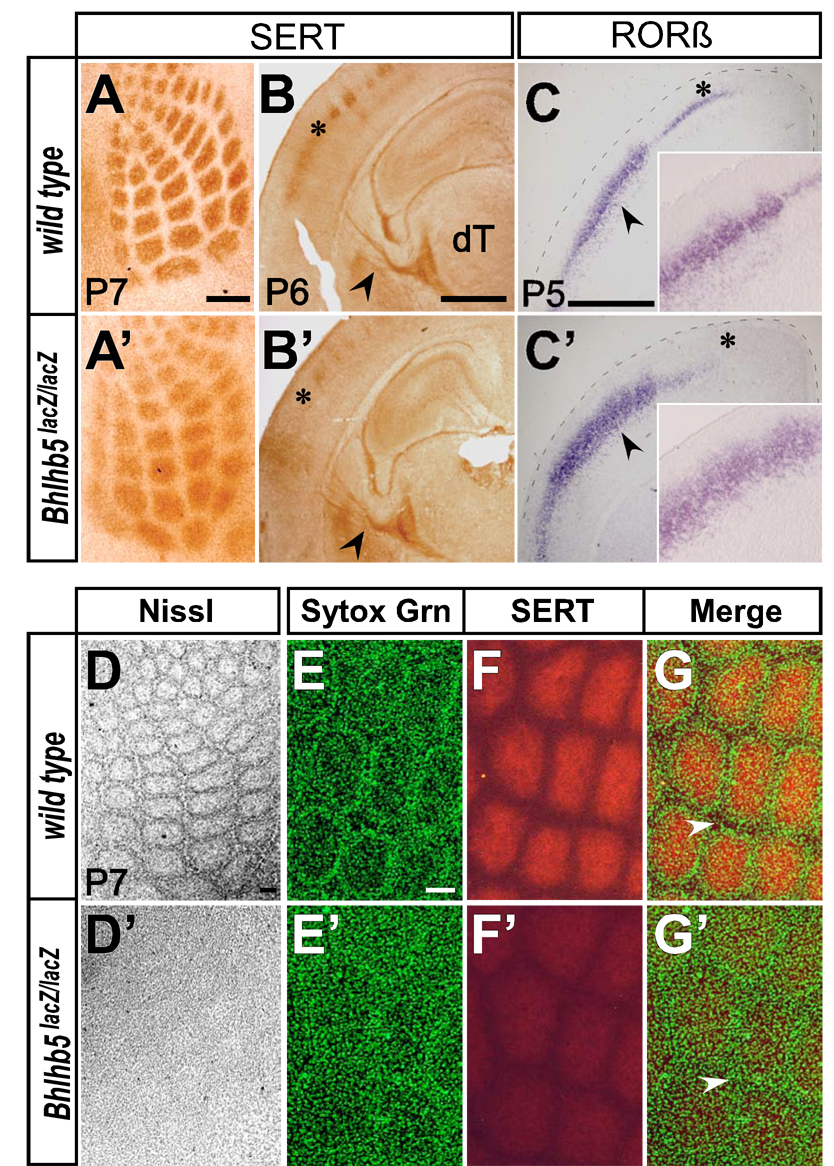
Figure 4. Loss of Bhlhb5 Function Disrupts Post-Synaptic Vibrissal Barrels in Somatosensory Cortex.
(A,A’) HRP-immunohistochemistry for SERT on P7 tangential cortical sections. Thalamocortical afferents segregate into vibrissal specific “hollows”, but with less intense staining and indistinct borders in Bhlhb5-nulls (wild type n=7; Bhlhb5-nulls n=7).
(B,B’) HRP-immunohistochemistry for SERT on P6 coronal sections reveals normal thalamocortical afferents in the internal capsule (black arrowheads), but not in barrel cortex (asterisks) of Bhlhb5-nulls (wild type n=3; Bhlhb5-nulls n=3).
(C,C’) In situ hybridization for RORβ on P5 coronal sections reveals absent expression medially in caudal motor cortex (asterisks), but disorganized expression laterally in the barrel region of the somatosensory cortex (black arrowheads; magnified views in insets) suggesting a post-synaptic disruption of vibrissal barrels in layer IV of Bhlhb5-nulls (wild type n=3; Bhlhb5-nulls n=3).
(D,D’) Nissl staining on tangential sections confirms robust disruption of cytoarchitectonic barrels with only a faint vibrissal pattern in Bhlhb5-nulls (wild type n=3; Bhlhb5-nulls n=3).
(E–G and E’–G’) Confocal microscopy on SERT immunolabeled sections counterstained with Sytox Green nucleic acid stain (Sytox Grn) reveals near absence of septa (white arrowheads) in Bhlhb5-nulls (wild type n=3; Bhlhb5-nulls n=3).
Scale Bars: 250 µm (A,A’), 1 mm (B,B’,C,C’), 100 µm (D–G and D’–G’). Insets in (C,C’) not to scale.
Cytochrome oxidase histochemistry in Bhlhb5-nulls reveals distinct vibrissal patterned barrels, though with slightly weaker intensity than in controls (Figures S5C and S5C’). Cytochrome oxidase, a mitochondrial enzyme, is present both presynaptically, in the TCA axon terminals, and post-synaptically, in the dendrites of layer IV neurons (Welker, 1976), making it difficult to distinguish the underlying defect with cytochrome oxidase staining alone. In contrast, serotonin transporter (SERT) aids visualization of the barrels specifically at the pre-synaptic level of VPM axonal terminals (Bruning and Liangos, 1997; Lebrand et al., 1996; Maier et al., 1999). SERT immunohistochemistry reveals vibrissal specific barrel “hollows” in Bhlhb5-null cortices that are less intensely stained and have indistinct borders (Figures 4A and 4A’). While the decrease in intensity of SERT labeling might be due to down regulation of the transporter on TCA axon terminals, the pattern of SERT staining clearly demonstrates the segregation of TCAs into vibrissal specific “hollows”. The segregation of TCAs into only slightly perturbed vibrissal “hollows” was predicted, since it is known that TCA axons segregate into rudimentary but topologically correct pattern in the subplate and layer VI, where Bhlhb5 is not expressed (Figure 2D), before extending axons radially to layer IV (Agmon et al., 1995; Agmon et al., 1993; O'Leary et al., 1994).
Bhlhb5’s expression in layer IV (Figure 2D), coupled with the disorganized RORβ expression in Bhlhb5-nulls (layer IV marker; Figures 4C and 4C’) in the barrel region of the cortex, motivated us to examine the post-synaptic barrel organization. While Nissl staining of tangential sections at P7 reveals the cytoarchitectonic barrels with distinct cell-dense walls and cell-sparse hollows in wild type (Figure 4D; n=3), a severe disruption with only an extremely faint vibrissal pattern is observed in Bhlhb5-nulls (Figure 4D’; n=3). To more deeply investigate this post-synaptic layer IV neuronal disorganization, we performed confocal microscopy on tangential sections immunolabeled for SERT (pre-synaptic) and counterstained with Sytox Green nucleic acid stain (post-synaptic) (Figures 4E–4G and 4E’–4G’). There is a striking disruption and near absence of septa in Bhlhb5-nulls, indicating a severe disruption of normal layer IV neuronal organization and aggregation. SERT immunohistochemistry reveals largely normal TCAs in the internal capsule in Bhlhb5-nulls (Figure 4B and 4B’). Taken together, along with the finding that Bhlhb5 is not expressed in any nucleus of the dorsal thalamus (including VPM) during development (Figure S1O–S1T), these data indicate that the post-synaptic disruption of barrels in Bhlhb5-nulls is autonomous to the cortex, and not due to abnormalities in the TCAs of dorsal thalamic projection neurons.
