Abstract
This article discusses cytokine patterns as potential biomarkers of vaginal inflammation, which are needed for the safety evaluation of topical microbicide products for the prevention of sexually transmitted HIV-1 infection. In order to be effective, the vaginal anti-HIV-1 microbicides should avoid proinflammatory responses that facilitate transepithelial viral penetration and replication. Pro-inflammatory and anti-inflammatory cytokines play bi-directional roles in HIV-1 pathogenesis, transmission, susceptibility and resistance. Previous research has shown that many of these key mediators of mucosal barrier function (e.g. IL-1, IL-1 receptor antagonist, IL-6, TNF-α, TNF-receptor II, transforming growth factor β, IL-10, IL-12, IL-8, macrophage inhibitory protein 1, etc.) can be detected in the vaginal secretions of healthy or infected individuals using non-invasive sampling techniques. As part of two microbicide trials, we measured IL-1α, IL-1β, IL-1 receptor antagonist, TNF-α and IL-8 in 291 cervicovaginal lavage samples obtained before product use and at the seventh and 14th day after product use. We showed that vaginal formulations, temperature and matrix-specific factors in the vaginal fluids may interfere with cytokine detection, and therefore specific protocols must be validated for various collection procedures and cytokine assays. Our results suggest that combined patterns of cytokine dynamics rather than individual measurements might distinguish proinflammatory product-related effects in microbicide safety trials. More research is needed to establish cytokine mucosal baselines and modulation by genetic factors, sexual intercourse, menstrual cycle, exercise, hormones, stress and infections before guidelines can be established for clinical trial enrollment criteria, the prediction of side/adverse events and ultimately microbicide benefit prognostication.
Keywords: Cervicovaginal lavage, cytokines, HIV-1, inflammation, microbicides, safety evaluation
Inflammatory Biomarkers in Drug Discovery: The Boost for Development
Objectively measured surrogate biochemical markers of normal function, pathological processes or drug response have long been sought to substitute for or complement invasive or subjective/descriptive clinical endpoints in the process of drug discovery to predict clinical benefit or harm. A currently accepted classification of biomarkers includes: type 0, disease markers to diagnose illness and suggest novel therapies; type I, drug effect markers to compare pre- and post-dosing and drug versus placebo; and type II, drug efficacy markers to compare responders versus non-responders, both pre- and post-dosing for benefit prognostication, prediction of side/adverse events, patient stratification, enrollment criteria and label expansion.1 Ubiquitous standards and automated detection methods are available for classic blood and urine type 0 and type I markers of cellular integrity and homeostasis, related to heart, liver, neuroendocrine, respiratory, circulatory and muscle function. Recent advances in genomics, proteomics and high throughput analysis techniques have expanded the list and facilitated the use of type II biomarkers of autoimmunity, allergy, infection, cancer and inflammation.
The current biochemical markers of systemic and mucosal inflammatory disease are particularly diverse. A better understanding of molecular crosstalk in inflammatory cascades and the emergence of anticytokine therapies against chronic diseases associated with immune dysregulation has facilitated the discovery of non-canonical inflammatory markers and immune response modifiers.2–5 Proposed indices of systemic and mucosal inflammation include endocrine/metabolic markers (arachidonic acids, nitric oxide, calcitonin precursors, leptin), neuroendocrine markers (adrenomedulin, monocyte migration inhibitor factor), cellular markers (heat shock proteins, nuclear factor kappa B, oxygen-free radicals) and immune system markers (acute phase proteins, cytokines, leukocyte elastase, adhesion molecules, kinins).6
Although numerous studies have characterized the levels of proinflammatory cytokines in trauma patients and systemic disease, the cytokine equilibrium at most mucosal surfaces has not been fully characterized under normal and pathological conditions. The rapid development of the concept of mucosally applied microbicides for the prevention of HIV-1 infection and other sexually transmitted disease (STD) has revitalized the field of mucosal immunology and interest in the genital tract inflammatory paradigm.7–12 This article discusses the use of inflammatory cytokines as type II biomarkers for the safety evaluation of vaginal anti-HIV-1 microbicides.
Cytokines for Microbicide Safety Evaluation: The Proof-of-Concept
Microbicides for vaginal application are currently regarded as a leading strategy against the heterosexual spread of HIV-1 and other STDs.7–13 The fact that the healthy mucosal environment provides an efficient natural barrier against HIV-1 and that these products are being developed for over-the-counter frequent use by healthy as well as HIV-1-infected individuals brings to the fore the need for adequate safety evaluation.
The sexual transmission of HIV-1 infection occurs via transepithelial viral penetration.14 It is intuitively obvious that effective anti-HIV-1 microbicides must preserve the mucosal immune function and their ability to ‘seal’ the cervicovaginal lumen from the subepithelial space. The inflammatory cytokine response cannot only erode epithelial continuity, but even in the absence of excessive lesions can cause macromolecular leaks and the migration of leukocytes via paracellular pathways lasting as long as 20 h after insult.15 Moreover, proinflammatory cytokines (e.g. IL-1, TNF-α, IL-6) can directly trigger HIV-1 replication in latently infected cells and may underlie the increased risk of HIV-1 infection associated with non-ulcerative inflammatory STDs.16–18 Cytokine activation of latent HIV-1 in mucosal reservoirs may increase sexual transmission in discordant couples or vertical mother-to-child transmission.19–22 Clinical and experimental evidence suggests that cytokine dysregulation after the frequent use of nonoxynol-9-based products may hamper their clinical anti-HIV-1 effectiveness despite potent anti-HIV-1 activity in vitro.10,23 It is thus imperative that anti-HIV-1 microbicides do not perturb the mucosal cytokine balance.
