Abstract
There is growing evidence that Rho-kinases (ROCKs), the immediate downstream targets of the small guanosine triphosphate-binding protein Rho, may contribute to cardiovascular disease. ROCKs play a central role in diverse cellular functions such as smooth muscle contraction, stress fiber formation and cell migration and proliferation. Overactivity of ROCKs is observed in cerebral ischemia, coronary vasospasm, hypertension, vascular inflammation, arteriosclerosis and atherosclerosis. ROCKs, therefore, may be an important and still relatively unexplored therapeutic target in cardiovascular disease. Recent experimental and clinical studies using ROCK inhibitors such as Y-27632 and fasudil have revealed a critical role of ROCKs in embryonic development, inflammation and oncogenesis. This review will focus on the potential role of ROCKs in cellular functions and discuss the prospects of ROCK inhibitors as emerging therapy for cardiovascular diseases.
Keywords: contraction, endothelium, hypertension, inflammation, leukocyte, remodeling, Rho-kinase, smooth muscle
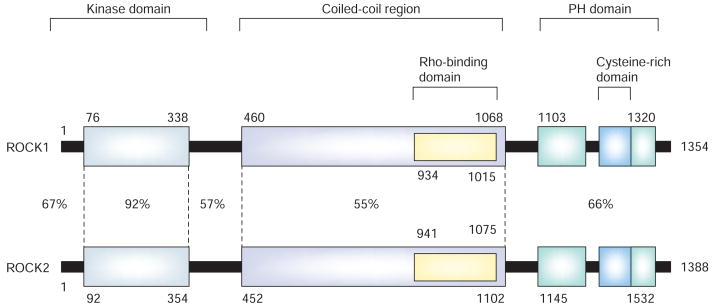
The small guanosine triphosphate (GTP)-binding proteins belonging to the Rho family regulate various aspects of cell shape, motility, proliferation and apoptosis [1]. Rho-kinases (ROCKs), which were one of the first downstream effectors of Rho to be discovered [2–4], were found to mediate RhoA-induced actin cytoskeletal changes through effects on myosin light-chain (MLC) phosphorylation [5,6]. ROCKs are protein serine/threonine kinases that share 45–50% homology with other actin cytoskeletal kinases such as myotonic dystrophy kinase (DMPK), myotonic dystrophy-related cdc42-binding kinase (MRCK) and citron kinase [1]. ROCKs consist of an amino-terminal kinase domain, followed by a mid-coiled-coil-forming region containing a Rho-binding domain (RBD) and a carboxy-terminal cysteine-rich domain (CRD) located within the pleckstrin homology (PH) motif (FIGURE 1). In mammalian systems, two ROCK isoforms have been identified. ROCK1, which is also known as ROKβ, and p160ROCK, which is located on chromosome 18 and encodes a 1354 amino acid protein [4,5]. ROCK2, which is also known as ROKα and sometimes confusingly called Rho-kinase, is located on chromosome 12 and contains 1388 amino acids [2,7,8]. ROCK1 and 2 share an overall 65% homology in amino acid sequence and 92% homology in their kinase domains [1]. Thus, pharmacologic inhibitors of ROCKs, such as Y-27632, fasudil (HA1077) and hydroxyfasudil, which target their ATP-dependent kinase domains, can inhibit both ROCK1 and 2. Furthermore, at higher concentrations, Y-27632 can also inhibit protein kinase C-related kinase (PRK)-2, protein kinase N and citron kinase [9], while fasudil can inhibit protein kinase A (PKA) and protein kinase C (PKC) [10]. Therefore, relying solely on the use of these ROCK inhibitors cannot distinguish the role of ROCK1 and 2, and could give misleading results, as they could nonspecifically inhibit other protein kinases as well. However, it should also be noted that Y-27632 or fasudil have a more potent inhibitory action for ROCK. In addition, H-1152P, a derivative of fasudil, is a highly potent and specific inhibitor of ROCK [11]. Thus, used properly, its action is inhibition of ROCK, although selectivity of the drug should be considered for interpretation of the results of the study.
Figure 1. Structures of ROCKs. ROCKs consist of the kinase domain, which is highly homologous between two isohorms, a coiled-coil region, and a PH domain.
Rho-binding domain and cysteine-rich domain located in the coiled-coil region and PH domain, respectively.
PH: Plecktrin-homology; ROCK: Rho-kinase.
ROCK1 and 2 are ubiquitously expressed in murine tissues from early embryonic development to adulthood. In particular, ROCK2 mRNA is most highly expressed in cardiac muscle and brain tissues, which indicates that this ROCK isoform may have a specialized role in these cell types. Immunolocalization and cell-fractionation studies of ROCK2 have shown that this protein is distributed mainly in the cytoplasm [2,8]. When activated by GTP-bound RhoA, ROCK2 partly translocates from the cytoplasm to membranes. Indeed, a small amount of ROCK2 has been found in the membrane fraction and some immunostaining is detectable at the cell periphery or membranes of growing cells [12]. In addition, ROCK2 has been localized to the cleavage furrow in late mitosis [13] and is partially associated with vimentin and actin stress fibers [14,15]. The determination of ROCK1 localization has been more difficult; however, a recent report suggests that this protein may colocalize to centrosomes [16].
The carboxy-terminal regions of ROCKs serve as an autoregulatory inhibitor of the amino-terminal kinase domain [17]. The interaction of the active GTP-bound form of Rho with RBD of ROCKs, increases ROCK activity through derepression of the carboxyl-terminal RBD–PH domains on the amino-terminal kinase domain, leading to an active open kinase domain. The open conformation could also be caused by the binding of arachidonic acid to the PH domain [18] or cleavage of the carboxyl-terminus by caspase-3 [19,20]. This closed-to-open conformation of ROCK activation is similar to that of DMPK and MRCK activation [15,17], and is consistent with studies demonstrating that overexpression of various carboxyl-terminal constructs of ROCK or kinase-defective forms of full-length ROCK, function as dominant–negative ROCK mutants [4,5,21]. ROCKs can also be activated independently of Rho through amino-terminal transphosphorylation caused by protein oligomerization [15,22] or inhibited by other small GTP-binding proteins such as Gem and Rad [23].
