Abstract
Objective
HMG-CoA reductase inhibitors (statins) broadly reduce cardiovascular events, effects that are only partly related to cholesterol lowering. Recent studies suggest important anti-inflammatory and antiproliferative properties of these drugs. The purpose of this study was to determine the influence of statin therapy on graft patency after autogenous infrainguinal arterial reconstructions.
Methods
A retrospective analysis of consecutive patients (1999–2001) who underwent primary autogenous infrainguinal reconstructions with a single segment of greater saphenous vein was performed. Patients were categorized according to concurrent use of a statin. Graft lesions (identified by duplex surveillance) and interventions were tabulated. Comparisons between groups were made by using the Fisher exact test for categorical variables and the Student t test for continuous variables. Patency, limb salvage, and survival were compared by log rank test. A stepwise Cox proportional hazards analysis was then employed to ascertain the relative importance of factors influencing graft patency.
Results
A total of 172 patients underwent 189 primary autogenous infrainguinal arterial reconstructions (94 statin, 95 control) during the study period. The groups were well matched for age, indication, and atherosclerotic risk factors. Procedures were performed primarily for limb salvage (92%), with 65% to an infrapopliteal target. Perioperative mortality (2.6%) and major morbidity (3.2%) were not different between groups. There was no difference in primary patency (74% ± 5% vs 69% ± 6%; P = .25), limb salvage (92% ± 3% vs 90% ± 4%; P = .37), or survival (69% ± 5% vs 63% ± 5%; P = .20) at 2 years. However, patients on statins had higher primary-revised (94% ± 2% vs 83% ± 5%; P < .02) and secondary (97% ± 2% vs 87% ± 4%; P < .02) graft patency rates at 2 years. Of all factors studied by univariate analysis, only statin use was associated with improved secondary patency (P = .03) at 2 years. This was confirmed by multivariate analysis. The risk of graft failure was 3.2-fold higher (95% confidence interval, 1.04–10.04) for the control group. Perioperative cholesterol levels (available in 47% of patients) were not statistically different between groups.
Conclusions
Statin therapy is associated with improved graft patency after infrainguinal bypass grafting with saphenous vein.
The efficacy and durability of autogenous vein bypass for the treatment of severe peripheral arterial disease have been well established.1–3 Despite advances in endovascular therapies, surgical revascularization remains the mainstay of treatment, and improving the long-term patency of infrainguinal arterial reconstructions is one of the most challenging problems facing modern vascular surgery. Failure of vein bypass grafts, which can occur in 30% to 50% of patients over 5 years, is a common occurrence that directly results in mortality, limb loss, additional interventions, and diminished quality of life. Despite more than 2 decades of investigation, no pharmacologic approach has yet demonstrated a significant influence on long-term vein graft function.
The use of 3-hydroxy-3-methylglutaryl-coenzyme A inhibitors (HMG-CoA reductase inhibitors, or statins) is increasing, as is our knowledge of their beneficial effects in vascular disease. These cholesterol-lowering agents have demonstrated salutary effects across the spectrum of cardiovascular disease, and a broad range of atheroprotective properties have been elucidated beyond their effects on lipid metabolism. Clinical studies have shown that statins are associated with a reduction in cardiovascular events in patients with coronary artery disease and have anti-inflammatory effects that are independent of their lipid-lowering effects.4–10 Statins have been shown to prevent new or worsening symptoms of claudication and were associated with improved walking distance, walking velocity, and overall ambulatory performance.11–14 In terms of vessel wall biology, statins have been demonstrated to improve endothelial function, reduce smooth muscle cell (SMC) proliferation and migration, inhibit neointimal inflammation, reduce plaque SMC and collagen content, and suppress the expression of tissue factor and matrix metalloproteinases that may influence plaque stability.15–19 Recent studies have suggested that statin therapy may be associated with a reduced incidence of graft failure following coronary artery bypass.20
The purpose of the current study was to determine the influence of statin therapy on the outcomes of autogenous vein bypass in lower extremity occlusive disease.