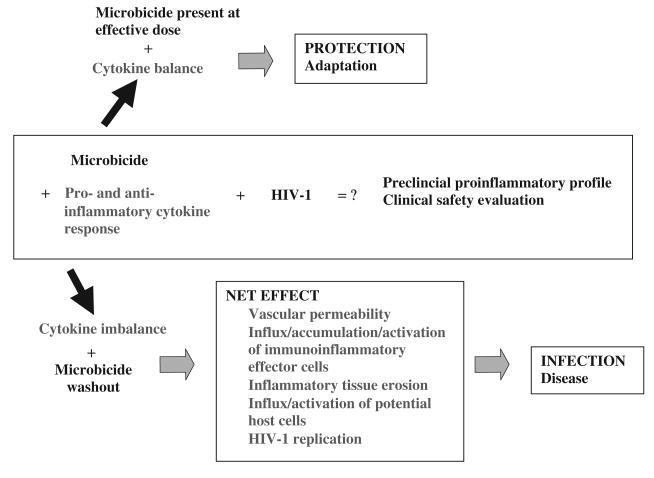
Various levels of proinflammatory and anti-inflammatory factors are physiologically present at equilibrium in the vaginal secretions of healthy individuals.10,24,25 As demonstrated by our nonoxynol-9 studies, vaginal products applied at the margin of cytotoxicity may cause an excessive release of IL-1 stores from membrane compromised keratinocytes or the direct activation of proinflammatory pathways that may turn pathogenic if they exceed the counterbalancing potential of the natural mucosal anti-inflammatory factors.10 On the other hand, it can be hypothesized that the transient inflammatory response may facilitate tissue repair and the adaptive immune function if the cytokine equilibrium is restored while the microbicide product still exerts its protective anti-HIV-1 action (Figure 1). Appropriate markers of vaginal inflammation and study designs need to be established that can distinguish between protective and increased-risk cytokine responses.
FIGURE 1.
The net effect of anti-HIV-1 microbicide products depends on the balance between the proinflammatory and anti-inflammatory host factors and the effective anti-HIV-1 dose. The magnitude, balance and timing of cytokine release should be targeted for clinical detection and prognostication.
The choice of cytokine markers of vaginal inflammation that can be monitored during clinical trials requires a good understanding of the dynamics of cytokine production at the mucosal site. Good candidates appear to be TNF-α, IL-1, IL-1 receptor agonist (RA), IL-6 and IL-8, which have shown a positive correlation with HIV-1 viral load or disease status.12,24,26–28 TNF-α, one of the most powerful primary pro-inflammatory mediators and activators of HIV-1 replication, has a very short half-life (minutes in blood), and its synthesis lasts only a short time (< 8 h) after initial stimulus.29 However, it may be stabilized by binding to soluble TNF receptors (TNF-RI, II) that are present in the vaginal secretions and might be increased after chemical insult.10,30 Clinical studies have shown correlation between TNFRII levels and the HIV-1 viral load in vaginal fluids.26 The IL-1 family includes several soluble members that can be found in the vaginal fluids: IL-1α (both precursor and mature forms are active), IL-1β (mature form), a signal-transducing receptor (IL-1RI) and one decoy receptor (IL-1RII) and endogenous IL-1RA.10,28,31 The initial burst of IL-1 after chemical insult can be partly neutralized by the release of preformed endogenous antagonists (IL-1RA and soluble IL-1RII) and their synthesis is upregulated by IL-1 in autocrine and paracrine fashion to balance the proinflammatory effect.10,31 Unlike TNF-α and IL-1, the secondary proinflammatory mediators IL-6 and IL-8 lack the tight self-regulatory feedback mechanism, and can thus demonstrate long-lasting (72 h) production after initial stimulus.29 Based on these dynamic patterns we postulate the possibility of lasting proinflammatory cytokine dysregulation in the microbicide washout period, which may activate latent HIV-1 reservoirs in the absence of protective microbicide, and thus pose an increased risk of HIV-1 infection and transmission despite or regardless of the patient's compliance with the product use.
Markers of Vaginal Inflammation and Confounding Variables: The Plausibility
Clinical variables and physiological conditions can modify the effect or confound the assessment of product-related cytokine dysregulation. Co-morbidity and genital tract infection can increase significantly the background cytokine ‘noise’ in the cervicovaginal secretions. Proinflammatory cytokines are abundantly present in the genital secretions of patients with HIV-1 infection, bacterial vaginosis and vaginitis.24,27,28,32 Furthermore, IL-8 levels in cervicovaginal lavages (CVLs) correlate with upper genital tract infections.33 More studies are needed to elucidate the impact of asymptomatic infection or chronic illness such as diabetes mellitus or other autoimmune diseases on the cytokine balance of genital tract mucosae.
More research is also needed to address the effect of endogenous and exogenous hormones that can affect cytokine production and stability in the cervicovaginal compartment. Variations of proinflammatory and anti-inflammatory cytokines (IL-1β, IL-6, IL-8, regulated upon activation: normal T cell expressed/secreted, macrophage-inflammatory protein 1α and 1β, transforming growth factor beta, IL-4, IL-10) in the cervical mucus or vaginal secretions have been reported by a few studies utilizing cytobrush or CVL collection at defined stages of the menstrual cycle of healthy subjects and HIV-1-infected individuals, as well as in cervical mucus during pregnancy and oral contraceptive use.26,34–36 A comparison of the cervical wick and CVL techniques showed a better reproducibility of cytokine results obtained with the CVL technique.37 It should be recognized that most of these studies include small numbers of subjects and different methodologies, which impairs the establishment of criteria for homeostatic cytokine values in healthy individuals.