Downstream cellular targets of ROCKs
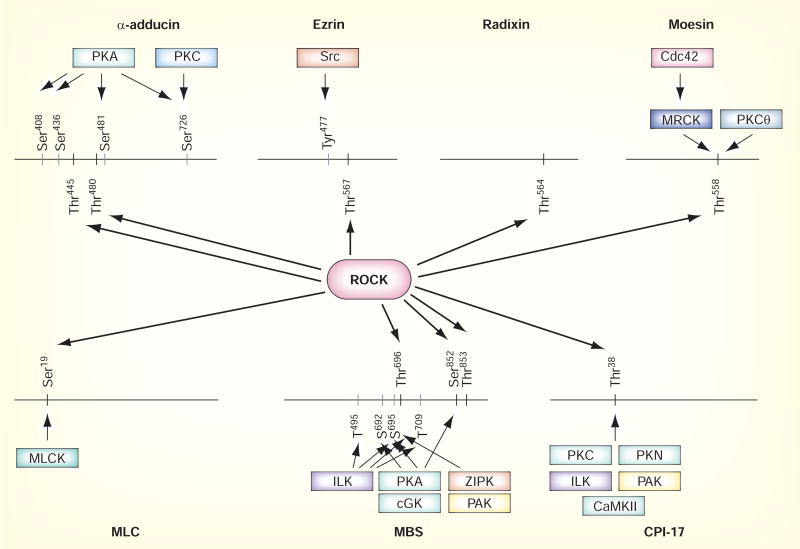
In response to activators of Rho, such as lysophosphatidic acid (LPA) or sphingosine-1 phosphate (S1P), which stimulate Rho-guanine nucleotide exchange factor (GEF) and lead to the formation of active GTP-bound Rho, ROCKs mediate a broad range of cellular responses that involve the actin cytoskeleton. For example, they control assembly of the actin cytoskeleton and cell contractility by phosphorylating a variety of proteins, such as MLC phosphatase, LIM-kinases, adducin and ezrin–radixin–moesin (ERM) proteins (FIGURE 2). The consensus amino acid sequences for phosphorylation are R/KXS/T or R/KXXS/T (R: arginine; K: lysine; X: any amino acid; S: serine; T: threonine) [24,25]. ROCKs can also be auto-phosphorylated [2,4], which may modulate their function. Specifically, ROCK2 phosphorylates Ser19 of MLC, the same residue that is phosphorylated by MLC-kinase (MLCK). Thus, ROCK2 can alter the sensitivity of smooth muscle cell contraction to Ca2+, since MLCK is Ca2+ sensitive [3]. In addition, ROCKs regulate MLC phosphorylation indirectly through the inhibition of MLC phosphatase (MLCP) activity. MLCP holoenzyme is composed of three subunits; a catalytic subunit (PP1cδ), a myosin-binding subunit (MBS) composed of a 58-kDa head and 32-kDa tail region, and a small noncatalytic subunit, M21. Depending on the species, ROCK2 phosphorylates MBS at Thr697, Ser854 and Thr855 [25]. Phosphorylation of Thr697 or Thr855 attenuates MLCP activity [26] and in some instances, the dissociation of MLCP from myosin [27]. ROCK2 also phosphorylates ERM proteins, namely Thr567 of ezrin, Thr564 of radixin and Thr558 of moesin [28]. ROCK2-mediated phosphorylation leads to the disruption of the head-to-tail association of ERM proteins and actin cytoskeletal reorganization. In contrast, ROCK1 phosphorylates LIM-kinase-1 at Thr508 [29] and LIM-kinase-2 at Thr505 [24], which enhance the ability of LIM-kinases to phosphorylate cofilin [30]. Since cofilin is an actin-binding and -depolymerizing protein that regulates the turnover of actin filaments, the phosphorylation of LIM-kinases by ROCK1 inhibits cofilin-mediated actin filament disassembly and leads to an increase in the number of actin filaments. Thus, despite having a similar kinase domain, ROCK1 and 2 may serve different functions and may have different downstream targets. Nevertheless, to date, most, if not all of the downstream targets of ROCKs are cellular proteins associated with the regulation of the actin cytoskeleton. As mentioned, the difficulty with studying the role of ROCKs is due to the relative lack of specificity of ROCK inhibitors, not only to distinguish ROCK isoforms but also to distinguish ROCK from other protein kinases such as PKA, PKC and citron kinase.
Figure 2. Downstream targets of ROCK.
ROCK phosphorylates serine and/or threonine residues of various proteins, which regulate actin-cytoskeleton assembly and cell contractility. Some phosphorylated sites are likely to be specifically phosphorylated by ROCK, whereas the others are phosphorylation targets of one or more other kinases such as PKA or PKC as well. Sequence in human homology.
CaMK II: Calmodulin-dependent kinase II; cGK: Cyclic GMP-dependent kinase; ILK: Integrin-linked kinase; MBS: Myosin-binding subunit of myosin phosphatase;
MLC: Myosin light chain; MLCK: Myosin light-chain kinase; MRCK: Myotonic dystrophy kinase-related Cdc42-binding kinase; PAK; p21-activated kinase;
PKA: Protein kinase A; PKC: Protein kinase C; PKN: Protein kinase N; Ser: Serine; Thr: Threonine; Tyr: Tyrosine; ZIPK: Zip kinase.
Cellular functions of ROCKs
ROCKs are important regulators of cellular growth, migration, metabolism and apoptosis, through control of the actin cytoskeletal assembly and cell contraction [1]. Although there is no evidence that ROCK isoforms have different functions, they are differentially expressed and regulated in specific tissues. For example, only ROCK1 is cleaved by caspase-3 during apoptosis [19,20]. Furthermore, ROCK1 expression tends to be more ubiquitous, while ROCK2 is most highly expressed in cardiac and brain tissues [7,31,32]. Thus, it is likely that using a genetic approach to dissecting the roles of ROCK isoforms (i.e., conditional ROCK deletion), distinct and novel cellular functions will be uncovered, which could be specifically ascribed to ROCK1 and 2.
Stimulation of tyrosine kinase and G-protein-coupled receptors leads to activation of Rho, the direct upstream activator of ROCKs, via recruitment and activation of Rho-GEF [33,34]. ROCKs are important effectors of Rho in regulating the actin cytoskeleton. Inhibitors of ROCKs, such as Y-27632 and fasudil, or overexpression of dominant–negative mutants of ROCKs, lead to the loss of stress fibers and focal adhesion complexes [4,35]. This is predominantly due to the phosphorylation and inhibition of MLCP by ROCK, which increases MLC phosphorylation and cellular contraction by facilitating interaction of myosin with F-actin. Thus, ROCKs regulate cell polarity and migration, predominantly through enhancing actomyosin contraction and focal adhesions. Indeed, increased ROCK activity is observed in tumor metastasis [36] and overexpression of constitutively activated ROCK promotes tumor invasion [37]. Conversely, invasion of rat hepatoma cells and migration of metastatic breast cancer cells are inhibited by overexpression of dominant–negative ROCK constructs or by the ROCK inhibitor, Y-27632 [38]. Treatment with Y-27632 reduces tumor cell dissemination in vivo, suggesting its potential use in cancer therapy [38]. In addition, ROCKs could also regulate macrophage phagocytic activity via actin cytoskeletal membrane protrusions and mediate endothelial cell permeability via affects on tight and adherens junctions [39,40]. ROCKs could inhibit insulin signaling via phosphorylation of insulin receptor substrate (IRS)-1, which uncouples the insulin receptor to phosphatidylinositol-3 kinase [41]. Conversely, it could also regulate cell size via enhancing insulin growth factor-induced cAMP responsive element-binding protein phosphorylation [42]. Indeed, this may be the underlying mechanism by which ROCK inhibitors reduce cardiac hypertrophy [43,44]. Finally, ROCKs may be involved in tissue differentiation from adipocytes to myocytes. In p190-B RhoGAP-deficient mice, which have high basal Rho/ROCK activity due to a lack of off switch for Rho, there is a defect in adipogenesis, with a predilection towards myogenesis [42,45]. Treatment of p190-B RhoGAP-deficient mice with Y27632 restores normal adipogenesis, suggesting that ROCKs are involved in the myogenesis differentiation program [45].
ROCK in hypertension
Abnormal and sustained increases in vascular tone are observed in hypertension. The molecular mechanisms underlying this increase in vascular resistance, however, are not completely understood. Contraction of vascular smooth muscle is triggered by an increase in cytosolic Ca2+ concentration. The binding of Ca2+ to calmodulin (CaM) activates MLCK, which, in turn, phosphorylates MLC, leading to a conformational change in MLC and its ability to interact with actin filaments. MLC phosphorylation can also be regulated by MLCP in a Ca2+-independent manner. The activity of MLCP is principally regulated by ROCK. ROCK phosphorylates and inhibits MLCP either directly or through CPI17 [46]. The phosphorylation of MLC and vascular smooth muscle contraction, therefore, are regulated in a Ca2+-dependent and -independent manner.