METHODS
We conducted a retrospective review of consecutive patients who underwent infrainguinal arterial reconstructions using autogenous saphenous vein at the Brigham and Women’s Hospital between January 1, 1999 and December 31, 2001. In order to reduce confounding variability in graft quality and type, only those patients who underwent primary reconstruction using a single segment of greater saphenous vein (SSGSV) as a conduit were included in the study. Data were retrieved from hospital charts (including medication history); electronic medical records; vascular laboratory records; clinic records; and a computerized registry in which patient demographics, risk factor profile, procedural details, perioperative events, and follow-up information have been prospectively entered for all vascular surgery patients at our institution since 1975. Following chart review, patients were divided into two groups: the statin group, which consisted of those patients who were taking a statin at the time of their initial procedure, and the control group, which consisted of those patients who were not taking a statin at the time of their operation. Patients who underwent bilateral procedures during the study period were included only if both procedures fell into the same treatment group (statin or control); in these cases (n = 17), each procedure was treated as an individual data point in the analysis. Perioperative serum cholesterol levels were defined as those that were obtained within 3 months before or after the index procedure.
All operations were performed by a surgical team composed of a staff vascular surgeon and either a vascular fellow or senior surgical resident using standard techniques of graft placement and vascular anastomosis that have previously been described.21,22 Operative reports were reviewed for indication, graft type, graft orientation, and proximal/distal anastomotic sites. Postoperative graft surveillance consisted of duplex ultrasound evaluation of grafts at 1, 3, 6, and 12 months, followed by annual evaluation thereafter. Surveillance duplex studies were reviewed to assess the incidence, location, and severity of vein graft lesions. For patients who had experienced a graft event, only the duplex evaluation that documented the event or prompted intervention was used in the analysis. For all other patients, we used the most recent duplex study available for analysis. Graft lesions were categorized by anatomic location and severity. Subcritical stenoses were defined by a peak systolic velocity (PSV) ratio between 2.0 and 3.5 corresponding to a 50% to 75% reduction in luminal diameter, and critical stenoses were defined as regions with a velocity ratio greater than 3.5, or PSV > 300 cm/s, corresponding to a ≥75% reduction in luminal diameter. Lesions identified by duplex evaluation were generally confirmed by arteriography in order to plan subsequent intervention(s).
Minor amputations were defined as those that resulted in a foot that was still usable for ambulation. Limb salvage was defined as freedom from major amputation. Primary, primary-assisted, and secondary patency were defined in accordance with the suggested reporting standards of the Ad Hoc Committee of the Society for Vascular Surgery and the North American Chapter of the International Society for Cardiovascular Surgery.23
Survival, graft patency, and limb salvage rates were calculated with the life-table method, and standard errors were calculated with the Greenwood method. Comparisons between groups were performed by using the Mantel-Cox log rank analysis. Categorical variables were compared by using univariate analysis, and individual comparisons were performed with the Fisher exact test. Continuous variables were compared with a two-tailed Student t test. A stepwise Cox proportional hazards analysis was then employed to ascertain the relative importance of factors influencing graft patency. The dependent variable in this analysis was time to occlusion. In all comparisons, a P value of <.05 was considered the threshold for statistical significance. Loss to follow-up was defined as no patient contact for more than 18 months.
RESULTS
Characterization of study cohort
During the study period, a total of 176 patients underwent 197 primary infrainguinal arterial reconstructions using SSGSV. A total of 21 patients underwent bilateral procedures during the study interval that met the inclusion criteria; however, 4 patients were excluded because they crossed over between the statin and control groups between operations. This resulted in a total of 172 patients and 189 grafts available for analysis (88 patients and 94 grafts in the statin group, 84 patients and 95 grafts in the control group). Follow-up was complete in 87% of patients, with a mean of 525 (range, 1–1,474) days. This was not significantly different between groups (statin group 543 days, control group 507 days).
Table I lists the demographic and risk factor profiles for the study patients. There was a greater prevalence of coronary artery disease and history of coronary artery bypass grafting in the statin group than in the control group. There was no difference between groups in gender, diabetes, smoking, hypertension, congestive heart failure, arrythmia, cerebrovascular disease, chronic pulmonary disease, or chronic renal disease.