The immunomodulatory impact of sexual intercourse and contact with semen constituents has been suggested by human in vitro and animal studies. Despite the extremely high anti-inflammatory properties of semen, epithelial cells elicit physiological proinflammatory cytokine responses upon contact with semen constituents, which are thought to be beneficial for the endometrial receptivity to embryo implantation.38 In vitro experiments have shown that human seminal plasma can induce an increase in the IL-10 : IL-12 ratio, which may interfere with natural killer and cytotoxic T lymphocyte responses to viral infection.39
Other factors that may contribute to the interindividual variation of baseline cytokine values include the genotype of the responder, age, exercise, stress, and circadian cycle. Several allotypes of human cytokine genes have been described recently that may be related to an inheritable capacity to produce cytokines under defined conditions.40,41 Increased plasma levels of IL-1β, IL-1RA, TNF-α, TNF-Rs, IL-8 and IL-10 and especially IL-6 have been associated with aging and strenuous exercise.42,43 Interestingly, highly trained individuals express higher baseline (pre-exercise) circulating IL-1 levels.42 Cytokine expression in the local cervicovaginal pool may vary with age and sexual maturation.44 On the other hand, monkey studies have shown that menopausal cytokine dysregulation may not be corrected by hormone replacement therapy, reinforcing the aging hypothesis.45 Stress and the circadian cycle have been associated with variations in cytokine expression by epithelial and immune cells and concentrations in various body fluids.46–48
The complexity of the cytokine network in the dynamic mucosal environment requires the establishment of clinically validated criteria for the separation of product-related changes from individual background levels.
Validation of Assays: Technical Caveats
A variety of methods and commercial assays are currently available for the quantitation of cytokines in body fluids, cellular exudates and biopsy specimens. These include bioassays and enzyme-linked immunosorbent assays (ELISAs) for soluble mediators, flow cytometry, enzyme-linked immunospot assays, and immunocytochemistry for detecting cytokines in the context of cell phenotype and tissue architecture, in-situ hybridization for cytokine messenger RNA in cells and tissues, and the microarray chip technologies for the simultaneous detection of hundreds of drug-induced genes (toxicogenomics) or proteins (proteomics).2,47,49 The choice of assays for the measurement of cytokines in body fluids is determined by the specificity, sensitivity and dynamic range, reproducibility, precision and utility of the assay. A number of pre- and post-sampling factors may interfere with cytokine detection and obscure data interpretation. Most commercial ELISAs are standardized for comparisons against World Health Organization accepted cytokine standards.50
Commercially available ELISAs often account for cytokine precursors, endogenous cytokine ‘trapping’ molecules such as a2-macroglobulin, autoantibodies, soluble receptors (sIL-1RII, TNFRI and II, IL6R), crossreactivity with competing cytokine subunits (IL-12p40). Polymorphism has been extensively studied for some proinflammatory cytokines (IL-1β, IL-RA, IL-6 and macrophage colony-stimulating factor), but has to be more adequately addressed by commercial assays.51
Sample processing on ice, the quick separation of fluid from cellular components, the use of low protein binding and endotoxin-free containers and tubing, and storage at a low temperature can be crucial to avoid false negative findings (cytokine degradation or binding to polymer or membrane receptors) or false positive results (cellular production and release after sampling).52 Matrix-specific factors such as hemoglobin, albumin, mucus, and pH may also interfere with the cytokine detection procedure.53
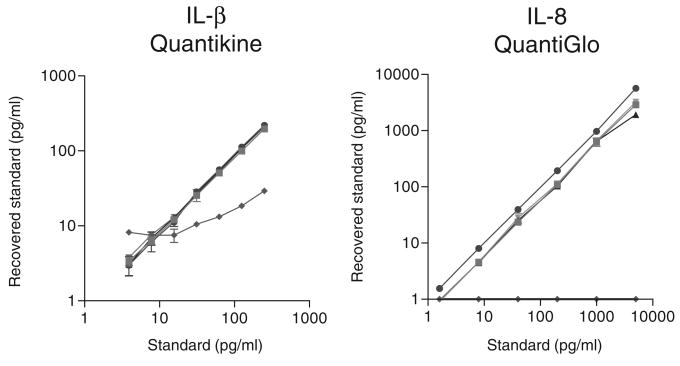
Although commercially available cytokine assays have been validated for serum, plasma (citrate, heparin, EDTA) and urine, and some have been qualified for amniotic fluid, sputum and bronchial lavage, most of the manufacturers do not provide information for genital tract fluids such as seminal plasma and vaginal lavage. Moreover, the presence of vaginal formulations in the lavage specimens obtained in microbicide clinical trials poses an additional confounding variable specific to this field (Figure 2).
FIGURE 2.
Examples of the recovery of cytokine standards spiked in 4% solutions of three microbicide gel formulations and two control formulations. One of the five formulations showed severe interference with commercial cytokine enzyme-linked immunosorbent assay kits represented by photometric Quantikine IL-1β and chemiluminescence QuantiGlo IL-8 kits available through R&D Systems.
Some Experience from Microbicide Clinical Trials: Pattern Interpretation
We established ELISA protocols and tested over a period of several months 291 CVL samples obtained from two CONRAD-funded microbicide studies. Volunteers for these studies applied either microbicide or control product intravaginally twice a day for 14 intermenstrual days. Lavage samples were obtained in 10 cc saline during three visits: visit 2 before product use; visit 3 and visit 4 at the seventh and 14th day of product use, respectively. Because the following results are presented before data analysis and study personnel unmasking, the treatment group and control group data are pooled.