Administration of the ROCK inhibitor Y-27632 decreases systemic blood pressure in spontaneously hypertensive rats (SHR), renal hypertensive rats and deoxycorticosterone acetate (DOCA)-salt hypertensive rats [35]. In contrast, Y-27632 causes only a slight and transient decrease in blood pressure in normotensive rats. These results suggest that ROCK may be involved in blood pressure regulation and that its activity may be increased in hypertension. Indeed, mRNA expression and activity of ROCK are elevated in SHR [47]. Interestingly, the increase in ROCK expression in carotid arteries of SHR precedes the onset of hypertension, suggesting that increased ROCK activity may be involved in the pathogenesis of hypertension rather than as a result of hypertension. However, other studies have demonstrated that the expression level of ROCK is comparable with that of normotensive rats, suggesting that activity, but not expression, may be elevated in hypertension [48]. Ex vivo experiments using isolated arteries demonstrated that contractile responses to phenylephrine and serotonin (5-HT) are augmented in SHR compared with control rats [47]. Exogenous addition of another ROCK inhibitor, hydroxyfasudil, attenuated these agonist-induced vasoconstrictions in SHR. In addition, SHR develop features of arteriosclerosis including medial thickening and perivascular fibrosis, following vascular injury. The formation of these vascular lesions was attenuated by long-term treatment of SHR with fasudil. In the clinical setting, fasudil-induced increases in forearm blood flow and decreases in forearm vascular resistance were greater in hypertensive patients than in normotensive subjects, whereas responses to sodium nitroprusside were comparable between these two groups [49].
The molecular mechanisms by which ROCK is activated in hypertension are not known. Vasoactive substances, such as angiotensin II, endothelin-1, 5-HT,α-adrenergic stimuli and reactive oxygen species may be involved. Activation of RhoA is observed in rat models of hypertension and angiotensin II infusion [48]. For example, angiotensin II type 1-receptor antagonists inhibit RhoA activation and lower blood pressure in stroke-prone spontaneously hypertensive rats [50]. Another potential candidate is cyclic GMP-dependent protein kinase I (cGKI), which is known to bind to and inhibit RhoA [51]. Indeed, the expression of cGKI is reduced in stroke-prone spontaneously hypertensive rats [50]. In the CNS, ROCK could also play a critical role in the regulation of blood pressure and heart rate [52–54]. The nucleus tractus solitarii (NTS) of the brain stem receives signals through afferent nerve fibers from arterial baroreceptors, chemoreceptors, cardiopulmonary receptors and other visceral receptors. In turn, NTS regulates blood pressure and heart rate through the sympathetic nerve system. In hypertensive rats, such as SHR and nitric oxide synthase (NOS) inhibitor L-NAME-treated rats, ROCK is activated in the NTS and microinjection of Y-27632 or a dominant–negative mutant of ROCK decreased systemic blood pressure. The extent of blood pressure decline was greater in SHR and L-NAME-treated rats compared with nor-motensive control rats, suggesting greater sensitivity to ROCK inhibition in hypertensive animals. However, it remains to be determined as to what extent ROCK activation in the NTS is involved in the pathogenesis of hypertension.
ROCK in acute coronary syndromes
Coronary artery spasms contribute to acute coronary syndrome and are characterized by inducible hypercontractility of vascular smooth muscle in response to acetylcholine or 5-HT [55]. Both acetylcholine and 5-HT activate ROCK, and ROCK inhibitors have been demonstrated to attenuate agonist-induced coronary vasospasm [56]. ROCK inhibitors also suppress vasospasms in porcine coronary artery induced by chronic treatment with the proinflammatory mediator, inter-leukin-1β [57,58]. Indeed, elevated ROCK expression and activity are observed in vasospastic segment [58]. Clinically, intracoronary administration of a ROCK inhibitor is effective in preventing coronary artery spasm and myocardial ischemia in patients with vasospastic and microvascular angina, as well as intractable severe coronary spasm following coronary artery bypass grafting [59–61]. Interestingly, the antianginal effects of ROCK inhibitors are not only seen in vasospastic angina but also in effort angina [62,63]. In the report of the multicenter Phase II study, the effect of oral administration of fasudil was examined in 45 or 125 Japanese patients with stable effort angina. Treatment with fasudil significantly improved exercise tolerance as demonstrated by prolonged maximum exercise duration and increased time to the onset of 1-mm ST segment depression on treadmill exercise test. In addition, the results of a multicenter Phase II study in the USA were reported [64]. In this Phase II, double-blind, placebo-controlled randomized trial, the effects of fasudil on total exercise duration and time to onset of myocardial ischemia were evaluated in patients with stable angina. A total of 84 patients received fasudil or matching placebo for 8 weeks with an antianginal medication, either a β-blocker or a calcium antagonist (41 and 43, fasudil and placebo groups, respectively). Both groups increased their exercise duration by 1.97 and 1.43 min (fasudil and placebo groups, respectively) and the time to onset of myocardial ischemia was delayed by 2.83 min in the fasudil group compared with the placebo group. These findings suggest that ROCK may be an important therapeutic target in patients with ischemic heart disease.
ROCK in vascular remodeling & atherosclerosis
ROCK is involved in vascular inflammation and remodeling. ROCK inhibitors attenuate the inflammatory response (i.e., macrophage accumulation) and vascular remodeling (medial thickening and perivascular fibrosis) in L-NAME-treated rats [65,66]. In addition, ROCK activity is increased in the neointima following balloon-induced vascular injury [67], which is suppressed by ROCK inhibitors or gene transfer of a dominant–negative mutant of ROCK [67–69]. Indeed, ROCK inhibitors prevent neointima formation following stent implantation in porcine coronary artery [69]. The mechanism contributing to decreased restenosis is, in part, due to inhibition of smooth muscle cell migration and proliferation [65], and increased smooth muscle cell apoptosis [68]. In addition, the differentiation of smooth muscle cells, which is recognized as alterations in smooth muscle cell phenotype, plays an important role in neointima formation. ROCK may also be involved in smooth muscle cell differentiation through the regulation of smooth muscle cell differentiation marker genes such as SM22 and SM α-actin [70].
ROCK inhibition reduces macrophage accumulation and collagen deposition, and enhances apoptosis [71]. Treatment of porcine coronary artery with monocyte chemoattractant protein (MCP)-1 and oxidized low-density lipoprotein (LDL) or interleukin-1β leads to increased ROCK activity, intimal thickening, constrictive remodeling and accumulation of macrophages in the adventitia. Inhibition of ROCK by a ROCK inhibitor or a dominant–negative ROCK mutant can ameliorate formation of vascular lesions and even induce a regression [72–74]. Recent studies demonstrated that inhibition of ROCK by an inhibitor or a dominant–negative ROCK results in suppression of cardiac allograft vasculopathy, characterized by intimal thickening and perivascular fibrosis in mice [75]. Thus, there is growing evidence that ROCK plays a critical role in vascular remodeling. However, it remains to be determined whether ROCK is involved in vascular remodeling in humans and if so, whether ROCK inhibitors could be effective therapies for vascular proliferative diseases.
ROCK is known to regulate the expression of genes involved in atherogenesis, such as MCP-1 [76], plasminogen activator inhibitor (PAI)-1 [77] and osteopontin [78]. ROCK regulates endothelial tissue factor expression, which is critical for atherothrombosis [79,80]. Indeed, treatment with Y-27632 decreased atherosclerotic lesion size by approximately 30% in LDL-receptor knockout mice [81]. This is associated with a decrease in T-lymphocyte accumulation and expression of the p65 subunit of nuclear factor (NF)-κB, suggesting that ROCK is proinflammatory and proatherogenic.