Bhlhb5 Regulates the Molecular Differentiation of Caudal CSMN and their Efferent Projections
We next investigated the disruption of areal identity in Bhlhb5-nulls at the level of projection neuron subtypes. We focused on subtypes of subcerebral projection neurons (SCPN) in layer Vb because 1) specification, initial differentiation and early survival of SCPN is autonomous to the cortex and independent of their distant targets in brainstem and spinal cord; 2) CTIP2 immunocytochemical analysis (labels all SCPN) indicates the essentially normal survival of SCPN in Bhlhb5-null mice (Figure S3G); 3) their molecular differentiation has been analyzed in substantial depth recently (Arlotta et al., 2005; Chen et al., 2005a; Chen et al., 2005b; Lai et al., 2008; Molyneaux et al., 2005; Molyneaux et al., 2007; Ozdinler and Macklis, 2006).
Two principal SCPN subtypes are CSMN and corticotectal projection neurons (CTPN), which send axons to the superior colliculus in the midbrain. CTPN are located caudally in the occipital cortex. The majority of CSMN are located in primary (M1) and secondary (M2) motor areas in the frontal cortex, while smaller numbers are found in the primary (S1) and secondary somatosensory (S2) areas in the parietal cortex. Within motor areas, more CSMN are located medial to S1 (termed caudal motor cortex), with fewer rostral to S1 (termed rostral motor cortex) (Arlotta et al., 2005; Donoghue and Wise, 1982; Polleux et al., 2001). Thus, medially along the rostrocaudal axis, fewer CSMN are located in rostral motor cortex, more CSMN are located in caudal motor cortex, and CTPN are located in occipital cortex (summarized in Figures 5A–C).
Bhlhb5 is expressed consistently at low levels in rostral motor cortex, with temporally regulated expression in caudal motor cortex, high at E15.5, followed by a postnatal decline (Figures 2L, 2O and 2P). This is likely the result of mediolateral downregulation of Bhlhb5 (Figure 1F) and is reflected in colabeling of Bhlhb5 with CTIP2 at E15.5 but not at P4 medially (Figures 5D and 5E), microarray analysis of Bhlhb5 in purified CSMN decreasing over time (Figure 5F), and absence of Bhlhb5 immunolabeling in retrogradely labeled medial CSMN in P6 caudal motor cortex (Figure S6).
To most rigorously investigate Bhlhb5 expression in SCPN along the rostrocaudal axis medially, we employed lineage tracing, a strategy to permanently mark neurons even transiently expressing Bhlhb5, by triggering continued GFP reporter production via a Cre–recombinase mediated excision of triple transcription termination/polyadenylation signal sequences upstream of GFP (Figure 5G). Bhlhb5cre/+ knock-in mice were generated and crossed with conditional GFP reporter Z/EG mice (Novak et al., 2000) to obtain Bhlhb5cre/+; Z/EG/+ heterozygotes (Figures S2A and S2B). Bhlhb5 directed GFP signal in the neocortex displays high fluorescence in caudal sensory cortex and low fluorescence in rostral motor cortex (Figure 5H and Figure S7A). This accurately reflects Bhlhb5’s cumulative spatiotemporal expression. Double immunolabeling of GFP and CTIP2 at P5 indicates that Bhlhb5 is expressed in most presumptive CTPN in occipital cortex and in CSMN located in caudal motor cortex, but not in CSMN in rostral motor cortex (Figure 5I). The presence of a few CTIP2+ GFP+ neurons in rostral motor cortex (Figure 5J) is likely due to the initial gradient of Bhlhb5 combined with high sensitivity of the excision event to low Cre-recombinase activity.
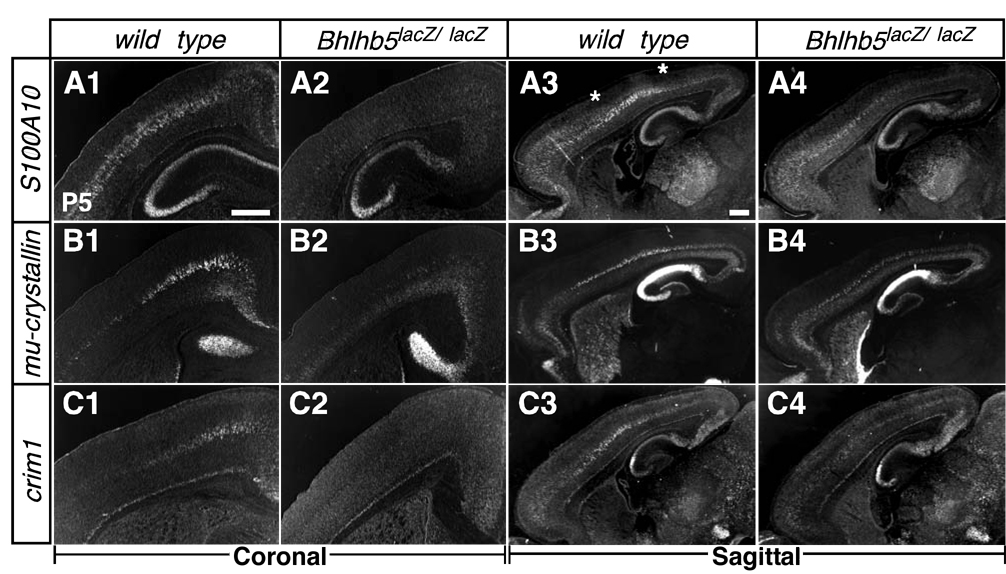
Taken together, these data suggested the hypothesis that Bhlhb5 is required for acquisition of CTPN fate by SCPN in the occipital cortex, and for CSMN fate by SCPN in caudal but not rostral motor cortex. We tested this hypothesis by evaluating several genes differentially expressed in distinct SCPN subtypes. The expression of S100A10, encoding a calcium binding protein expressed broadly in layer V SCPN, including CSMN (Arlotta et al., 2005), is drastically reduced in somatosensory and caudal motor cortices (Figures 6A1–6A4), without change in rostral motor cortex. The thyroid hormone binding protein Mu-crystallin (Figures 6B1–6B4) and the transmembrane BMP regulator Crim1 (Figures 6C1–6C4), both specifically expressed in SCPN within neocortex (Arlotta et al., 2005), exhibit dramatically decreased levels of expression in caudal motor cortex without significant changes in rostral motor cortex. Ten_m3, a type II transmembrane glycoprotein expressed in CTPN in occipital cortex (Leamey et al., 2007), is unaltered in Bhlhb5-nulls, consistent with largely preserved occipital gene expression observed in the absence of Bhlhb5 function (Figure S4C). Taken together, these data indicate that Bhlhb5 regulates the acquisition of important SCPN and CSMN molecular identity in caudal but not rostral motor cortex.
Figure 6. Loss of Bhlhb5 Function Results in Loss of SCPN and CSMN Molecular Features in Caudal Motor Cortex.
In situ hybridization on coronal (20 µm thick) and sagittal (30 µm thick) brain sections of P5 wild type and Bhlhb5lacZ/lacZ (nulls) for subcerebral projection neuron markers, (A1–A4) S100A10, (B1–B4) mu-crystallin and (C1–C4) Crim1, reveals markedly reduced expression of these markers in caudal but not rostral motor cortex (wild type n=3; Bhlhb5-nulls n=3). Scale bars: 500 µm.