Table I.
Patient demographic and risk factor profiles*
| Group |
||||
|---|---|---|---|---|
| Characteristic | Overall | Statin | Control | P |
| Limbs treated | 189 | 94 | 95 | |
| Men/women | 101/71 | 54/34 | 47/37 | .47 |
| Age (y) | 69 ± 10 | 67 ± 10 | 70 ± 11 | <.04 |
| Diabetes | 104 | 58 | 46 | .14 |
| Smoking | 35 | 17 | 18 | .73 |
| Hypertension | 114 | 54 | 60 | .16 |
| Coronary artery disease | 94 | 59 | 35 | <.01 |
| Prior coronary artery bypass grafting | 42 | 28 | 14 | <.02 |
| Congestive heart failure | 26 | 13 | 13 | .90 |
| Arrythmia/atrial fibrillation | 24 | 10 | 14 | .27 |
| Cerebrovascular disease | 27 | 13 | 14 | .73 |
| Chronic pulmonary disease | 16 | 8 | 8 | .92 |
| Chronic renal disease | 49 | 20 | 29 | .09 |
| Hemodialysis-dependent | 31 | 11 | 20 | .05 |
| Perioperative total cholesterol (mg/dL) | 170 ± 60 (n = 80) | 174 ± 66 (n = 38) | 168 ± 54 (n = 42) | .67 |
| Statin type (n = 88) | ||||
| Atorvastatin | 28 (30%) | |||
| Cerivastatin | 1 (1%) | |||
| Lovastatin | 3 (3%) | |||
| Pravastatin | 2 (2%) | |||
| Simvastain | 60 (64%) | |||
Data shown as either coded raw data (n) or summary data (mean ± SD).
Perioperative cholesterol levels were available for analysis in 47% of patients. The mean cholesterol level was 170 ± 60 mg/dL and was not significantly different between groups. Statin drugs used included atorvastatin (30%), cerivastatin (1%), lovastatin (3%), pravastatin (2%), and simvastatin (64%).
Overall, 90% of study patients were taking some form of antithrombotic agent, including aspirin (63%), warfarin sodium (6%), clopidogrel (1.7%), pentoxifylline (1.3%), or some combination of these (18%). There was no difference in the incidence or type of antithrombotic agent use between groups.
Overall, 179 of 189 (95%) operations performed on patients during the study interval were performed by three staff vascular surgeons. There was an equal distribution among surgeons in both the number and type of procedure performed, as well as the group to which the patients were assigned (statin vs control).
Forty-six percent of the operations were performed under general anesthesia alone, and 54% were performed with a combination of regional and general anesthetic techniques. There was no difference in the anesthetic method used between groups.
Table II summarizes the operative indications and graft characteristics of all procedures in the study. Of the 172 procedures, 8% were for claudication and 92% were for limb salvage, of which 24% were for rest pain and 68% for tissue loss. The proximal anastomosis arose from the common femoral artery (CFA) in 53%, the superficial femoral artery (SFA) in 25%, and the popliteal artery in 22% of cases. Grafts more commonly arose from the SFA in the control group than in the statin group (P < .03). The distal anastomosis was created in the above-knee popliteal artery in 12%, the below-knee popliteal artery in 22%, the tibioperoneal trunk in 0.5%, the anterior tibial artery in 16%, the posterior tibial artery in 21%, the peroneal artery in 13%, and the dorsalis pedis artery in 15%. Graft orientation consisted of 32% in situ, 52% nonreversed, and 16% reversed saphenous vein grafts. There was no difference in operative indication, distal anastomotic site, or graft orientation between groups.
Table II.