We established detailed protocols and quality control procedures for the measurement of IL-1α, IL-1β, IL-8, IL-1RA and TNF-α in the CVLs using commercial photometric or luminescence ELISAs (R&D Systems, Minneapolis, MN, USA, and Endogen-Pierce, Rockford, IL, USA), a Multilabel Microplate Counter Victor 2 (Perkin Elmer Life Sciences, Boston, MA, USA) and WorkOut Version 1.5 Wallace Software (DAZDAQ Ltd., Ringmer, East Sussex, UK). The intra-assay and inter-assay coefficients of variation were determined from duplicate measurements in at least three independent assay runs for each assay. For this purpose we used standard spiking in pooled CVLs.
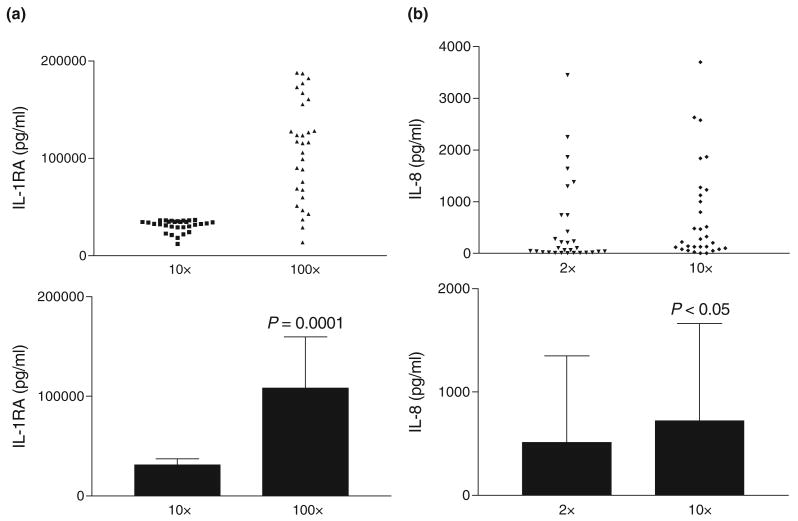
We determined the optimal dilutions of CVLs that avoid matrix interference and allow reproducible measurements within the best-fit detection range. To neutralize the acidic pH of the CVLs, which interferes with some cytokine detection systems, we recommend the dilution of samples at least two times with the assay's sample or calibrator diluent (usually protein/serum base with preservatives which may contain 0.01% Thimerosal). For IL-8 quantitation in 10 cc CVLs we recommend a screening dilution of 10× and for IL-1RA – 100× (Figure 3). Higher dilutions (40× and, respectively, 400×) are applied to samples showing cytokine values at the top of the assay's dynamic range.
FIGURE 3.
Cytokine detection in human cervicovaginal lavages at different sample dilutions. (a) The assay dynamic range and cervicovaginal lavage (CVL) matrix require a 100× screening dilution; (b) CVL matrix interference requires a 10× dilution with assay diluent. The upper panel represents the average of duplicate measurements in baseline CVLs (no drug exposure). The lower panel represents the mean and SD of the same samples.
Using our quality control procedure and taking the dilution factors into consideration we established the following cut-off levels for highly reproducibly quantifiable cytokine levels in human CVLs (10 cc saline): IL-1α 20 pg/ml; IL-1β 10 pg/ml; IL-8 256 pg/ml; IL-1RA 4690 pg/ml; TNF-α 22 pg/ml. These values are close to experimentally established ED50s for these cytokines. Lower concentrations can be measurable in high sensitivity assays; however, the coefficient of variation for duplicate measurements is usually greater than 30%, and the biological significance of such levels is questionable.
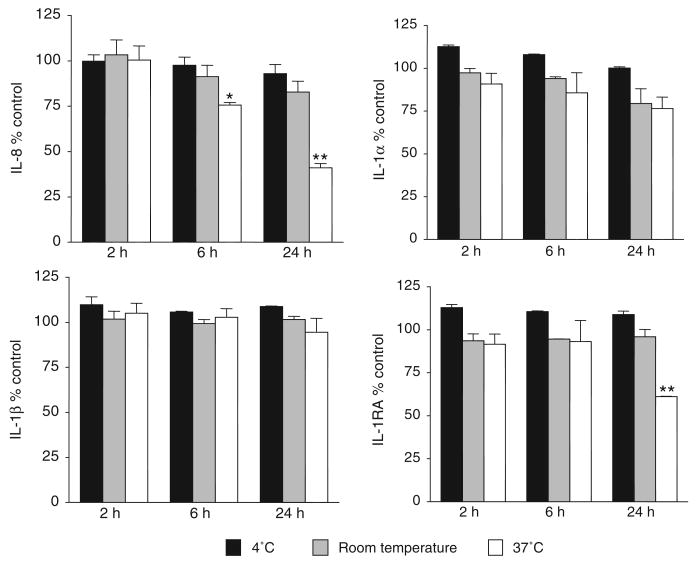
To establish the stability of endogenous cytokines detectable in the saline CVLs we pooled CVLs collected before product use and measured cytokine levels by ELISA after different lengths of exposure to various temperature conditions. The results from four independent experiments with different CVL pools showed that IL1α, IL-1β, IL-8 and IL-1RA were stable for 2 h at temperatures as high as 37°C and that most of them were stable at 4°C for up to 24 h, suggesting that CVLs for cytokine monitoring could be collected in field conditions or low resource clinical settings (Figure 4).
FIGURE 4.