CSMN in Caudal Motor Cortex Incorrectly Target their Axons in Bhlhb5-null Mice
To investigate whether aberrant molecular differentiation of CSMN in caudal motor cortex in Bhlhb5-nulls affects their axonal targeting to the spinal cord, the most critical and defining CSMN characteristic, we examined the trajectory of the corticospinal tract. CSMN and other SCPN axons descend from the cortex into the internal capsule, and then through the cerebral peduncle into the midbrain and pons. CSMN axons travel through the pyramidal tract in the ventral medulla, and decussate before descending within the dorsal funiculus of the spinal cord. Initially, all SCPN axons extend transiently toward to the spinal cord, but only CSMN retain projections to it (Molyneaux et al., 2007; O'Leary and Koester, 1993; Polleux et al., 2001; Weimann et al., 1999).
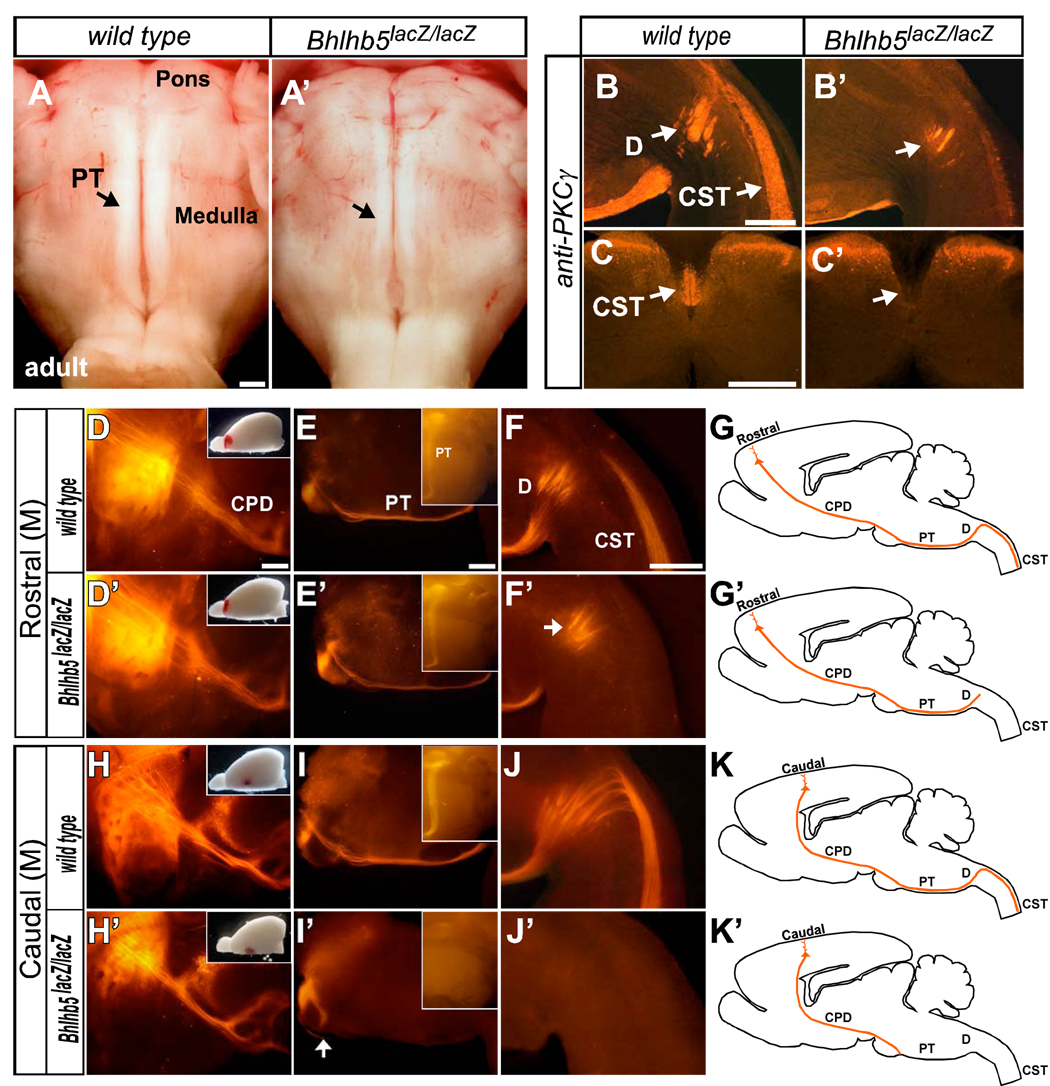
Strikingly, whole mount preparations of adult brainstem reveal dramatic reduction in the size of the medullary pyramidal tract in Bhlhb5-nulls (n=5), compared with wild types and heterozygotes (n=5), (Figures 7A and 7A’). In contrast, proximally at P0, L1-CAM immunohistochemistry (Fujimori et al., 2000) shows no abnormality in the internal capsule or cerebral peduncle in Bhlhb5-nulls (data not shown). Intriguingly, while a small number of fibers of the dramatically reduced pyramidal tract enter the pyramidal decussation in the medulla, as seen in whole mounts and with immunostaining for PKCγ, a late-postnatal corticospinal tract marker (Mori et al., 1990), there is an absence of the spinal portion of the corticospinal tract in Bhlhb5-nulls (Figures 7B, 7B’, 7C and 7C’).
Figure 7. Rostral and Caudal CSMN have Distinct Failures of Spinal Cord Targeting.
(A and A’) Ventral views of whole mount adult brainstem show dramatic reduction of the medullary pyramidal tract (PT) in Bhlhb5lacZ/lacZ (null) adults (wild type n=5; Bhlhb5-nulls n=5).
(B–C’) PKCγ immunohistochemistry reveals CSMN axons in the medullary pyramidal decussation (B and B’) but not in the ventral dorsal funiculus of the spinal cord (C and C’), suggesting loss of the spinal segment of the corticospinal tract (CST) in Bhlhb5-null adults (wild type n=5; Bhlhb5-nulls n=5).
(D–G’) DiI anterograde tracing of the CST from P10 rostral motor cortex (wild type n=4; Bhlhb5-nulls n=4). Sagittal views show DiI labeled axons entering (D and D’) the cerebral peduncle, (E and E’) medullary pyramidal tract, and (F and F’) pyramidal decussation, but not in the ventral dorsal funiculus of the spinal cord in Bhlhb5-nulls. Thus, rostral CSMN axons stop at the pyramidal decussation in Bhlhb5-nulls (G and G’; schematics).
(H–K’) DiI anterograde tracing of the CST from P10 caudal motor cortex (wild type n=3; Bhlhb5-nulls n=3). DiI labeled axons enter (H and H’) the cerebral peduncle, but (I and I’) stop at the base of the pons with no fibers entering the medullary pyramidal tract, (J and J’) pyramidal decussation, or ventral dorsal funiculus of the spinal cord. Thus, caudal CSMN axons do not extend beyond the base of the pons in Bhlhb5-nulls (K and K’; schematics).
Insets in: (D,D’,H,H’) show DiI injection site; (E,E’,I,I’) are ventral views of whole mount brainstem.
CPD, cerebral peduncle; PT, medullary pyramidal tract; D, decussation; CST, spinal segment of the corticospinal tract.
Scale bars: 500 µm; Insets not to scale.