Operative indications and graft characteristics*
| Group |
||||
|---|---|---|---|---|
| Characteristic | Overall | Statin | Control | P |
| Operative indication | ||||
| Claudication | 16 | 10 | 6 | .29 |
| Limb salvage (combined) | 173 | 84 | 89 | .29 |
| Rest pain | 44 | 22 | 22 | .97 |
| Tissue loss | 129 | 62 | 67 | .50 |
| Proximal anastomotic site | ||||
| Common femoral artery | 101 | 55 | 46 | .16 |
| Superficial femoral artery | 47 | 17 | 30 | <.03 |
| Popliteal artery | 41 | 22 | 19 | .57 |
| Distal anastomotic site | ||||
| Popliteal artery | 65 | 36 | 29 | .26 |
| Tibial/pedal arteries | 124 | 58 | 66 | .26 |
| Graft orientation | ||||
| In situ saphenous vein | 61 | 33 | 28 | .41 |
| Non-reversed saphenous vein | 98 | 45 | 53 | .28 |
| Reversed saphenous vein | 30 | 16 | 14 | .67 |
Data shown as coded raw data (n).
Perioperative events
Postoperative (30-day) mortality and morbidity are summarized in Table III. Thirty-day operative mortality occurred in 5 patients (2.6%)—3 from multisystem failure, 1 from lung cancer, and 2 from unknown cause—and was not significantly different between groups (2 deaths in the statin group, 3 deaths in the control group; P = .51). The overall postoperative morbidity rate was 21%, and was not significantly different between groups. Early graft thrombosis (<30 days) occurred in 3.7% of grafts and was not significantly different between groups (P = .06).
Table III.
Postoperative mortality and morbidity*
| Group |
||||
|---|---|---|---|---|
| Characteristic | Overall (%) | Statin | Control | P |
| 30-day mortality | 5 (2.6) | 2 | 3 | .51 |
| Overall morbidity | 40 (21) | 15 | 25 | .08 |
| Major morbidity | 6 (3.2) | 3 | 3 | .65 |
| Myocardial infarction | 3 (1.6) | 2 | 1 | .55 |
| Stroke | 0 (0) | 0 | 0 | N/A |
| Renal failure | 2 (1.1) | 0 | 2 | .25 |
| Pulmonary complications | 1 (0.5) | 1 | 0 | .50 |
| Other morbidities | ||||
| Early graft thrombosis | 7 (3.7) | 1 | 6 | .06 |
| Early amputation | 1 (0.5) | 0 | 1 | .32 |
| Cardiovascular (other) | ||||
| Congestive heart failure | 3 (1.6) | 2 | 1 | .50 |
| Arrythmia | 7 (3.7) | 3 | 4 | .51 |
| Gastrointestinal | ||||
| Cholecystitis | 2 (1) | 0 | 2 | .25 |
| Ischemic colitis | 2 (1) | 0 | 2 | .25 |
| Pancreatitis | 1 (0.5) | 0 | 1 | .32 |
| Deep venous thrombosis | 1 (0.5) | 1 | 0 | .50 |
| Wound infection | 15 (6.8) | 5 | 10 | .15 |
| Hematoma/seroma | 3 (1.6) | 1 | 2 | .50 |
All events occurred within 30 days of surgery.
Data shown as coded raw data (n) and percent.
Long-term outcomes
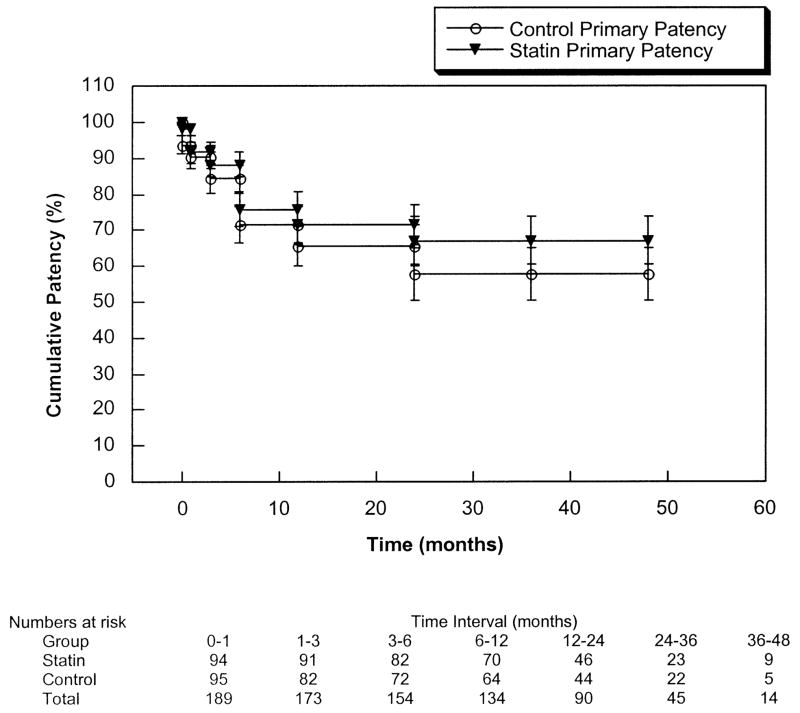
Overall 2-year cumulative patient survival was 66% ± 4% and was not significantly different between groups (statin group 69% ± 5%, control group 63% ± 5%; P = .20). Table IV, and Figs 1 and 2 depict the primary, primary-revised, and secondary patency rates and limb salvage rates for the entire patient cohort. Combined primary patency at 2 years was 72% ± 4% and was not significantly different between groups (statin group 74% ± 5%, control group 69% ± 6%; P = .25). Two-year primary-revised patency was 89% ± 3% (statin group 94% ± 2%, control group 83% ± 5%; P < .02), and secondary patency was 92% ± 2% (statin group 97% ± 2%, control group 87% ± 4%; P < .02). Both primary-revised and secondary patencies were significantly greater in the statin group. The overall risk of graft failure was 3.2-fold higher (95% confidence interval, 1.04–10.04) for the control group. Limb salvage at 2 years was 91% ± 3% and was not significantly different between groups.