The recovery of endogenous cytokines from pooled cervicovaginal lavages collected at baseline. The results represent the mean and SEM from duplicate measurements from two independent experiments for each cytokine. Cervicovaginal lavage pools were divided into five equal portions. One portion (control) was frozen immediately at −70°C. One portion was subjected to repeated freeze–thaw cycles, and the remaining three portions were incubated at 4°C, room temperature or 37°C for 2, 6 and 24 h. Cytokine concentrations were measured by Endogen IL-8 and IL-1α and by R&D Systems QuantiGlo IL-1β and Quantikine IL-1RA. *P < 0.05; **P < 0.001.
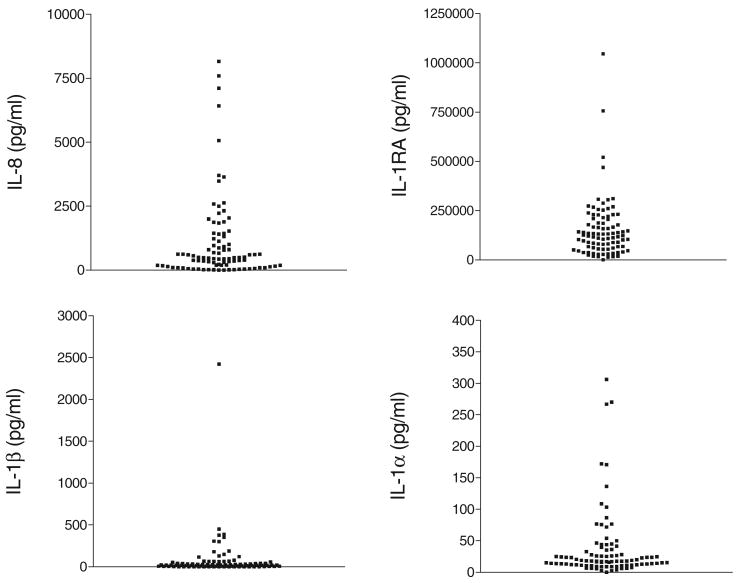
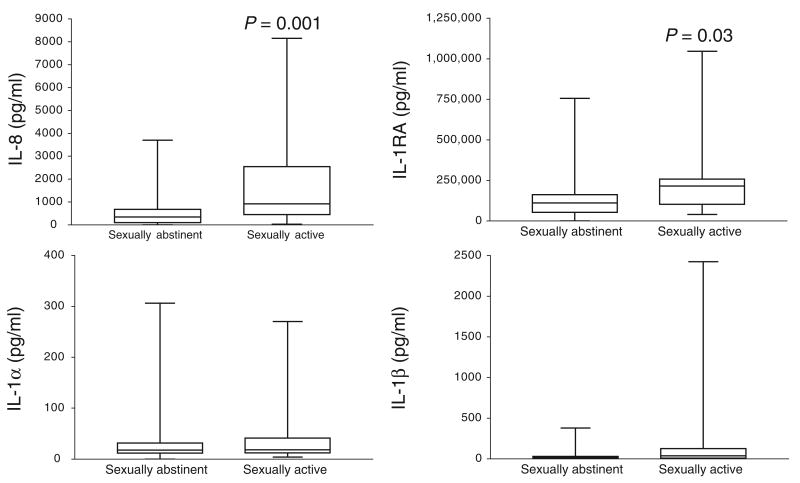
Our results confirmed the expected considerable variation of cytokine concentrations at baseline, especially for IL-8 and IL-1RA (Figure 5). The subject stratification based on sexual abstinence, however, showed significantly higher values and baseline ranges in the sexually active cohorts as compared with sexually abstinent cohorts (Figure 6). The cytokine profiling of subjects before and after product use revealed three major patterns, each of them underscoring some important caveats of interpretation.
FIGURE 5.
Baseline cytokine variability in human cervicovaginal lavages collected from healthy volunteers (n = 90) in CONRAD-sponsored clinical trials. Each dot represents the average of duplicate measurements of individual cervicovaginal lavage samples.
FIGURE 6.
Baseline IL-8 and IL-1RA values are significantly elevated in the cervicovaginal lavages of sexually active versus sexually abstinent women (n = 90).
In the most common pattern, all five cytokines detected in the CVL samples post treatment (visits 3 and 4) were within the range of normal baseline variation for the sexually abstinent or sexually active cohorts, and showed no significant difference from the pre-dosing cytokine background for the individual subject. As interference with the cytokine detection assay was ruled out for the products tested in this study, a conclusion can be made that this pattern is associated with a lack of product effects on the cytokine equilibrium.
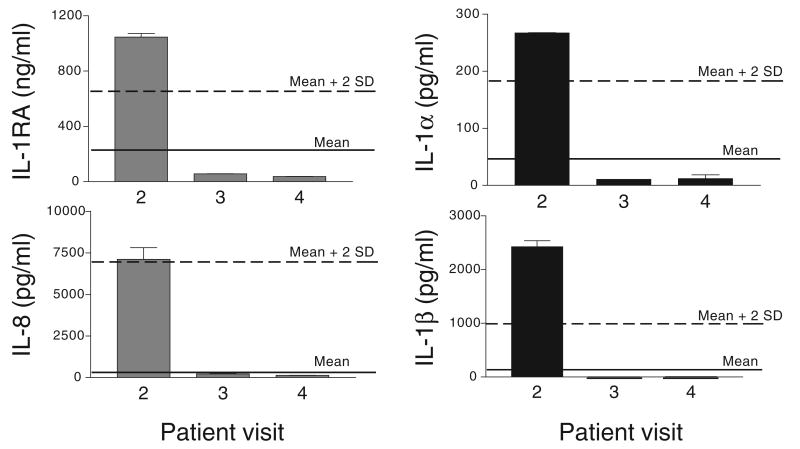
The second pattern is illustrated by the case of a sexually active healthy volunteer (Figure 7). The CVL collected at baseline (pre-treatment visit 2) showed traces of blood. At the same time, the baseline sample showed markedly increased cytokine levels. Whereas the IL-1RA and IL-8 values were consistent with the baseline values observed in the sexually active cohort, the higher IL-1α and IL-1β levels could be explained with the presence of blood in this sample.