To further investigate this interesting and complex phenotype, we assessed: 1) rostral vs caudal CSMN axonal targeting to the spinal cord; 2) Bhlhb5-expressing CSMN axonal targeting to the spinal cord; 3) whether the reduced supraspinal pyramidal tract and absent intraspinal corticospinal tract in Bhlhb5-null adults, is a function of either aberrant developmental targeting or subsequent degeneration of CSMN axons. We first performed anterograde tracing of the corticospinal tract via injections of 1’-dioctadecyl-3,3,3’,3’-tetra-methylindocarbocyanine perchlorate (DiI ) at rostral (n=4 per group) and caudal (n=3 per group) locations in the motor cortex of wild type and Bhlhb5-null mice at P10. In Bhlhb5-nulls, DiI labeled CSMN axons from rostral motor cortex pass through the internal capsule, cerebral peduncle, and brainstem pyramidal tract but stop at the medullary pyramidal decussation, with none descending into the ventral dorsal funiculus of the spinal cord (Figures 7D–7G and 7D’–7G’). Even more strikingly, DiI labeled axons from the caudal motor cortex in two of three Bhlhb5-nulls passed through the internal capsule, and cerebral peduncle, but stop at the base of the pons, with no fibers entering the medullary pyramidal tract, pyramidal decussation, or ventral dorsal funiculus (Figures 7H–7K and 7H’–7K’). In the third Bhlhb5-null, very few DiI labeled axons from caudal motor cortex entered the medullary pyramidal tract, though not as far as the pyramidal decussation (data not shown). Thus, the internal capsule and cerebral peduncle appear normal in Bhlhb5-nulls; axons from both rostral and caudal motor areas descend through them. In striking contrast, the failure of caudal CSMN to extend axons into the medullary pyramidal tract results in its substantially reduced size in Bhlhb5-nulls, and confirms that Bhlhb5 is required for proper CSMN differentiation.
To further investigate axonal outgrowth and targeting of Bhlhb5-expressing CSMN, we performed genetic axonal tracing, taking advantage of GFP diffusion throughout the entire length of CSMN axons (Figures S7B and S7C). To visualize Bhlhb5-expressing CSMN axons in Bhlhb5-null mice, we crossed Bhlhb5cre/+; Z/EG/+ and Bhlhb5lacz/+ heterozygotes to obtain Bhlhb5cre/lacZ; Z/EG/+ nulls (Figures S2A and S2B). The data indicate that the reduced pyramidal tract in Bhlhb5-nulls results from aberrant developmental targeting, rather than subsequent degeneration of Bhlhb5-expressing CSMN axons. At P0, the internal capsule and cerebral peduncle in Bhlhb5-nulls (n=10) were indistinguishable from the heterozygotes (n=4), suggesting normal extension of SCPN axons from the cortex into the midbrain (Figures 8A–8D). In contrast, while GFP+ SCPN axons in heterozygotes enter the pons and extend into the medullary pyramidal tract and pyramidal decussation, no axons extend beyond pons in Bhlhb5-nulls, even using GFP immunohistochemical amplification (Figures 8E–8H).
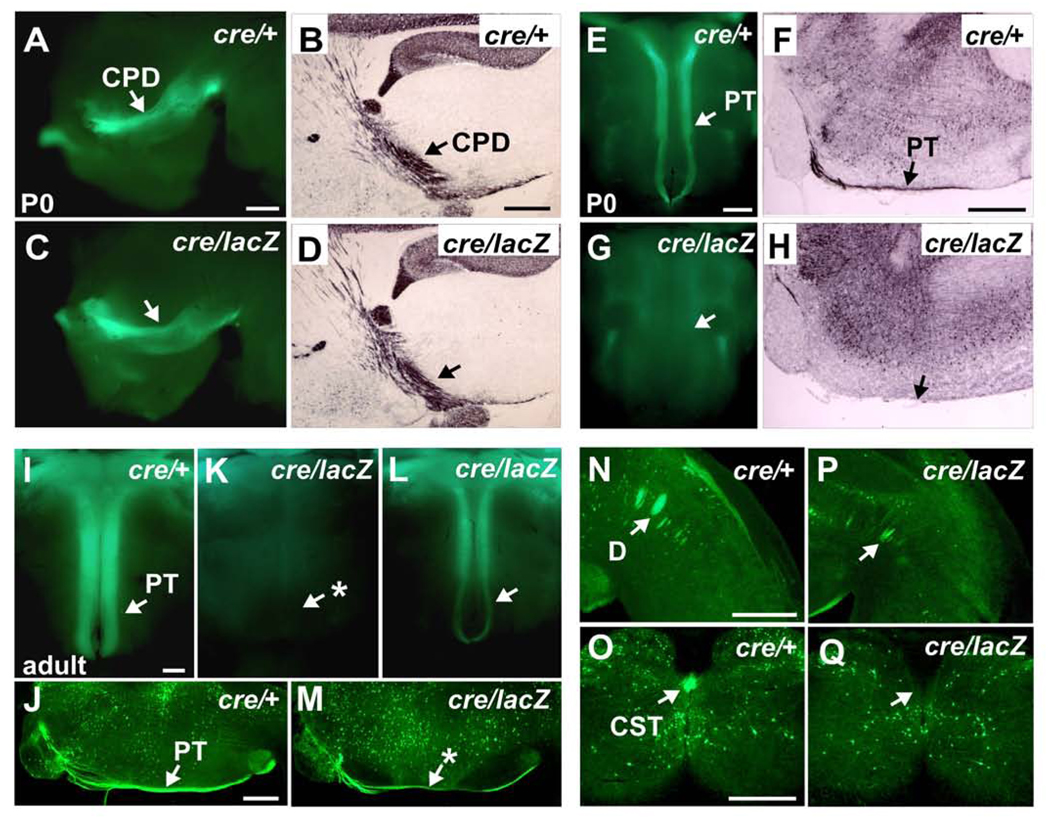
Figure 8. Loss of Bhlhb5 Function Causes Failure of Developmental Axon Targeting by Bhlhb5-expressing CSMN.
(A–Q) Genetic lineage axon tracing in Bhlhb5cre/+; Z/EG/+ heterozygote controls (cre/+) and Bhlhb5cre/lacZ; Z/EG/+ nulls (cre/lacZ).
(A–D) At P0, the cerebral peduncle appears normal in Bhlhb5-nulls via endogenous GFP fluorescence in side view of whole mount brains (A and C), and via GFP HRP-immunohistochemistry of sagittal brain sections (B and D).
(E–H) At P0, no Bhlhb5-expressing fibers are detected in the medullary pyramidal tract in Bhlhb5-nulls via endogenous GFP fluorescence in ventral views of whole mount brainstem (E and G), or via GFP immunohistochemistry of sagittal brainstem sections (F and H).
(I–M) In adults, very few Bhlhb5-expressing fibers are observed in the medullary pyramidal tracts of 10 of 17 Bhlhb5-nulls via endogenous GFP fluorescence (I and L). Bhlhb5-expressing fibers can be visualized in the other 7 (K) via GFP immunohistochemistry (J and M).
(N–Q) Bhlhb5-expressing fibers are observed in the medullary pyramidal decussation (N and P) but not in the ventral dorsal funiculus of the spinal cord (O and Q) in any Bhlhb5-nulls via GFP immunohistochemistry.