Table IV.
Two-year outcomes of survival, primary-revised, and secondary patencies and limb salvage*
| Group |
||||
|---|---|---|---|---|
| Characteristic | Overall | Statin | Control | P |
| Survival | 66 ± 4 | 69 ± 5 | 63 ± 5 | .20 |
| Primary patency | 72 ± 4 | 74 ± 5 | 69 ± 6 | .25 |
| Primary-revised patency | 89 ± 3 | 94 ± 2 | 83 ± 5 | <.02 |
| Secondary patency | 92 ± 2 | 97 ± 2 | 87 ± 4 | <.02 |
| Limb salvage | 91 ± 3 | 92 ± 3 | 90 ± 4 | .37 |
All data shown as percent ± SE of the mean.
Fig 1.
Cumulative primary patency of the statin and control groups (P = .25).
Fig 2.
Cumulative secondary patency of the statin and control groups (P < .02).
By univariate analysis, only statin use (P = .03) had a significant association with secondary graft patency among the risk factors analyzed (smoking, diabetes, hypertension, coronary artery disease, history of coronary artery bypass grafting, chronic obstructive pulmonary disease, chronic renal disease, proximal or distal anastomotic sites). When all variables were examined in a stepwise Cox proportional hazards model, the association with statins alone remained significant (P < .03). Although the groups were not well matched for hemodialysis dependence, there was no observable impact of renal disease on graft patency in this series. Four dialysis patients (2 statin, 2 control) underwent graft revisions that maintained patency and only two permanent graft failures occurred in patients with chronic renal disease, both of whom were not hemodialysis-dependent. Consistent with other series, a reduced survival of the dialysis-dependent cohort (29% ± 9% at 2 years) was observed, thereby limiting the potential effect of this comorbidity on long-term graft function. Nonetheless, even when hemodialysis patients are excluded from the analysis, there remains a statistically significant improvement in secondary patency at 2 years associated with statin use (statin group 94% ± 3% vs control group 81% ± 5%, P < .02).
Characterization of graft lesions, events, and interventions
Postoperative duplex examinations were available for analysis in 146 of 189 grafts (77%). The incidence and type of graft lesions, events, and subsequent interventions are summarized in Table V. Of the 189 grafts included in the study, 64 (34%) had no significant lesions on the last available duplex examination, 23 (12%) developed subcritical lesions, 38 (20%) developed critical lesions, 21 (11%) occluded, and 43 (23%) had no duplex examination available for evaluation. Of the 61 grafts that developed a significant, nonoccluding lesion, 7 (11%) occurred in the inflow vessel, 22 (36%) occurred at the proximal or distal anastomosis, 17 (28%) occurred within the graft, 6 (10%) occurred in the outflow vessel, and 9 (15%) developed stenoses in multiple sites. There was no difference in either the location or severity of stenoses between groups. Four study patients, one in the statin group and three in the control group, had critical stenoses identified by duplex examination that did not lead to an intervention. Of these patients, two died (one statin patient, one control patient) with a patent graft and two patients continue under clinical observation.