ROCK in pulmonary hypertension
Pulmonary hypertension results from abnormal pulmonary vasoconstriction and vascular remodeling. Although drugs such as prostacyclin analogs and endothelin receptor antagonists are potential therapies for pulmonary hypertension, their efficacy is limited. Thus, understanding the pathologic mechanism underlying pulmonary hypertension is of great clinical importance in the development of effective therapies to improve survival. Recent studies indicate that treatment with ROCK inhibitors improves monocrotaline-induced pulmonary hypertension in rats [82] and hypoxia-induced pulmonary hypertension in mice [83]. Furthermore, oral administration of fasudil improved survival of monocrotaline-injected rats even when therapy was initiated after the development of pulmonary hypertension. Indeed, ROCK inhibitors improved pulmonary hypertension, right ventricular remodeling and pulmonary vascular remodeling. Alternatively, inhaled delivery of ROCK inhibitors was also able to reduce pulmonary artery pressure in animal models of pulmonary hypertension [84]. Clinically, treatment with fasudil lowered pulmonary artery vascular resistance in patients with pulmonary hypertension [85]. In this study, three men and seven women (26–73 years of age) with severe pulmonary hypertension were studied (three with primary pulmonary hypertension and seven with secondary causes of pulmonary hypertension). Each patient was treated with intravenous fasudil, sublingual nifedipine, inhalation oxygen or NO, using a pulmonary arterial catheter. The hemodynamic parameters of the pulmonary and systemic circulation were evaluated before and after each treatment. Treatment with fasudil reduced pulmonary artery vascular resistance, while nifedipine, oxygen and NO did not. These studies suggest that ROCK is involved in the pathogenesis of pulmonary hypertension and that treatment with ROCK inhibitors might be a novel therapeutic strategy for this disease.
ROCK & endothelial nitric oxide synthase
NO, constitutively produced by endothelial NOS (eNOS), is an essential regulator of vascular tone, integrity and homeostasis. The biologic effects of NO include mediating vasorelaxation, inhibiting vascular smooth muscle cell proliferation and migration, attenuating leukocyte adhesion and preventing platelet aggregation. These actions of NO may be cardiovascular protective, as administration of NO donor compounds, stimulation of endogenous production by supplementation of L-arginine, a precursor of NO, or gene transfer of eNOS is protective. In contrast, systemic administration of NOS inhibitors or gene-targeting deletion of eNOS accelerates atherosclerotic lesion formation and severity of diseases. Recent studies indicate that RhoA/ROCK signaling inversely regulates eNOS expression and activity [86–88]. Hypoxia and thrombin stimulate ROCK activity, leading to the downregulation of eNOS expression via destabilization of eNOS mRNA [87,88]. Inhibition of ROCK prevents downregulation of eNOS expression by inhibiting destabilization of eNOS mRNA.
ROCK can also negatively regulate eNOS activity via the phosphatidylinositol 3-kinase/Akt-dependent pathway [89,90]. Activation of ROCK leads to the inhibition of eNOS phosphorylation at Ser1177, one of the critical phosphorylation sites necessary for eNOS activity [89]. Indeed, inhibition of ROCK by ROCK inhibitors results in the activation of Akt through phosphatidylinositol-3-kinase [90]. The molecular mechanisms by which ROCK regulates phosphatidylinositol-3-kinase and/or Akt activities, however, remain to be determined.
ROCK in erectile dysfunction
The prevalence of erectile dysfunction increases with age and is often secondary to diseases such as diabetes and hypertension. Cavernosal vasodilation and vasoconstriction plays a central role in the regulation of penile erection. ROCK is expressed in the corpus cavernosum, and inhibition of endogenous ROCK activity by injection of Y-27632 resulted in increased corpus cavernosum pressure, indicating an important role of endogenous ROCK activity in erectile dysfunction [91]. This effect of Y-27632 was independent of NO because inhibition of NOS by L-NAME did not alter erectile response. In the diabetic state, expression of RhoA and ROCK in corporal tissue is elevated. Increased ROCK activity plays a role in erectile dysfunction through the downregulation of eNOS expression in streptozotocin-induced diabetic rat penis [92]. Inhibition of RhoA/ROCK activity improved erectile responses in association with restoring eNOS expression and activity to levels in control rats. These results suggest that the ROCK inhibitors are promising for the treatment of erectile dysfunction.
ROCK in heart diseases
ROCK is involved in the pathogenesis of not only vascular disorders but also of heart diseases. Although several previous studies have demonstrated the involvement of RhoA/ROCK signaling in cardiac hypertrophy in vitro, transgenic mice overexpressing RhoA in the heart do not develop cardiac hypertrophy [93]. Nevertheless, some studies suggest an important role of ROCK in cardiac hypertrophy [94,95]. For example, in angiotensin II-infused rat models, oral administration of fasudil suppressed left ventricular (LV) hypertrophy. This was accompanied by decreased perivascular fibrosis and endothelial superoxide anion production, probably through the inhibition of NAD(P)H oxidase expression [94]. Similarly, in Dahl salt-sensitive rats fed a high salt diet, Y-27632 inhibited the development of LV hypertrophy and improved cardiac contractile dysfunction [95].
ROCK is also activated in murine hearts following myocardial ischemia-reperfusion injury [96] and infarction [97]. Ischemia-reperfusion injury increased RhoA expression and ROCK activity in ischemic myocardium and treatment with Y-27632 protects against ischemia-reperfusion injury [96]. Y-27632 decreased infarct size and improved cardiac function by inhibiting myocardial apoptosis and inflammatory responses, in part by preventing Bcl-2 downregulation and blocking proinflammatory cytokine production. Another ROCK inhibitor, fasudil, also decreased myocardial infarct size in rats and this cardioprotective effect was blocked by cotreatment with phosphatidylimositol 3-kinase inhibitors or the NOS inhibitor, L-NAME [90]. Similarly, hydroxyfasudil reduced myocardial infarct size in dogs after ischemia-reperfusion injury, with an improved vasodilator response via preservation of the eNOS expression [98]. Moreover, treatment with fasudil improved LV remodeling and inhibited the inflammatory response in mice irrespective of infarction size or time of treatment [97].
Clinical implications & limitations
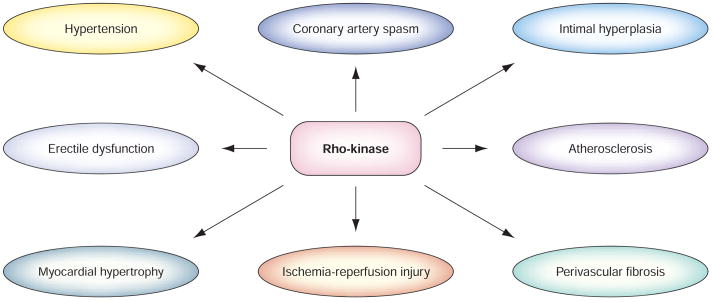
There is growing evidence from both animal and clinical studies that ROCK plays a central role in the pathogenesis of various cardiovascular diseases (FIGURE 3) [99]. Indeed, many of the so-called pleiotropic or cholesterol-independent effects of statins may be due to their ability to block isoprenoid synthesis and inhibit the Rho/ROCK pathway [100]. Inhibition of ROCK leads to the stabilization of eNOS mRNA [86,101] and the rapid phosphorylation and activation of eNOS via the phosphatidylinositol-3-kinase/protein kinase Akt pathway [90,102]. Thus, targeting ROCK with specific inhibitors may be an important therapeutic strategy for decreasing cardiovascular events. It is also likely that ROCK inhibitors will have therapeutic benefits that extend beyond the cardiovascular arena to include rheumatologic, oncologic and neurologic diseases. Although statin therapy has provided numerous benefits for patients with cardiovascular diseases, statins are often combined with another class of drugs – fibric acid derivatives – to lower both cholesterol and trigly-ceride levels. Myopathy, such as myalgia and rhabdomyolysis, is known to be a rare, but serious side effect of statin therapy. Thus far, only fasudil is available clinically for the treatment of vasospasm following hemorrhagic stroke. No serious side effect of fasudil has been observed.