FIGURE 7.
Case interpretation: concentrations of cytokines in cervicovaginal lavage samples obtained from a sexually active healthy volunteer. Bars represent means ± SD of duplicate cytokine measurements by enzyme-linked immunosorbent assay. The solid line represents the average baseline cytokine level obtained for sexually active women (n = 34), participating in two CONRAD-funded microbicide safety studies. The dashed line represents the mean plus 2 SD for these cohorts.
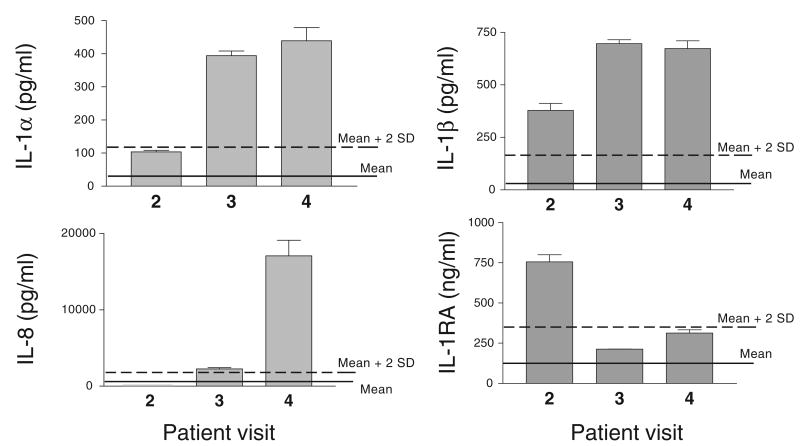
The third cytokine pattern is illustrated by the case of a sexually abstinent woman (Figure 8). In this case, IL-1α and IL-8 were progressively increased at visits 3 and 4 compared with the baseline visit 2, with the baseline values being within the normal variation for sexually abstinent cohorts (Figure 8). If no other cytokines had been measured in these CVLs, a product-initiated inflammatory response would have been suspected in this woman. However, the evaluation of the early response inflammatory mediators IL-1β and IL-RA demonstrates a marked increase of both cytokines at baseline (Figure 8), which is significantly higher than the normal range for the sexually abstinent cohorts. These findings reverse the interpretation pattern, suggesting the presence of an inflammatory background condition before product use (most likely asymptomatic as the subject was enrolled in the study). The cytokine data interpretation in the context of the complete clinical analysis may provide further clues as to whether the dynamics of this cytokine profile have been exacerbated by product use.
FIGURE 8.
Case interpretation: dynamic of cytokine concentrations in cervicovaginal lavages obtained from a sexually abstinent woman before microbicide product use (visit 2) and after microbicide product use (visits 2 and 3). Bars represent means ± SD of duplicate cytokine measurements by enzyme-linked immunosorbent assay. The solid line represents the average baseline cytokine level obtained for sexually abstinent women (n = 56), participating in two CONRAD-funded microbicide safety studies. The dashed line represents the mean plus 2 SD for these cohorts.
Conclusion
The monitoring of vaginal proinflammatory cytokines during clinical trials using standardized techniques and interpretation tools can provide valuable information about the mechanism of action of vaginal products and accelerate the bench-to-bedside transition of safe and efficacious anti-HIV-1 microbicides. More research is needed to establish the range of homeostatic cytokine values in cervicovaginal secretions under different physiological and pathological conditions.
Acknowledgments
Support for this study (subproject MSA-02-304) was provided by CONRAD, Eastern Virginia Medical School, under a cooperative agreement (HRN-A-00-98-00020-00) with the US Agency for International Development (USAID). The views expressed by the author do not necessarily reflect the views of USAID and CONRAD. The cytokine work for the safety evaluation of vaginal microbicides is also partly supported by National Institutes of Health P01 HD041761.