Table V.
Graft lesions, events, and interventions*
| Group |
||||
|---|---|---|---|---|
| Characteristic | Overall (%) | Statin | Control | P |
| Graft disease burden | ||||
| No significant disease | 64 (34) | 33 | 31 | .72 |
| Subcritical stenosis | 23 (12) | 12 | 11 | .80 |
| Critical stenosis | 38 (20) | 24 | 14 | .06 |
| Occlusion | 21 (11) | 7 | 14 | .11 |
| Unknown | 43 (23) | 18 | 25 | .24 |
| Clinical events | ||||
| Overall | 49 (100) | 22 | 27 | .43 |
| Elective revision | 28 (57) | 15 | 13 | .66 |
| Graft occlusion | 21 (43) | 7 | 14 | .11 |
| Interventions | ||||
| Overall | 42 (100) | 20 | 22 | .76 |
| Thrombolysis/Thrombectomy | 4 (10) | 1 | 3 | .32 |
| Angioplasty | 10 (25) | 7 | 3 | .19 |
| Interposition/Jump graft | 13 (31) | 6 | 7 | .79 |
| Combination/Multiple | 3 (7) | 2 | 1 | .55 |
| Amputation | 3 (7) | 2 | 1 | .55 |
| New graft constructed | 9 (21) | 2 | 7 | .09 |
| Time to intervention (days) | ||||
| Mean | 230 ± 228 | 225 ± 203 | 234 ± 251 | .90 |
| Median | 176 | 182 | 152 | |
Data shown as either coded raw data (n) with percent or summary data (mean ± SD).
The mean time to intervention was 230 ± 228 days with a median of 176 days and was not significantly different between groups. Of the 42 interventions performed on study patients, there were 4 (10%) thrombolysis/thrombectomies, 10 (24%) angioplasties (1 percutaneous, 9 open), 13 (31%) interposition and jump grafts, 3 (7%) combination procedures, 3 (7%) occluded grafts that resulted in major amputations, and 9 (21%) new grafts constructed to replace occluded grafts. There was no significant difference in the incidence or type of interventions performed between groups.
Twenty-one grafts developed occlusion during the study interval. Six grafts occluded within the first postoperative week, only one of which was salvaged by thrombectomy. Thirteen grafts presented occluded without prior duplex evidence of graft stenosis, and two grafts had pre-occlusive lesions identified on duplex surveillance prior to the occlusion event. In these two patients, the time between lesion identification and graft occlusion was 3 and 11 months respectively.
DISCUSSION
Our results suggest that statin therapy may have a beneficial effect on the patency of autogenous infrainguinal bypass grafts. The observed difference in outcomes was almost exclusively related to a lower incidence of graft occlusions in the statin group and a correspondingly greater number of unsalvageable grafts in the control group. Primary patency rates were not different, nor were there any significant differences in the incidence or severity of occult graft stenoses identified on duplex surveillance.
This retrospective study has significant limitations and its findings should be interpreted with some caution. First, data on statin use was obtained retrospectively from patient records and may be incomplete; thus we are unable to determine patient compliance or duration of therapy. Available data on serum cholesterol was also limited to approximately half of the cohort, limiting the power to analyze the influence of this variable. The two groups identified from this consecutive cohort of primary bypasses were relatively well matched in number and risk factor prevalence, and multivariate analysis did not demonstrate an association between any of the other variables tested and graft patency. Nonetheless, the relatively small sample size limits the statistical power for analysis of potentially confounding variables. The apparent beneficial influence of statins (3.2-fold risk reduction) was statistically significant, but the wide confidence interval demonstrates the limited robustness of a small series. A multicenter, prospective randomized trial to examine the question appears to be indicated but may be difficult to execute due to the increasing prevalence of statin use and potential concerns with removing patients from therapy prior to randomization. Despite the limitations noted, when taken in the context of the available evidence on statins and peripheral arterial disease, this report adds further rationale to support a broader use of these agents in advanced limb ischemia patients.