Figure 3. Targeting Rho-kinase in therapy for cardiovascular diseases.
Rho-kinase is involved in various aspects of cardiovascular diseases.
As ROCKs are involved in various aspects of vascular function and disease, understanding their role in the vascular wall may provide key insights into how the vasculature as a whole is regulated under normal and pathophysiologic conditions. However, despite a growing number of reports demonstrating that ROCK activity is increased under a variety of pathologic conditions, little is known regarding the molecular mechanisms that contribute to increased ROCK activity or what the downstream targets for ROCK are. Furthermore, determining the precise role of ROCKs in the vascular wall is limited by pharmacologic inhibitors, which cannot discriminate between ROCK isoforms or the role of ROCKs in individual component cells. Hence, a genetic approach with tissue-specific gene targeting of ROCK deletion to individual components of the vascular wall offers the greatest likelihood of success in dissecting the role of ROCKs in vascular disease. Despite such promise, embryonic lethality could also present a major obstacle in studying ROCK gene deletion. Indeed, approximately 90% of ROCK2-deficient mice die in utero from placental insufficiency due to extensive thrombus formation in the labyrinth layer [103]. Further investigations using inducible and conditional ROCK gene deletions offer the greatest hope in understanding the in vivo pathophysiologic role of ROCK isoforms in adult animals. In humans, epidemiologic studies in patients with polymorphism of the ROCK gene may help to clarify whether ROCK is an important mediator or marker of cardiovascular disease.
Expert opinion
Accumulating evidence suggests that ROCK plays an important role in the pathogenesis of cardiovascular disease. Increased ROCK activity is associated with cardiovascular risk factors such as hypertension and diabetes, and abnormal ROCK activity in vascular and nonvascular cells may contribute, either directly or indirectly, to the development of vascular disease. Consequently, therapeutic strategies that specifically target and inhibit ROCK in the vascular wall and inflammatory cells may have clinical benefits in improving cardiovascular outcomes. Several ROCK inhibitors are commercially available, but currently, only one is approved for clinical use in alleviating vasospasm following hemorrhagic stroke. As most ROCK inhibitors target the catalytic ATPase domain, which shares homology to other protein serine-threonine kinases, they are not entirely specific and can potentially produce untoward side effects. Thus, the main limitations of ROCK inhibitors for clinical use are specificity and toxicity. Furthermore, it is not entirely clear whether ROCK inhibitors would be superior to statins, which could also inhibit the Rho/ROCK pathway and have been demonstrated to be well tolerated. Nevertheless, cardiovascular diseases, such as vasospastic angina and pulmonary hypertension, have limited therapeutic options, and may benefit from treatment with ROCK inhibitors. Clinical trials will have to be performed to determine whether ROCK inhibitors are superior in terms of efficacy and safety compared with existing drugs for cardiovascular disease such as statins, calcium channel blockers, angiotensin-converting enzyme inhibitors and angiotensin II-receptor blockers.
Five-year view
Current on-going studies will provide further insights into the role of ROCK in cardiovascular disease. Most of these studies will involve the use of ROCK inhibitors, which will be limited by their lack of specificity and in clinical studies, perhaps, by their toxicity. Further development of specific ROCK inhibitors that could target the regulatory region (i.e., carboxy-terminus) of ROCK, may prove to be useful in separating the role of ROCK isoforms in the vascular wall. Indeed, basic studies with mutant mice harboring tissue-specific ROCK deletions would have the greatest chance of increasing our understanding of the function of specific ROCK isoforms in various tissues. This increase in knowledge is likely to broaden and extend the therapeutic benefits of ROCK inhibitors beyond cardiovascular disease to other disciplines of medicine such as oncology, endocrinology and rheumatology.
Key issues
Rho-kinases (ROCKs) play a central role in diverse cellular functions such as smooth muscle contraction, stress fiber formation and cell migration and proliferation.
Recent experimental and clinical studies have demonstrated that overactivity of ROCKs contributes to cardiovascular disease, such as cerebral ischemia and coronary vasospasm, hypertension, vascular inflammation, atherosclerosis, erectile dysfunction and cardiac hypertrophy, suggesting that ROCKs may be an important therapeutic target in cardiovascular disease.
Why ROCK activity is increased, what are the downstream targets for ROCKs and which ROCK isoform plays a role in cardiovascular cells are questions that remain to be answered.
Epidemiologic studies in patients with polymorphism of the ROCK gene may help clarify whether ROCK is an important mediator or marker of cardiovascular disease.
Contributor Information
Yoshiyuki Rikitake, Brigham and Women’s Hospital and Harvard Medical School, Vascular Medicine Research Unit, Cardiovascular Division, Department of Medicine, Boston, MA 02115, USA, Tel.: +617 768 8409, Fax: +617 768 8421, yrikitake@rics.bwh.harvard.edu.