CPD, cerebral peduncle; PT, medullary pyramidal tract; D, decussation; CST, spinal segment of the corticospinal tract. P0 (controls n=4; Bhlhb5-nulls n=10); Adults (controls n=6; Bhlhb5-nulls n=17). Scale bars: 500 µm.
To investigate the possibility of delayed medullary entry rather than total failure of entry, we assessed the pyramidal tracts via endogenous GFP fluorescence at P2 (n=3 per group) and P5 (n=2 per group). We observed only rare GFP fibers in the pyramidal tracts of three of the five nulls, and none in the other two nulls (data not shown). To investigate whether Bhlhb5-expressing fibers in the pyramidal tract increase after P5, we analyzed six heterozygotes and seventeen nulls at several stages beyond P14, when the corticospinal tract is considered mature. While a normal GFP+ pyramidal tract is observed via endogenous fluorescence in all heterozygotes (Figure 8I), substantially reduced numbers of GFP+ fibers were seen in ten of seventeen nulls via endogenous fluorescence (Figure 8L and 8K). However, GFP+ fibers were observed in the pyramidal tract and decussation of the other seven nulls using more sensitive GFP immunolabeling thereby suggesting incomplete penetrance and variation in the number of Bhlhb5-expressing CSMN axons in the pyramidal tract of Bhlhb5-nulls (Figures 8J, 8M, 8N and 8P). Interestingly, we found that Bhlhb5 regulates its own stoichiometry via autofeedback inhibition (Figure S8). Thus, absence of Bhlhb5 results in increased production of Cre-recombinase and triggers GFP reporter expression in a greater number of neurons in Bhlhb5-null cortex (Figure S8A–S8D). This suggests that some or all of the reduced number of GFP+ axons observed in the pyramidal tract of the Bhlhb5cre/lacZ;Z/EG/+ nulls might originate not only from the few GFP+ CSMN of rostral motor cortex (as in heterozygotes), but also from CSMN that express normally undetectable levels of Bhlhb5 during development. Further, no GFP+ fibers are observed in the dorsal funiculus of the spinal cord in any nulls, even using GFP immunohistochemistry (Figures 8O and 8Q). Taken together, both anterograde DiI tracing and genetic axonal labeling reveal dramatically reduced numbers of SCPN axons even entering the medullary pyramidal tract, which appear to originate from the few GFP+ rostral CSMN. There is striking failure of pyramidal tract entry by caudal CSMN axons. In the absence of Bhlhb5 function, there are no CST fibers in the dorsal funiculus of the spinal cord, thus complete absence of the corticospinal tract.
Together, our data indicate that Bhlhb5 functions centrally in post-mitotic acquisition of areal identity in somatosensory and caudal motor cortices. The absence of Bhlhb5 function critically disrupts rostrocaudal and mediolateral molecular areal boundaries within cortex; proper establishment of somatosensory barrel cortex; and proper differentiation and corticospinal tract connectivity of caudal CSMN. Thus, Bhlhb5 plays a central and critical role in executing and refining the cytoarchitecture and both afferent and efferent connectivity of the neocortex.
DISCUSSION
The experiments described here demonstrate Bhlhb5’s central function in the post-mitotic acquisition of somatosensory and caudal motor areal identity in layers II–V of the developing neocortex. Bhlhb5 has a progressively patterned expression, consistent with these functions, in post-mitotic excitatory neurons of neocortical layers II–V. Bhlhb5 exhibits an initial high caudomedial to low rostrolateral gradient that transforms into a sharp border between sensory and rostral motor cortices during cortical plate establishment. Analysis of Bhlhb5-null mice reveals severely abnormal molecular development of somatosensory and caudal motor cortices. Further, the cytoarchitectionic barrels are disrupted in somatosensory cortex, and CSMN in caudal motor cortex fail to differentiate correctly or completely. These data and others demonstrate that Bhlhb5 plays a critical role in the acquisition of the defining molecular and anatomical properties of somatosensory and caudal motor cortices.
Specification and Acquisition of Areal Identities
These experiments elucidate the distinct processes that occur in progenitors versus post-mitotic neurons during arealization, and importantly extend the concept of arealization beyond that of specification of areal/positional identities in progenitors. Prior work has shown that manipulations of signaling molecules that affect progenitors such as FGF8, and progenitor expressed TFs such as Emx2, Pax6, COUP-TF1 and Sp8 result in re-specification of progenitor areal/positional identities. Such re-specification is primarily reflected in rostral or caudal shifts of gene expression patterns, rostral or caudal expansions, and/or changes in cortical maps and area-specific TCA targeting (O'Leary et al., 2007). Here, we report that the post-mitotically expressed Bhlhb5 acts further downstream in the genetic hierarchy that regulates neocortical arealization. Aberrant somatosensory and caudal motor chemoarchitecture, and disrupted anatomical features such as vibrissal barrels, indicate errors during post-mitotic acquisition of areal identities (even though these are correctly specified in Bhlhb5-nulls). Thus, Bhlhb5 appears to translate positional information generated by TF gradients in progenitors into area-specific properties in post-mitotic neurons (Figures 9A–9C).
Bhlhb5 might be a downstream target of Emx2, Pax6, and/or COUP-TF1, since its expression in the cortical plate parallels or partially overlaps their progenitor gradients respectively (interestingly, Bhlhb5 might also be upstream of COUP-TF1 in layer IV somatosensory neurons; Figure 3B). While microarray studies have revealed Bhlhb5 as a possible downstream target of Pax6 (Holm et al., 2007), in-depth spatiotemporal analysis of Bhlhb5 expression in Emx2, Pax6, and COUP-TF1-nulls would be important in better defining their regulatory relationships. It will be important to determine if Bhlhb5 is an immediate downstream target, or if there are intermediate regulatory elements in post-mitotic neurons. Such intermediate regulatory elements, downstream of progenitor-restricted factors such as Emx2, but upstream of post-mitotic factors such as Bhlhb5, would be predicted to result in re-specification of areal identity if manipulated. Possible candidates for such intermediate regulators might be members of the Ten_m family, which exhibit anterior and posterior shifts in the cortical plate in Emx2 and Pax6-null mice, respectively (Li et al., 2006), and COUP-TF1 in post-mitotic neurons (Liu et al., 2000). It would be intriguing to observe whether deletion of COUP-TF1 specifically in post-mitotic neurons might result in the same substantial re-specification of sensory to frontal identity that occurs following its deletion in progenitors (Armentano et al., 2007), or a disruption of sensory identity and refinement similar to that in Bhlhb5-nulls.
The similarity in the high caudal to low rostral expression of the progenitor restricted Emx2 and the post-mitotically expressed Bhlhb5 in developing neocortex, and Emx2’s importance in visual cortex development, raises the question of whether Bhlhb5 plays a substantial role in visual areal identity. Of the many area-specific markers that we analyzed, only EphA7 expression is disrupted in the visual cortex of Bhlhb5-null mice. This indicates that Bhlhb5 does not play a pivotal role in visual cortex development, but also suggests that progenitor restricted TFs such as Emx2 might recruit other post-mitotic TFs critical for acquisition of other aspects of visual cortex identity.