The potential mechanisms by which statins may influence vein graft disease remain speculative. Statins have been shown to have a variety of anti-inflammatory and antiatherosclerotic properties, and several large clinical series have established that statins reduce the risk of coronary events24,25 and stroke.6,24–27 Many studies have also demonstrated a predictive association between serum concentrations of C-reactive protein (CRP), an acute-phase reactant, and the long-term risk of cardiovascular events.28–30 Even in healthy, symptomless adults, a single CRP level in the high normal range predicts an increased long-term risk of cardiovascular events including angina pectoris, myocardial infarction, and death. Statins have been shown to decrease the serum concentration of CRP in a manner independent of serum lipid concentration reduction.31,32 Basic investigations have demonstrated a significant inflammatory response in the vein graft wall33–36; however, to date there is limited clinical evidence directly linking inflammation with graft disease or failure.37 This is a subject of ongoing studies.
Recent studies have provided insight into the mechanisms by which statins exert their beneficial effects on inflammation and atherogenesis. Statins inhibit lipopolysaccharide-stimulated production of inflammatory cytokines (eg, tumor necrosis factor–α and interleukin-6) by leukocytes,38,39 and inhibit T cell activation and antigen-presenting functions.40,41 An in vivo study demonstrated a significant reduction in inflammation in a mouse model with a 1 hour predose of simvastatin to a degree that was comparable to indomethacin.10 The same study demonstrated a reduction in atherosclerosis in apolipoprotein E-deficient mice after 6 weeks of simvastatin administration—an effect independent of cholesterol lowering. A clinical study of patients with familial hypercholesterolemia demonstrated a significant reduction in carotid and femoral artery intima-media thickness after 2 years of treatment with simvastatin.42 In studies of intimal hyperplasia, statins have been shown to inhibit smooth muscle proliferation and migration,43,44 stimulate SMC apoptosis,45 and possibly affect monocyte recruitment by inhibiting expression of monocyte chemotactic protein-1.46 These studies provide strong evidence for the anti-inflammatory and antiatherogenic properties of the statin agents.
Statins have potent, beneficial effects on endothelial function that are directly relevant to the vascular injury response. Statins inhibit Rho/Rho-kinase activity, thereby leading to an increase in the expression and activity of endothelial nitric oxide synthase (eNOS).47,48 Statins have been shown to inhibit ischemic strokes in normal mice, but not in eNOS-deficient mice.49 Recent studies have demonstrated that statins increase the number and differentiation of circulating endothelial progenitor cells,50–52 which may be involved in local re-endothelialization at sites of injury (eg, vein graft). Finally, there is also evidence of a positive effect of statins on the endothelial-based fibrinolytic system by a reduction in the level of plasminogen activator inhibitor type-1 and increase in the levels of tissue plasminogen activator, suggesting a potential mechanism for antithrombotic effects.53–55
In the present study, an improvement in secondary patency without a reduction in occult stenoses was demonstrated in the statin group. This leads us to speculate that statins may have either reduced the overall virulence of the graft hyperplasia response, or perhaps exerted a primary antithrombotic effect. Further studies are clearly indicated to confirm the validity of these clinical findings and to examine the mechanisms by which statins may influence vein graft disease.
Acknowledgments
The authors would like to thank Ms. Julie Lombara for her assistance in maintenance of the vascular registry, data retrieval, and statistical analysis. Peter Gaccione, MA (Department of Health Care Policy, Harvard Medical School) provided biostatistical consultation and performed the multivariate analysis.
Footnotes
Competition of interest: none.
Presented at the Thirtieth Annual Meeting of The New England Society for Vascular Surgery, Newport, RI, Sep 19–21, 2003.