James K Liao, Brigham and Women’s Hospital and Harvard Medical School, Vascular Medicine Research Unit, Cardiovascular Division, Department of Medicine, Boston, MA 02115, USA, Tel.: +617 768 8424, Fax: +617 768 8425, jliao@rics.bwh.harvard.edu.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Riento K, Ridley AJ. ROCKs: multifunctional kinases in cell behaviour. Nature Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 2.Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995;270:29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- 3.Amano M, Ito M, Kimura K, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 4.Ishizaki T, Maekawa M, Fujisawa K, et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- 5.Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK-α is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, Rho-kinase and protein phosphatase to smooth muscle and nonmuscle myosin II. J Physiol. 2000;522:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawa O, Fujisawa K, Ishizaki T, et al. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189–193. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- 8.Matsui T, Amano M, Yamamoto T, et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- 9.Ishizaki T, Uehata M, Tamechika I, et al. Pharmacological properties of Y-27632, a specific inhibitor of Rho-associated kinases. Mol Pharmacol. 2000;57:976–983. [PubMed] [Google Scholar]
- 10.Ikenoya M, Hidaka H, Hosoya T, Suzuki M, Yamamoto N, Sasaki Y. Inhibition of Rho-kinase-induced myristoylated alanine-rich C kinase substrate (MARCKS) phosphorylation in human neuronal cells by H-1152, a novel and specific Rho-kinase inhibitor. J Neurochem. 2002;81:9–16. doi: 10.1046/j.1471-4159.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki Y, Suzuki M, Hidaka H. The novel and specific Rho-kinase inhibitor (S)-(+)-2-methyl-1-[(4-methyl-5-isoquinoline)sulfonyl]-homopiperazine as a probing molecule for Rho-kinase-involved pathway. Pharmacol Ther. 2002;93:225–232. doi: 10.1016/s0163-7258(02)00191-2. [DOI] [PubMed] [Google Scholar]
- 12.Kimura K, Ito M, Amano M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 13.Inada H, Goto H, Tanabe K, et al. Rho-associated kinase phosphorylates desmin, the myogenic intermediate filament protein, at unique amino-terminal sites. Biochem Biophys Res Commun. 1998;253:21–25. doi: 10.1006/bbrc.1998.9732. [DOI] [PubMed] [Google Scholar]
- 14.Katoh K, Kano Y, Amano M, et al. Rho-kinase-mediated contraction of isolated stress fibers. J Cell Biol. 2001;153:569–584. doi: 10.1083/jcb.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen XQ, Tan I, Ng CH, Hall C, Lim L, Leung T. Characterization of RhoA-binding kinase ROK-α implication of the pleckstrin homology domain in ROK-α function using region-specific antibodies. J Biol Chem. 2002;277:12680–12688. doi: 10.1074/jbc.M109839200. [DOI] [PubMed] [Google Scholar]
- 16.Chevrier V, Piel M, Collomb N, et al. The Rho-associated protein kinase p160ROCK is required for centrosome positioning. J Cell Biol. 2002;157:807–817. doi: 10.1083/jcb.200203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amano M, Chihara K, Nakamura N, Kaneko T, Matsuura Y, Kaibuchi K. The COOH terminus of Rho-kinase negatively regulates Rho-kinase activity. J Biol Chem. 1999;274:32418–32424. doi: 10.1074/jbc.274.45.32418. [DOI] [PubMed] [Google Scholar]
- 18.Feng J, Ito M, Kureishi Y, et al. Rho-associated kinase of chicken gizzard smooth muscle. J Biol Chem. 1999;274:3744–3752. doi: 10.1074/jbc.274.6.3744. [DOI] [PubMed] [Google Scholar]
- 19.Sebbagh M, Renvoize C, Hamelin J, et al. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nature Cell Biol. 2001;3:346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- 20.Coleman ML, Sahai EA, Yeo M, et al. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nature Cell Biol. 2001;3:339–345. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- 21.Amano M, Chihara K, Kimura K, et al. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 22.Turner MS, Fen Fen L, Trauger JW, Stephens J, LoGrasso P. Characterization and purification of truncated human Rho-kinase II expressed in Sf-21 cells. Arch Biochem Biophys. 2002;405:13–20. doi: 10.1016/s0003-9861(02)00249-7. [DOI] [PubMed] [Google Scholar]
- 23.Ward Y, Yap SF, Ravichandran V, et al. The GTP-binding proteins Gem and Rad are negative regulators of the Rho-Rho kinase pathway. J Cell Biol. 2002;157:291–302. doi: 10.1083/jcb.200111026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumi T, Matsumoto K, Nakamura T. Specific activation of LIM-kinase-2 via phosphorylation of threonine 505 by ROCK, a Rho-dependent protein kinase. J Biol Chem. 2001;276:670–676. doi: 10.1074/jbc.M007074200. [DOI] [PubMed] [Google Scholar]
- 25.Kawano Y, Fukata Y, Oshiro N, et al. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol. 1999;147:1023–1038. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng J, Ito M, Ichikawa K, et al. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem. 1999;274:37385–37390. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- 27.Velasco G, Armstrong C, Morrice N, Frame S, Cohen P. Phosphorylation of the regulatory subunit of smooth muscle protein phosphatase 1M at Thr850 induces its dissociation from myosin. FEBS Lett. 2002;527:101–104. doi: 10.1016/s0014-5793(02)03175-7. [DOI] [PubMed] [Google Scholar]
- 28.Matsui T, Maeda M, Doi Y, et al. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem. 2000;275:3577–3582. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- 30.Maekawa M, Ishizaki T, Boku S, et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 31.Di Cunto F, Imarisio S, Hirsch E, et al. Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron. 2000;28:115–127. doi: 10.1016/s0896-6273(00)00090-8. [DOI] [PubMed] [Google Scholar]
- 32.Wei L, Roberts W, Wang L, et al. Rho kinases play an obligatory role in vertebrate embryonic organogenesis. Development. 2001;128:2953–2962. doi: 10.1242/dev.128.15.2953. [DOI] [PubMed] [Google Scholar]
- 33.Hart MJ, Jiang X, Kozasa T, et al. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Gα 13. Science. 1998;280:2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- 34.Kozasa T, Jiang X, Hart MJ, et al. p115 RhoGEF, a GTPase activating protein for Gα 12 and Gα 13. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- 35•.Uehata M, Ishizaki T, Satoh H, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. Characterizes Y-27632, the most widely used pharmacologic as a specific Rho-kinase (ROCK) inhibitor. [DOI] [PubMed] [Google Scholar]
- 36.Bourguignon LY, Zhu H, Shao L, Zhu D, Chen YW. Rho-kinase (ROK) promotes CD44v(3,8–10)-ankyrin interaction and tumor cell migration in metastatic breast cancer cells. Cell Motil Cytoskeleton. 1999;43:269–287. doi: 10.1002/(SICI)1097-0169(1999)43:4<269::AID-CM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Yoshioka K, Nakamori S, Itoh K. Overexpression of small GTP-binding protein RhoA promotes invasion of tumor cells. Cancer Res. 1999;59:2004–2010. [PubMed] [Google Scholar]
- 38.Itoh K, Yoshioka K, Akedo H, Uehata M, Ishizaki T, Narumiya S. An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nature Med. 1999;5:221–225. doi: 10.1038/5587. [DOI] [PubMed] [Google Scholar]
- 39.Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vasc Pharmacol. 2002;39:187–199. doi: 10.1016/s1537-1891(03)00008-9. [DOI] [PubMed] [Google Scholar]
- 40.Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114:1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- 41.Farah S, Agazie Y, Ohan N, Ngsee JK, Liu XJ. A Rho-associated protein kinase, ROKα, binds insulin receptor substrate-1 and modulates insulin signaling. J Biol Chem. 1998;273:4740–4746. doi: 10.1074/jbc.273.8.4740. [DOI] [PubMed] [Google Scholar]