References
- 1.Hargreaves R, Frank R. Clinical biomarkers in drug discovery and development. Nat Rev Drug Discov. 2003;2:566–580. doi: 10.1038/nrd1130. [DOI] [PubMed] [Google Scholar]
- 2.Kingsmore SF, Patel DD. Multiplexed protein profiling on antibody-based microarrays by rolling circle amplification. Curr Opin Biotech. 2003;14:74–81. doi: 10.1016/s0958-1669(02)00019-8. [DOI] [PubMed] [Google Scholar]
- 3.Zagury D, Le Buanec H, Bizzini B, et al. Active versus passive anti-cytokine antibody therapy against cytokine-associated chronic diseases. Cytokine Growth Factor Rev. 2003;247:1–15. doi: 10.1016/s1359-6101(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 4.Stewart TA. Neutralizing interferon alpha as a therapeutic approach to autoimmune diseases. Cytokine Growth Factor Rev. 2003;242:1–16. doi: 10.1016/s1359-6101(02)00088-6. [DOI] [PubMed] [Google Scholar]
- 5.Lipshultz SE, Fisher SD, Lai WW, et al. Cardiovascular risk factors, monitoring, and therapy for HIV-infected patients. AIDS. 2003;17(Suppl 1):S96–S122. doi: 10.1097/00002030-200304001-00014. [DOI] [PubMed] [Google Scholar]
- 6.Nylen ES, Alarifi AA. Humoral markers of severity and prognosis of critical illness. Best Pract Res. 2001;15:553–573. doi: 10.1053/beem.2001.0169. [DOI] [PubMed] [Google Scholar]
- 7.Stone A. Microbicides: a new approach to preventing HIV and other sexually transmitted infections. Nat Rev Drug Discov. 2002;1:977–985. doi: 10.1038/nrd959. [DOI] [PubMed] [Google Scholar]
- 8.Turpin JA. Considerations and development of topical microbicides to inhibit the sexual transmission of HIV. Expert Opin Investig Drugs. 2002;11:1077–1097. doi: 10.1517/13543784.11.8.1077. [DOI] [PubMed] [Google Scholar]
- 9.Mauck C, Rosenberg Z, Van Damme L. Recommendations for the clinical development of topical microbicides: an update. AIDS. 2001;15:857–868. doi: 10.1097/00002030-200105040-00006. [DOI] [PubMed] [Google Scholar]
- 10.Fichorova RN, Tucker L, Anderson DJ. The molecular basis of Nonoxynol-9 induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J Infect Dis. 2001;184:418–428. doi: 10.1086/322047. [DOI] [PubMed] [Google Scholar]
- 11.Keller MJ, Klotman ME, Herold BC. Rigorous pre-clinical evaluation of topical microbicides to prevent transmission of human immunodeficiency virus. J Antimicrob Chemother. 2003;51:1099–1102. doi: 10.1093/jac/dkg214. [DOI] [PubMed] [Google Scholar]
- 12.Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 13.Doncel GF. Barrier contraceptives current status and future prospects. In: Mauck C, Gabelnick HL, Spieler JM, Rivera R, editors. Chemical vaginal contraceptive preclinical evaluation. New York: Wiley-Liss; 1994. pp. 147–162. [Google Scholar]
- 14.Greenhead P, Hayes P, Watts P, et al. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol. 2000;74:5577–5586. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkos CA, Colgan SP, Madara JL. Interactions of neutrophils with epithelial cells: lessons from the intestine. J Am Soc Nephrol. 1994;5:138–152. doi: 10.1681/ASN.V52138. [DOI] [PubMed] [Google Scholar]
- 16.Alfano M, Poli G. The cytokine network in HIV infection. Curr Molec Med. 2002;2:579–592. doi: 10.2174/1566524023361925. [DOI] [PubMed] [Google Scholar]
- 17.Royce RA, Sena A, Cates W, Jr, et al. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 18.Gayle HD, Hill GL. Global impact of human immunodeficiency virus and AIDS. Clin Microb Rev. 2001;14:327–335. doi: 10.1128/CMR.14.2.327-335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Blanco M, Cullen B. Molecular basis of latency in pathogenic human viruses. Science. 1991;254:815–820. doi: 10.1126/science.1658933. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Dornadula G, Beumont M, et al. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med. 1998;339:1803–1809. doi: 10.1056/NEJM199812173392502. [DOI] [PubMed] [Google Scholar]
- 21.Shearer GM, Clerici M. Protective immunity against HIV infection: has nature done the experiment for us? Immunol Today. 1996;17:21–24. doi: 10.1016/0167-5699(96)80564-0. [DOI] [PubMed] [Google Scholar]
- 22.Coedert JJ, Duliege AM, Amos CI, et al. High risk of HIV infection for first-born twins. The International Registry of HIIV-Exposed Twins Lancet. 1991;338:1471–1475. doi: 10.1016/0140-6736(91)92297-f. [DOI] [PubMed] [Google Scholar]
- 23.Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–977. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 24.Belec L, Gherardi R, Payan C, et al. Proinflammatory cytokine expression in cervicovaginal secretions of normal and HIV-infected women. Cytokine. 1995;7:568–574. doi: 10.1006/cyto.1995.0077. [DOI] [PubMed] [Google Scholar]
- 25.Fichorova RN, Anderson DJ. Cytokines in Reproduction. New York: Wiley-Liss; 2000. Cytokines in the cervical vaginal environment. [Google Scholar]
- 26.Al-Harthi L, Kovacs A, Coombs RW, et al. A menstrual cycle pattern for cytokine levels exists in HIV vaginal and plasma shedding. AIDS. 2001;15:1535–1543. doi: 10.1097/00002030-200108170-00011. [DOI] [PubMed] [Google Scholar]
- 27.Anderson DJ, Politch JA, Tucker LD, et al. Quantitation of mediators of inflammation and immunity in genital tract secretions and their relevance to HIV transmission. AIDS Res Hum Retrovirus. 1998;14(Suppl 1):S43–S49. [PubMed] [Google Scholar]