Bhlhb5 as a Candidate Disease Gene for Hereditary Spastic Paraplegia
The corticospinal tract is dramatically evolutionarily expanded, and is critical for a far wider range of fine and precise voluntary movements in humans and other primates than in rodents. Bhlhb5-null mice exhibit clasping and shuffling of their forelimbs, and body tremors when suspended by their tails (data not shown). However, as expected given the relatively limited role of the corticospinal tract in rodents (Whishaw et al., 1998), Bhlhb5-nulls display grossly normal cage behavior and locomotion despite their lack of a corticospinal tract. The corticospinal tract functions in rodents primarily for finer aspects of voluntary paw grasping and manipulation, and for precision of voluntary gait and swimming movements (Whishaw et al., 1998); the corticospinal tract is not required for basic locomotion in rodents, with the rubrospinal tract of primary importance for gross gait. Normal gross locomotion despite the absence of the corticospinal tract is also observed in mice null for genes such as Fezf2 (Chen et al., 2005a; Molyneaux et al., 2005).
Human BHLHB5 maps to Chromosome 8q13, an autosomal recessive locus associated with hereditary spastic paraplegia (Hentati et al., 1994; Klebe et al., 2007; Xu et al., 2002). HSP is a motor neuron disorder related to amyotrophic lateral sclerosis, but typically affecting only CSMN (“upper motor neurons”), in particular those controlling the lower limbs (Reid, 2003). Given Bhlhb5’s chromosomal position in an HSP candidate locus, and its important role in CSMN differentiation, Bhlhb5 is a reasonable candidate for investigation as an HSP causative and/or modifying gene, including the possibility of mutations of associated noncoding promoter or enhancer regions.
Control by Bhlhb5 over area-specific differentiation of CSMN in the caudal, but not rostral, motor cortex is accompanied by failure of caudal CSMN to extend axons through the pyramidal tract. In addition, despite the absence of Bhlhb5 expression in most rostral CSMN, rostral CSMN axons still fail to enter into the spinal cord in Bhlhb5-nulls, resulting in a totally absent corticospinal tract (Figures 7D’–7F’). This suggests the possibility of an additional, non-cell autonomous contribution to the absence of the corticospinal tract. Bhlhb5 is expressed in cells of the pyramidal decussation and spinal cord (Figures 8N and 8O) that might provide CSMN axons with guidance, elongation, and/or maintenance signals. Another possibility could be the failure of early pioneer CSMN axons to reach the pyramidal decussation. Analysis of chimeric or conditional Bhlhb5-knock-in mice with cortex specific deletion might elucidate some of these alternative hypotheses.
Bhlhb5 Provides Further Evidence for Transcriptional Combinatorial Codes in Neocortical Development
Due to their patterns of expression and post-mitotic action, it is likely that post-mitotic transcriptional regulators act in a combinatorial fashion to control acquisition of areal identity, rather than any single TF controlling areal identity, i.e., controlling all area-specific features in all layers and projection neuron subtypes in an area. Most TFs expressed post-mitotically in an area-specific manner are also restricted to one or more lamina. Thus, unlike regulatory genes expressed by progenitors, which can potentially specify areal identities in all layers, post-mitotic TFs likely function increasingly specifically in acquisition of area identities. By acting combinatorially, a regulatory gene might be indispensable for acquisition of identity for some areas but not others despite expression in each. For example, RORβ is expressed along the rostrocaudal axis in layer IV, but in Bhlhb5-nulls, is specifically downregulated in caudal motor cortex, but not in occipital cortex, where Bhlhb5 is highly expressed (Figures 3D and 3E). Further, downregulation of Ephrin-A5 occurs in layers IV–V but not layers II–III of somatosensory cortex in Bhlhb5-nulls (Figure 3C), despite Bhlhb5’s expression in layers II–V. This result demonstrates that, even within an area, a regulatory molecule may be responsible for acquisition of area-specific properties in some layers but not others. Taken together, these results reinforce the concept that combinatorial interactions of multiple TFs generate area-specific properties at the level of lamina and specific projection neuron subtypes.
A long-standing question in the field of arealization is how abrupt borders (cytoarchitectural and molecular) that demarcate area transitions are generated (O'Leary and Nakagawa, 2002; Rash and Grove, 2006; Sur and Rubenstein, 2005). In the absence of individual TFs restricted to single areas, and building on known genetic mechanisms of Drosophila segmentation, and spinal cord development, it has been proposed that combinatorial activities of partially overlapping transcriptional activators and repressors might generate abrupt borders of genes observed in the cortical plate (Liu et al., 2000; O'Leary and Nakagawa, 2002; Small et al., 1992; Stanojevic et al., 1991). The disruption of gene expression patterns in Bhlhb5-nulls provides evidence for this combinatorial code model within the neocortex. For example, Cadherin 8, expressed in layers II–III of motor and visual but not somatosensory cortex, normally delineates both the motor-somatosensory and somatosensory-visual transitions. Bhlhb5-nulls exhibit ectopic expression of Cadherin 8 in layers II–III of somatosensory cortex (Figure 3A). Thus, Bhlhb5 appears to be part of a TF combinatorial code that generates Cadherin 8 borders by suppressing Cadherin 8 expression in the somatosensory cortex (Figure 9D). Identification of additional regulators of neocortical arealization will increasingly elucidate the manner by which combinatorial TF codes generate abrupt areal transitions during neocortical development.
EXPERIMENTAL PROCEDURES
Mice
Bhlhb5-lacZ knock-in mice were generated previously (Feng et al., 2006). Bhlhb5-cre knock-in mice were generated using a similar strategy (see Supplemental Data). The Z/EG conditional enhanced GFP reporter mice were purchased from the Jackson Laboratory (Stock Number: 003920). GAD67-GFP knock-in mice were generated by Tamamaki and colleagues (Tamamaki et al., 2003). Embryos were considered as E0.5 at noon on the day at which vaginal plugs were observed. The day of birth was designated P0.
Immunocytochemistry, In Situ Hybridization, and other Histology
Standard protocols for immunohistochemistry (Arlotta et al., 2005), non-radioactive in situ hybridization, X-Gal staining (Feng et al., 2006), and cytochromeoxidase histochemistry (Wong-Riley and Welt, 1980) were used as described; see Supplemental Data for details.
Anterograde Axonal Tracing, Retrograde Labeling, and Affymetrix Microarray Analysis of CSMN
For anterograde corticospinal tracing, P10 mice were anesthetized with Avertin and placed in a stereotaxic apparatus for surgery. 0.3–0.5 µl of 0.5% DiI in DMF was injected 0.5 mm deep in either a rostral or caudal site in the motor cortex. Mice were perfused with 4% PFA at P13, and brains and spinal cords were sectioned sagitally at 180µm. Retrograde labeling, CSMN purification, and microarray analysis have been described previously (Arlotta et al., 2005). Investigation of Bhlhb5 expression in CSMN by microarray was performed by analyzing previously published microarray data (Arlotta et al., 2005). To investigate Bhlhb5 protein expression in CSMN, retrograde labeling was performed via FluoroGold (Hydroxystilbamidine; Molecular Probes) injection into the spinal cord (C4/5 level) at P4. Mice were perfused at P6, and brains were processed for immunolabeling with anti-Bhlhb5.