- 42.Sordella R, Classon M, Hu KQ, et al. Modulation of CREB activity by the Rho GTPase regulates cell and organism size during mouse embryonic development. Dev Cell. 2002;2:553–565. doi: 10.1016/s1534-5807(02)00162-4. [DOI] [PubMed] [Google Scholar]
- 43.O’Cochlain DF, Perez-Terzic C, Reyes S, et al. Transgenic overexpression of human DMPK accumulates into hypertrophic cardiomyopathy, myotonic myopathy and hypotension traits of myotonic dystrophy. Hum Mol Genet. 2004;13:2505–2518. doi: 10.1093/hmg/ddh266. [DOI] [PubMed] [Google Scholar]
- 44.Pan J, Singh US, Takahashi T, et al. PKC mediates cyclic stretch-induced cardiac hypertrophy through Rho family GTPases and mitogen-activated protein kinases in cardiomyocytes. J Cell Physiol. 2005;202:536–553. doi: 10.1002/jcp.20151. [DOI] [PubMed] [Google Scholar]
- 45.Sordella R, Jiang W, Chen GC, Curto M, Settleman J. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell. 2003;113:147–158. doi: 10.1016/s0092-8674(03)00271-x. [DOI] [PubMed] [Google Scholar]
- 46.Niiro N, Koga Y, Ikebe M. Agonist-induced changes in the phosphorylation of the myosin-binding subunit of myosin light chain phosphatase and CPI17, two regulatory factors of myosin light chain phosphatase, in smooth muscle. Biochem J. 2003;369:117–128. doi: 10.1042/BJ20021040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mukai Y, Shimokawa H, Matoba T, et al. Involvement of Rho-kinase in hypertensive vascular disease: a novel therapeutic target in hypertension. FASEB J. 2001;15:1062–1064. doi: 10.1096/fj.00-0735fje. [DOI] [PubMed] [Google Scholar]
- 48.Seko T, Ito M, Kureishi Y, et al. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res. 2003;92:411–418. doi: 10.1161/01.RES.0000059987.90200.44. [DOI] [PubMed] [Google Scholar]
- 49.Masumoto A, Hirooka Y, Shimokawa H, Hironaga K, Setoguchi S, Takeshita A. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension. 2001;38:1307–1310. doi: 10.1161/hy1201.096541. [DOI] [PubMed] [Google Scholar]
- 50.Moriki N, Ito M, Seko T, et al. RhoA activation in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. Hypertens Res. 2004;27:263–270. doi: 10.1291/hypres.27.263. [DOI] [PubMed] [Google Scholar]
- 51.Surks HK, Mochizuki N, Kasai Y, et al. Regulation of myosin phosphatase by a specific interaction with cGMP-dependent protein kinase Iα. Science. 1999;286:1583–1587. doi: 10.1126/science.286.5444.1583. [DOI] [PubMed] [Google Scholar]
- 52.Ito K, Hirooka Y, Sakai K, et al. Rho/Rho-kinase pathway in brain stem contributes to blood pressure regulation via sympathetic nervous system: possible involvement in neural mechanisms of hypertension. Circ Res. 2003;92:1337–1343. doi: 10.1161/01.RES.0000079941.59846.D4. [DOI] [PubMed] [Google Scholar]
- 53.Ito K, Hirooka Y, Kishi T, et al. Rho/Rho-kinase pathway in the brainstem contributes to hypertension caused by chronic nitric oxide synthase inhibition. Hypertension. 2004;43:156–162. doi: 10.1161/01.HYP.0000114602.82140.a4. [DOI] [PubMed] [Google Scholar]
- 54•.Ito K, Hirooka Y, Sagara Y, et al. Inhibition of Rho-kinase in the brainstem augments baroreflex control of heart rate in rats. Hypertension. 2004;44:478–483. doi: 10.1161/01.HYP.0000143120.24612.68. This paper, [52] and [53] show that ROCK is involved in the central regulatory mechanism of blood pressure. [DOI] [PubMed] [Google Scholar]
- 55.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2) N Engl J Med. 1992;326:310–318. doi: 10.1056/NEJM199201303260506. [DOI] [PubMed] [Google Scholar]
- 56.Shimokawa H. Rho-kinase as a novel therapeutic target in treatment of cardiovascular diseases. J Cardiovasc Pharmacol. 2002;39:319–327. doi: 10.1097/00005344-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 57.Shimokawa H, Seto M, Katsumata N, et al. Rho-kinase-mediated pathway induces enhanced myosin light-chain phosphorylations in a swine model of coronary artery spasm. Cardiovasc Res. 1999;43:1029–1039. doi: 10.1016/s0008-6363(99)00144-3. [DOI] [PubMed] [Google Scholar]
- 58.Kandabashi T, Shimokawa H, Miyata K, et al. Inhibition of myosin phosphatase by upregulated Rho-kinase plays a key role for coronary artery spasm in a porcine model with interleukin-1β. Circulation. 2000;101:1319–1323. doi: 10.1161/01.cir.101.11.1319. [DOI] [PubMed] [Google Scholar]
- 59.Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105:1545–1547. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- 60.Mohri M, Shimokawa H, Hirakawa Y, Masumoto A, Takeshita A. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischaemia in patients with coronary microvascular spasm. J Am Coll Cardiol. 2003;41:15–19. doi: 10.1016/s0735-1097(02)02632-3. [DOI] [PubMed] [Google Scholar]
- 61.Inokuchi K, Ito A, Fukumoto Y, et al. Usefulness of fasudil, a Rho-kinase inhibitor, to treat intractable severe coronary spasm after coronary artery bypass surgery. J Cardiovasc Pharmacol. 2004;44:275–277. doi: 10.1097/01.fjc.0000134775.76636.3f. [DOI] [PubMed] [Google Scholar]
- 62.Utsunomiya T, Satoh S, Ikegaki I, et al. Antianginal effects of hydroxyfasudil, a Rho-kinase inhibitor, in a canine model of effort angina. Br J Pharmacol. 2001;134:1724–1730. doi: 10.1038/sj.bjp.0704410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63••.Shimokawa H, Hiramori K, Iinuma H, et al. Antianginal effect of fasudil, a Rho-kinase inhibitor, in patients with stable effort angina: a multicenter study. J Cardiovasc Pharmacol. 2002;40:751–761. doi: 10.1097/00005344-200211000-00013. This report of a Phase II clinical trial in Japan shows the usefulness of oral administration of fasudil in patients with stable effort angina. [DOI] [PubMed] [Google Scholar]
- 64••.Vicari R, Chaitman B, Keefe D, et al. American College of Cardiology 53rd Annual Scientific Session. New Orleans, LA, USA: 2004. A randomized double-blind placebo-controlled Phase II study on the efficancy and safety of fasudil in patients with stable angina. This report of a Phase II clinical trial in the USA shows the usefulness of oral administration of fasudil in patients with effort angina. [Google Scholar]
- 65.Kataoka C, Egashira K, Inoue S, et al. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension. 2002;39:245–250. doi: 10.1161/hy0202.103271. [DOI] [PubMed] [Google Scholar]
- 66.Ikegaki I, Hattori T, Yamaguchi T, et al. Involvement of Rho-kinase in vascular remodeling caused by long-term inhibition of nitric oxide synthesis in rats. Eur J Pharmacol. 2001;427:69–75. doi: 10.1016/s0014-2999(01)01181-5. [DOI] [PubMed] [Google Scholar]
- 67.Sawada N, Itoh H, Ueyama K, et al. Inhibition of Rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation. 2000;101:2030–2033. doi: 10.1161/01.cir.101.17.2030. [DOI] [PubMed] [Google Scholar]
- 68.Shibata R, Kai H, Seki Y, et al. Role of Rho-associated kinase in neointima formation after vascular injury. Circulation. 2001;103:284–289. doi: 10.1161/01.cir.103.2.284. [DOI] [PubMed] [Google Scholar]
- 69.Eto Y, Shimokawa H, Hiroki J, et al. Gene transfer of dominant–negative Rho-kinase suppresses neointimal formation after balloon injury in pigs. Am J Physiol Heart Circ Physiol. 2000;278:H1744–H1750. doi: 10.1152/ajpheart.2000.278.6.H1744. [DOI] [PubMed] [Google Scholar]
- 70.Mack CP, Somlyo AV, Hautmann M, Somlyo AP, Owens GK. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J Biol Chem. 2001;276:341–347. doi: 10.1074/jbc.M005505200. [DOI] [PubMed] [Google Scholar]