- 28.Sha BE, D'Amico RD, Landay AL, et al. Evaluation of immunologic markers in cervicovaginal fluid of HIV-infected and uninfected women: implications for the immunologic response to HIV in the female genital tract. J Acquir Immune Defic Syndr. 1997;16:161–168. doi: 10.1097/00042560-199711010-00004. [DOI] [PubMed] [Google Scholar]
- 29.Volk H, Reinke P, Docke W. Immunological monitoring of the inflammatory process: which variables? when to assess? Eur J Surg. 1999;584:70–72. doi: 10.1080/11024159950188600. [DOI] [PubMed] [Google Scholar]
- 30.Aderka D, Engelmann H, Maor Y, et al. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med. 1992;175:323–329. doi: 10.1084/jem.175.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 32.Imseis HM, Greig PC, Livengood C, et al. Characterization of the inflammatory cytokines in the vagina during pregnancy and labor and with bacterial vaginosis. J Soc Gynecol Invest. 1997;4:90–94. [PubMed] [Google Scholar]
- 33.Hitti J, Hillier SL, Agnew KJ, et al. Vaginal indicators of amniotic fluid infection in preterm labor. Obstet Gynecol. 2001;97:211–219. doi: 10.1016/s0029-7844(00)01146-7. [DOI] [PubMed] [Google Scholar]
- 34.Al-Harthi L, Wright DJ, Anderson D, et al. The impact of the ovulatory cycle on cytokine production: evaluation of systemic, cervicovaginal, and salivary compartments. J Interferon Cytokine Res. 2000;20:719–724. doi: 10.1089/10799900050116426. [DOI] [PubMed] [Google Scholar]
- 35.Anderson D, Fichorova R, Haimovici F, et al. Cytokine levels in cervicovaginal secretions of women throughout the menstrual cycle. J Reprod Immunol. 1997;34:41. [Google Scholar]
- 36.Kutteh WH, Franlin RD. Quantification of immunoglobulinms and cytokines in human cervical mucus during each trimester of pregnancy. Am J Obstet Gynecol. 2001;184:865–872. doi: 10.1067/mob.2001.113853. [DOI] [PubMed] [Google Scholar]
- 37.Snowhite IV, Jones WE, Dumestre J, et al. Comparative analysis of methods for collection and measurement of cytokines and immunoglobulins in cervical and vaginal secretions of HIV and HPV infected women. J Immunol Methods. 2002;263:85–95. doi: 10.1016/s0022-1759(02)00038-8. [DOI] [PubMed] [Google Scholar]
- 38.Robertson SA, Mau VJ, Hudson SN, et al. Cytokine-leukocyte networks and the establishment of pregnancy. Am J Immunol. 1997;37:438–442. doi: 10.1111/j.1600-0897.1997.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 39.Kelly RW, Carr GG, Critchley HO. A cytokine switch induced by human seminal plasma: an immune modulation with implications for sexually transmitted diseases. Hum Reprod. 1997;12:677–681. doi: 10.1093/humrep/12.4.677. [DOI] [PubMed] [Google Scholar]
- 40.Lio D, Caruso C, Di Stefano R, et al. IL-10 and TNF-alpha polymorphisms and the recovery from HCV infection. Hum Immunol. 2003;64:674–680. doi: 10.1016/s0198-8859(03)00080-6. [DOI] [PubMed] [Google Scholar]
- 41.Gentile DA, Doyle WJ, Zeevi A, et al. Cytokine gene polymorphisms moderate illness severity in infants with respiratory syncytial virus infection. Hum Immunol. 2003;64:338–344. doi: 10.1016/s0198-8859(02)00827-3. [DOI] [PubMed] [Google Scholar]
- 42.Pedersen BK, Bruunsgaard H, Ostrowski K, et al. Cytokines in aging and exercise. Int J Sports Med. 2000;21(Suppl 1):S4–S9. doi: 10.1055/s-2000-1444. [DOI] [PubMed] [Google Scholar]
- 43.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on aging. Ann NY Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 44.Shrier LA, Bowman FP, Lin M, et al. Mucosal immunity of the adolescent female genital tract. J Adolesc Health. 2003;32:183–186. doi: 10.1016/s1054-139x(02)00536-0. [DOI] [PubMed] [Google Scholar]
- 45.Roberts JA, Kaack MB, Harrison RM, et al. Bladder infection in the menopausal monkey. J Urol. 1999;162:254–257. doi: 10.1097/00005392-199907000-00077. [DOI] [PubMed] [Google Scholar]
- 46.Haimovici F, Fichorova R, Anderson J, et al. Psychopathology and cytokines in IVF patients. American Psychiatry Association, Annual Meeting; Chicago, IL. May, 2000. [Google Scholar]
- 47.Foster JR. The functions of cytokines and their uses in toxicology. J Exp Pathol. 2001;82:171–192. doi: 10.1046/j.1365-2613.2001.iep0082-0171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiecolt-Glaser JK, Glaser R. Depression and immune function. Central pathways to morbidity and mortality. J Psychosom Res. 2002;53:873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 49.Bienvenu JAD, Monneret G, Gutowski MC, et al. Cytokine assays in human sera and tissues. Toxicology. 1998;129:55–61. doi: 10.1016/s0300-483x(98)00063-8. [DOI] [PubMed] [Google Scholar]
- 50.Mire-Sluis AR, Das RG, Padilla A. WHO cytokine standardization: facilitating the development of cytokines in research, diagnosis and as therapeutic agents. J Immunol Methods. 1998;216:103–116. doi: 10.1016/s0022-1759(98)00073-8. [DOI] [PubMed] [Google Scholar]
- 51.Atamas SP. Alternative splice variant of cytokines: making a list. Life Sci. 1997;61:1105–1112. doi: 10.1016/s0024-3205(97)00243-9. [DOI] [PubMed] [Google Scholar]
- 52.Riches P, Gooding R, Millar BC, et al. Influence of collection and separation of blood samples on plasma IL-1, IL-6, and TNFa concentrations. J Immunol Methods. 1992;153:125–131. doi: 10.1016/0022-1759(92)90314-j. [DOI] [PubMed] [Google Scholar]
- 53.Ginsburg KA, Wolf NA, Fidel PL. Potential effects of midcycle cervical mucus on mediators of immune reactivity. Fertil Steril. 1997;67:46–50. doi: 10.1016/s0015-0282(97)81854-7. [DOI] [PubMed] [Google Scholar]