Supplementary Material
The supplemental data for this article can be found online at http://www.neuron.org/cgi/content/full/
ACKNOWLEDGMENTS
We thank Drs. J. Rubenstein, R. Hevner, S. McConnell, T. Mori, T. Rabbitts, M. Tsai, C. Leamy, and K-F. Lee for generous sharing of antibodies and cDNA clones; Y. Yanagawa, V. Cuzon and H. Yeh for providing GAD67-GFP knock-in brains; J. Zhang, A. Majewska, and K.Billmers for technical help with corticospinal tracing. This work was supported by NIH grants EY013426 and EY015551 to L.G., NIH grants NS45523 and NS49553 to J.D.M., the Research to Prevent Blindness challenge grant to the Department of Ophthalmology at the University of Rochester (L.G.), the Harvard Stem Cell Institute (J.D.M.), the Spastic Paraplegia Foundation (J.D.M.), and the ALS Association (J.D.M.). B.J.M. was supported by the Harvard M.S.T.P. and United Sydney Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agmon A, Yang LT, Jones EG, O'Dowd DK. Topological precision in the thalamic projection to neonatal mouse barrel cortex. J Neurosci. 1995;15:549–561. doi: 10.1523/JNEUROSCI.15-01-00549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agmon A, Yang LT, O'Dowd DK, Jones EG. Organized growth of thalamocortical axons from the deep tier of terminations into layer IV of developing mouse barrel cortex. J Neurosci. 1993;13:5365–5382. doi: 10.1523/JNEUROSCI.13-12-05365.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Armentano M, Chou SJ, Tomassy GS, Leingartner A, O'Leary DD, Studer M. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10:1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Goudreau G, O'Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Rubenstein JL, O'Leary DD. Distinct actions of Emx1, Emx2, and Pax6 in regulating the specification of areas in the developing neocortex. J Neurosci. 2002;22:7627–7638. doi: 10.1523/JNEUROSCI.22-17-07627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning G, Liangos O. Transient expression of the serotonin transporter in the developing mouse thalamocortical system. Acta Histochem. 1997;99:117–121. doi: 10.1016/S0065-1281(97)80016-5. [DOI] [PubMed] [Google Scholar]
- Bulchand S, Subramanian L, Tole S. Dynamic spatiotemporal expression of LIM genes and cofactors in the embryonic and postnatal cerebral cortex. Dev Dyn. 2003;226:460–469. doi: 10.1002/dvdy.10235. [DOI] [PubMed] [Google Scholar]
- Bulfone A, Smiga SM, Shimamura K, Peterson A, Puelles L, Rubenstein JL. T-brain-1: a homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron. 1995;15:63–78. doi: 10.1016/0896-6273(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Chen B, Schaevitz LR, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci U S A. 2005a;102:17184–17189. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JG, Rasin MR, Kwan KY, Sestan N. Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. Proc Natl Acad Sci U S A. 2005b;102:17792–17797. doi: 10.1073/pnas.0509032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP, Wise SP. The motor cortex of the rat: cytoarchitecture and microstimulation mapping. J Comp Neurol. 1982;212:76–88. doi: 10.1002/cne.902120106. [DOI] [PubMed] [Google Scholar]
- Faedo A, Tomassy GS, Ruan Y, Teichmann H, Krauss S, Pleasure SJ, Tsai SY, Tsai MJ, Studer M, Rubenstein JL. COUP-TFI Coordinates Cortical Patterning, Neurogenesis, and Laminar Fate and Modulates MAPK/ERK, AKT, and {beta}-Catenin Signaling. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Xie X, Joshi PS, Yang Z, Shibasaki K, Chow RL, Gan L. Requirement for Bhlhb5 in the specification of amacrine and cone bipolar subtypes in mouse retina. Development. 2006;133:4815–4825. doi: 10.1242/dev.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori KE, Takeuchi K, Yazaki T, Uyemura K, Nojyo Y, Tamamki N. Expression of L1 and TAG-1 in the corticospinal, callosal, and hippocampal commissural neurons in the developing rat telencephalon as revealed by retrograde and in situ hybridization double labeling. J Comp Neurol. 2000;417:275–288. doi: 10.1002/(sici)1096-9861(20000214)417:3<275::aid-cne2>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Emx2 patterns the neocortex by regulating FGF positional signaling. Nat Neurosci. 2003;6:825–831. doi: 10.1038/nn1093. [DOI] [PubMed] [Google Scholar]
- Gao PP, Yue Y, Zhang JH, Cerretti DP, Levitt P, Zhou R. Regulation of thalamic neurite outgrowth by the Eph ligand ephrin-A5: implications in the development of thalamocortical projections. Proc Natl Acad Sci U S A. 1998;95:5329–5334. doi: 10.1073/pnas.95.9.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel S, Huffman KJ, Rubenstein JL. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130:1903–1914. doi: 10.1242/dev.00416. [DOI] [PubMed] [Google Scholar]
- Grove EA, Fukuchi-Shimogori T. Generating the cerebral cortical area map. Annu Rev Neurosci. 2003;26:355–380. doi: 10.1146/annurev.neuro.26.041002.131137. [DOI] [PubMed] [Google Scholar]
- Gulisano M, Broccoli V, Pardini C, Boncinelli E. Emx1 and Emx2 show different patterns of expression during proliferation and differentiation of the developing cerebral cortex in the mouse. Eur J Neurosci. 1996;8:1037–1050. doi: 10.1111/j.1460-9568.1996.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Hamasaki T, Leingartner A, Ringstedt T, O'Leary DD. EMX2 regulates sizes and positioning of the primary sensory and motor areas in neocortex by direct specification of cortical progenitors. Neuron. 2004;43:359–372. doi: 10.1016/j.neuron.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Hentati A, Pericak-Vance MA, Hung WY, Belal S, Laing N, Boustany RM, Hentati F, Ben Hamida M, Siddique T. Linkage of 'pure' autosomal recessive familial spastic paraplegia to chromosome 8 markers and evidence of genetic locus heterogeneity. Hum Mol Genet. 1994;3:1263–1267. doi: 10.1093/hmg/3.8.1263. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JL. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- Holm PC, Mader MT, Haubst N, Wizenmann A, Sigvardsson M, Gotz M. Loss- and gain-of-function analyses reveal targets of Pax6 in the developing mouse telencephalon. Mol Cell Neurosci. 2007;34:99–119. doi: 10.1016/j.mcn.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Kashani AH, Qiu Z, Jurata L, Lee SK, Pfaff S, Goebbels S, Nave KA, Ghosh A. Calcium activation of the LMO4 transcription complex and its role in the patterning of thalamocortical connections. J Neurosci. 2006;26:8398–8408. doi: 10.1523/JNEUROSCI.0618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Gunnersen J, Augustine C, Tan SS. Region-specific expression of the helix-loop-helix gene BETA3 in developing and adult brains. Mech Dev. 2002;114:125–128. doi: 10.1016/s0925-4773(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Klebe S, Durr A, Bouslam N, Grid D, Paternotte C, Depienne C, Hanein S, Bouhouche A, Elleuch N, Azzedine H, et al. Spastic paraplegia 5: Locus refinement, candidate gene analysis and clinical description. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:854–861. doi: 10.1002/ajmg.b.30518. [DOI] [PubMed] [Google Scholar]