- 71.Matsumoto Y, Uwatoku T, Oi K, et al. Long-term inhibition of Rho-kinase suppresses neointimal formation after stent implantation in porcine coronary arteries: involvement of multiple mechanisms. Arterioscler Thromb Vasc Biol. 2004;24:181–186. doi: 10.1161/01.ATV.0000105053.46994.5B. [DOI] [PubMed] [Google Scholar]
- 72.Miyata K, Shimokawa H, Kandabashi T, et al. Rho-kinase is involved in macrophage-mediated formation of coronary vascular lesions in pigs in vivo. Arterioscler Thromb Vasc Biol. 2000;20:2351–2358. doi: 10.1161/01.atv.20.11.2351. [DOI] [PubMed] [Google Scholar]
- 73.Shimokawa H, Morishige K, Miyata K, et al. Long-term inhibition of Rho-kinase induces a regression of arteriosclerotic coronary lesions in a porcine model in vivo. Cardiovasc Res. 2001;51:169–177. doi: 10.1016/s0008-6363(01)00291-7. [DOI] [PubMed] [Google Scholar]
- 74.Morishige K, Shimokawa H, Eto Y, et al. Adenovirus-mediated transfer of dominant–negative Rho-kinase induces a regression of coronary arteriosclerosis in pigs in vivo. Arterioscler Thromb Vasc Biol. 2001;21:548–554. doi: 10.1161/01.atv.21.4.548. [DOI] [PubMed] [Google Scholar]
- 75.Hattori T, Shimokawa H, Higashi M, et al. Long-term treatment with a specific Rho-kinase inhibitor suppresses cardiac allograft vasculopathy in mice. Circ Res. 2004;94:46–52. doi: 10.1161/01.RES.0000107196.21335.2B. [DOI] [PubMed] [Google Scholar]
- 76.Funakoshi Y, Ichiki T, Shimokawa H, et al. Rho-kinase mediates angiotensin II-induced monocyte chemoattractant protein-1 expression in rat vascular smooth muscle cells. Hypertension. 2001;38:100–104. doi: 10.1161/01.hyp.38.1.100. [DOI] [PubMed] [Google Scholar]
- 77.Takeda K, Ichiki T, Tokunou T, et al. Critical role of Rho-kinase and MEK/ERK pathways for angiotensin II-induced plasminogen activator inhibitor type-1 gene expression. Arterioscler Thromb Vasc Biol. 2001;21:868–873. doi: 10.1161/01.atv.21.5.868. [DOI] [PubMed] [Google Scholar]
- 78.Kawamura H, Yokote K, Asaumi S, et al. High glucose-induced upregulation of osteopontin is mediated via Rho/Rho-kinase pathway in cultured rat aortic smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:276–281. doi: 10.1161/01.ATV.0000112012.33770.2a. [DOI] [PubMed] [Google Scholar]
- 79.Ishibashi T, Sakamoto T, Ohkawara H, et al. Integral role of RhoA activation in monocyte adhesion-triggered tissue factor expression in endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:681–687. doi: 10.1161/01.ATV.0000065194.00822.C7. [DOI] [PubMed] [Google Scholar]
- 80.Eto M, Kozai T, Cosentino F, Joch H, Luscher TF. Statin prevents tissue factor expression in human endothelial cells: role of Rho/Rho-kinase and Akt pathways. Circulation. 2002;105:1756–1759. doi: 10.1161/01.cir.0000015465.73933.3b. [DOI] [PubMed] [Google Scholar]
- 81.Mallat Z, Gojova A, Sauzeau V, et al. Rho-associated protein kinase contributes to early atherosclerotic lesion formation in mice. Circ Res. 2003;93:884–888. doi: 10.1161/01.RES.0000099062.55042.9A. [DOI] [PubMed] [Google Scholar]
- 82.Abe K, Shimokawa H, Morikawa K, et al. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res. 2004;94:385–393. doi: 10.1161/01.RES.0000111804.34509.94. [DOI] [PubMed] [Google Scholar]
- 83.Fagan KA, Oka M, Bauer NR, et al. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287:L656–L664. doi: 10.1152/ajplung.00090.2003. [DOI] [PubMed] [Google Scholar]
- 84.Nagaoka T, Fagan KA, Gebb SA, et al. Inhaled Rho-kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med. 2005;171:494–499. doi: 10.1164/rccm.200405-637OC. [DOI] [PubMed] [Google Scholar]
- 85•.Fukumoto Y, Hirakawa Y, Abe K, et al. Intravenous administration of a rho-kinase inhibitor, fasudil, reduces pulmonary vascular resistance in patients with pulmonary hypertension. The 13th Intnatl Symp. Atheroscler; Kyoto, Japan. 2003. First demonstrated that fasudil effectively reduces pulmonary artery resistance in patients with severe pulmonary hypertension. [Google Scholar]
- 86.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 87.Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 88.Eto M, Barandier C, Rathgeb L, et al. Thrombin suppresses endothelial nitric oxide synthase and upregulates endothelin-converting enzyme-1 expression by distinct pathways: role of Rho/ROCK and mitogen-activated protein kinase. Circ Res. 2001;89:583–590. doi: 10.1161/hh1901.097084. [DOI] [PubMed] [Google Scholar]
- 89•.Ming XF, Viswambharan H, Barandier C, et al. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22:8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. Demonstrates that ROCK negatively regulates not only endothelial nitric oxide synthase (eNOS) expression but also eNOS phosphorylation and thereby inhibits nitric oxide production by endothelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wolfrum S, Dendorfer A, Rikitake Y, et al. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24:1842–1847. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chitaley K, Wingard CJ, Clinton Webb R, et al. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nature Med. 2001;7:119–122. doi: 10.1038/83258. [DOI] [PubMed] [Google Scholar]
- 92.Bivalacqua TJ, Champion HC, Usta MF, et al. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci USA. 2004;101:9121–9126. doi: 10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sah VP, Minamisawa S, Tam SP, et al. Cardiac-specific overexpression of RhoA results in sinus and atrioventricular nodal dysfunction and contractile failure. J Clin Invest. 1999;103:1627–1634. doi: 10.1172/JCI6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Higashi M, Shimokawa H, Hattori T, et al. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res. 2003;93:767–775. doi: 10.1161/01.RES.0000096650.91688.28. [DOI] [PubMed] [Google Scholar]
- 95.Satoh S, Ueda Y, Koyanagi M, et al. Chronic inhibition of Rho-kinase blunts the process of left ventricular hypertrophy leading to cardiac contractile dysfunction in hypertension-induced heart failure. J Mol Cell Cardiol. 2003;35:59–70. doi: 10.1016/s0022-2828(02)00278-x. [DOI] [PubMed] [Google Scholar]
- 96.Bao W, Hu E, Tao L, et al. Inhibition of Rho-kinase protects the heart against ischaemia/reperfusion injury. Cardiovasc Res. 2004;61:548–558. doi: 10.1016/j.cardiores.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 97.Hattori T, Shimokawa H, Higashi M, et al. Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation. 2004;109:2234–2239. doi: 10.1161/01.CIR.0000127939.16111.58. [DOI] [PubMed] [Google Scholar]
- 98.Yada T, Shimokawa H, Hiramatsu O, et al. Beneficial effect of hydroxyfasudil, a specific Rho-kinase inhibitor, on ischaemia/reperfusion injury in canine coronary microcirculation in vivo. J Am Coll Cardiol. 2005;45:599–607. doi: 10.1016/j.jacc.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 99.Hirooka Y, Shimokawa H. Therapeutic potential of Rho-kinase inhibitors in cardiovascular diseases. Am J Cardiovasc Drugs. 2005;5:31–39. doi: 10.2165/00129784-200505010-00005. [DOI] [PubMed] [Google Scholar]
- 100.Liao JK. Isoprenoids as mediators of the biological effects of statins. J Clin Invest. 2002;110:285–288. doi: 10.1172/JCI16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 102.Wolfrum S, Jensen KS, Liao JK. Endothelium-dependent effects of statins. Arterioscler Thromb Vasc Biol. 2003;23:729–736. doi: 10.1161/01.ATV.0000063385.12476.A7. [DOI] [PubMed] [Google Scholar]
- 103.Thumkeo D, Keel J, Ishizaki T, et al. Targeted disruption of the mouse Rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol. 2003;23:5043–5055. doi: 10.1128/MCB.23.14.5043-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]