- Lai T, Jabaudon D, Molyneaux BJ, Azim E, Arlotta P, Menezes JR, Macklis JD. SOX5 Controls the Sequential Generation of Distinct Corticofugal Neuron Subtypes. Neuron. 2008;57:232–247. doi: 10.1016/j.neuron.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Leamey CA, Glendining KA, Kreiman G, Kang ND, Wang KH, Fassler R, Sawatari A, Tonegawa S, Sur M. Differential Gene Expression between Sensory Neocortical Areas: Potential Roles for Ten_m3 and Bcl6 in Patterning Visual and Somatosensory Pathways. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm031. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Li H, Bishop KM, O'Leary DD. Potential target genes of EMX2 include Odz/Ten-M and other gene families with implications for cortical patterning. Mol Cell Neurosci. 2006;33:136–149. doi: 10.1016/j.mcn.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Liu Q, Dwyer ND, O'Leary DD. Differential expression of COUP-TFI, CHL1, and two novel genes in developing neocortex identified by differential display PCR. J Neurosci. 2000;20:7682–7690. doi: 10.1523/JNEUROSCI.20-20-07682.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackarehtschian K, Lau CK, Caras I, McConnell SK. Regional differences in the developing cerebral cortex revealed by ephrin-A5 expression. Cereb Cortex. 1999;9:601–610. doi: 10.1093/cercor/9.6.601. [DOI] [PubMed] [Google Scholar]
- Maier DL, Mani S, Donovan SL, Soppet D, Tessarollo L, McCasland JS, Meiri KF. Disrupted cortical map and absence of cortical barrels in growth-associated protein (GAP)-43 knockout mice. Proc Natl Acad Sci U S A. 1999;96:9397–9402. doi: 10.1073/pnas.96.16.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallamaci A, Stoykova A. Gene networks controlling early cerebral cortex arealization. Eur J Neurosci. 2006;23:847–856. doi: 10.1111/j.1460-9568.2006.04634.x. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Mori M, Kose A, Tsujino T, Tanaka C. Immunocytochemical localization of protein kinase C subspecies in the rat spinal cord: light and electron microscopic study. J Comp Neurol. 1990;299:167–177. doi: 10.1002/cne.902990204. [DOI] [PubMed] [Google Scholar]
- Mori T, Wanaka A, Taguchi A, Matsumoto K, Tohyama M. Localization of novel receptor tyrosine kinase genes of the eph family, MDK1 and its splicing variant, in the developing mouse nervous system. Brain Res Mol Brain Res. 1995;34:154–160. doi: 10.1016/0169-328x(95)00154-k. [DOI] [PubMed] [Google Scholar]
- Muzio L, Mallamaci A. Emx1, emx2 and pax6 in specification, regionalization and arealization of the cerebral cortex. Cereb Cortex. 2003;13:641–647. doi: 10.1093/cercor/13.6.641. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- O'Leary DD, Chou SJ, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, Koester SE. Development of projection neuron types, axon pathways, and patterned connections of the mammalian cortex. Neuron. 1993;10:991–1006. doi: 10.1016/0896-6273(93)90049-w. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, Nakagawa Y. Patterning centers, regulatory genes and extrinsic mechanisms controlling arealization of the neocortex. Curr Opin Neurobiol. 2002;12:14–25. doi: 10.1016/s0959-4388(02)00285-4. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, Ruff NL, Dyck RH. Development, critical period plasticity, and adult reorganizations of mammalian somatosensory systems. Curr Opin Neurobiol. 1994;4:535–544. doi: 10.1016/0959-4388(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Ozdinler PH, Macklis JD. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat Neurosci. 2006;9:1371–1381. doi: 10.1038/nn1789. [DOI] [PubMed] [Google Scholar]
- Park HT, Kim YJ, Yoon S, Kim JB, Kim JJ. Distributional characteristics of the mRNA for retinoid Z receptor beta (RZR beta), a putative nuclear melatonin receptor, in the rat brain and spinal cord. Brain Res. 1997;747:332–337. doi: 10.1016/s0006-8993(96)01320-0. [DOI] [PubMed] [Google Scholar]
- Peyton M, Stellrecht CM, Naya FJ, Huang HP, Samora PJ, Tsai MJ. BETA3, a novel helix-loop-helix protein, can act as a negative regulator of BETA2 and MyoD-responsive genes. Mol Cell Biol. 1996;16:626–633. doi: 10.1128/mcb.16.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Dehay C, Goffinet A, Kennedy H. Pre- and post-mitotic events contribute to the progressive acquisition of area-specific connectional fate in the neocortex. Cereb Cortex. 2001;11:1027–1039. doi: 10.1093/cercor/11.11.1027. [DOI] [PubMed] [Google Scholar]
- Rash BG, Grove EA. Area and layer patterning in the developing cerebral cortex. Curr Opin Neurobiol. 2006;16:25–34. doi: 10.1016/j.conb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Reid E. Science in motion: common molecular pathological themes emerge in the hereditary spastic paraplegias. J Med Genet. 2003;40:81–86. doi: 10.1136/jmg.40.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Anderson S, Shi L, Miyashita-Lin E, Bulfone A, Hevner R. Genetic control of cortical regionalization and connectivity. Cereb Cortex. 1999;9:524–532. doi: 10.1093/cercor/9.6.524. [DOI] [PubMed] [Google Scholar]
- Simeone A, Acampora D, Gulisano M, Stornaiuolo A, Boncinelli E. Nested expression domains of four homeobox genes in developing rostral brain. Nature. 1992;358:687–690. doi: 10.1038/358687a0. [DOI] [PubMed] [Google Scholar]
- Small S, Blair A, Levine M. Regulation of even-skipped stripe 2 in the Drosophila embryo. Embo J. 1992;11:4047–4057. doi: 10.1002/j.1460-2075.1992.tb05498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanojevic D, Small S, Levine M. Regulation of a segmentation stripe by overlapping activators and repressors in the Drosophila embryo. Science. 1991;254:1385–1387. doi: 10.1126/science.1683715. [DOI] [PubMed] [Google Scholar]
- Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Suzuki SC, Inoue T, Kimura Y, Tanaka T, Takeichi M. Neuronal circuits are subdivided by differential expression of type-II classic cadherins in postnatal mouse brains. Mol Cell Neurosci. 1997;9:433–447. doi: 10.1006/mcne.1997.0626. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Weimann JM, Zhang YA, Levin ME, Devine WP, Brulet P, McConnell SK. Cortical neurons require Otx1 for the refinement of exuberant axonal projections to subcortical targets. Neuron. 1999;24:819–831. doi: 10.1016/s0896-6273(00)81030-2. [DOI] [PubMed] [Google Scholar]
- Welker C. Receptive fields of barrels in the somatosensory neocortex of the rat. J Comp Neurol. 1976;166:173–189. doi: 10.1002/cne.901660205. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Gorny B, Sarna J. Paw and limb use in skilled and spontaneous reaching after pyramidal tract, red nucleus and combined lesions in the rat: behavioral and anatomical dissociations. Behav Brain Res. 1998;93:167–183. doi: 10.1016/s0166-4328(97)00152-6. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Welt C. Histochemical changes in cytochrome oxidase of cortical barrels after vibrissal removal in neonatal and adult mice. Proc Natl Acad Sci U S A. 1980;77:2333–2337. doi: 10.1073/pnas.77.4.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA, Dierker ML, Wann DF. Mouse SmI cortex: qualitative and quantitative classification of golgi-impregnated barrel neurons. Proc Natl Acad Sci U S A. 1975;72:2165–2169. doi: 10.1073/pnas.72.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Xu ZP, Dutra A, Stellrecht CM, Wu C, Piatigorsky J, Saunders GF. Functional and structural characterization of the human gene BHLHB5, encoding a basic helix-loop-helix transcription factor. Genomics. 2002;80:311–318. doi: 10.1006/geno.2002.6833. [DOI] [PubMed] [Google Scholar]
- Zhou C, Tsai SY, Tsai MJ. COUP-TFI: an intrinsic factor for early regionalization of the neocortex. Genes Dev. 2001;15:2054–2059. doi: 10.1101/gad.913601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplemental data for this article can be found online at http://www.neuron.org/cgi/content/full/