References
- 1.Taylor LM, Jr, Edwards JM, Porter JM. Present status of reversed vein bypass grafting: five-year results of a modern series. J Vasc Surg. 1990;11:193–206. doi: 10.1067/mva.1990.17235. [DOI] [PubMed] [Google Scholar]
- 2.Belkin M, Conte MS, Donaldson MC, Mannick JA, Whittemore AD. The impact of gender on the results of arterial bypass with in-situ greater saphenous vein. Am J Surg. 1995;170:97–102. doi: 10.1016/s0002-9610(99)80263-3. [DOI] [PubMed] [Google Scholar]
- 3.Shah DM, Darling RC, III, Chang BB, Fitzgerald KM, Paty PS, Leather RP. Long-term results of in situ saphenous vein bypass: analysis of 2058 cases. Ann Surg. 1995;222:438–48. doi: 10.1097/00000658-199510000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. Cholesterol and Recurrent Events Trial investigators The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 5.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 6.Scandinavian Simvastin Survival Study Group. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 7.Chan AW, Bhatt DL, Chew DP, Reginelli J, Schneider JP, Topol EJ, et al. Relation of inflammation and benefit of statins after percutaneous coronary interventions. Circulation. 2003;107:1750–6. doi: 10.1161/01.CIR.0000060541.18923.E9. [DOI] [PubMed] [Google Scholar]
- 8.Albert MA, Danielson E, Rifai N, Ridker P for the PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Rifai N, Cearfield M, Downs JR, Weis SE, Miles JS, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–65. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 10.Sparrow CP, Burton CA, Hernandez M, Mundt S, Hassing H, Patel S, et al. Simvastatin has antiinflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Aterioscler Thromb Vasc Biol. 2001;21:115–21. doi: 10.1161/01.atv.21.1.115. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen TR, Kjekshus J, Pyörälä K, Olsson AG, Cook TJ, Musliner TA, et al. Effect of simvastatin on ischemic signs and symptoms in the Scandinavian Simvastatin Survival Study (4S) Am J Cardiol. 1998;81:333–5. doi: 10.1016/s0002-9149(97)00904-1. [DOI] [PubMed] [Google Scholar]
- 12.McDermott MM, Guralnik JM, Greenland P, Pearce WH, Criqui MH, Liu K, et al. Statin use and leg functioning in patients with and without lower-extremity peripheral arterial disease. Circulation. 2003;107:757–61. doi: 10.1161/01.cir.0000050380.64025.07. [DOI] [PubMed] [Google Scholar]
- 13.Mondillo S, Ballo P, Barbati R, Guerrini F, Ammaturo T, Agricola E, et al. Effects of simvastatin on walking performance and symptoms of intermittent claudication in hypercholesterolemic patients with peripheral vascular disease. Am J Med. 2003;114:359–64. doi: 10.1016/s0002-9343(03)00010-x. [DOI] [PubMed] [Google Scholar]
- 14.Mohler ER, III, Hiatt WR, Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003:108. doi: 10.1161/01.CIR.0000090686.57897.F5. [DOI] [PubMed] [Google Scholar]
- 15.Bustos C, Hernandez-Presa MA, Ortega M, Tunon J, Ortega L, Perez F, et al. HMG-CoA reductase inhibition by atorvastatin reduces neointimal inflammation in a rabbit model of atherosclerosis. J Am Coll Cardiol. 1998;32:2057–64. doi: 10.1016/s0735-1097(98)00487-2. [DOI] [PubMed] [Google Scholar]
- 16.Fukumoto Y, Libby P, Rabkin E, Hill CC, Makoto E, Hirouchi Y, et al. Statins alter smooth muscle cell accumulation and collagen content in established atheroma of watanabe heritable hyperlipidemic rabbits. Circulation. 2001;103:993–9. doi: 10.1161/01.cir.103.7.993. [DOI] [PubMed] [Google Scholar]
- 17.Bellosta S, Via D, Canavesi M, Pfister P, Fumagalli R, Paoletti R, et al. HMG-CoA reductase inhibitors reduce MMP-9 secretion by macrophages. Arterioscler Thomb Vasc Biol. 1998;18:1671–8. doi: 10.1161/01.atv.18.11.1671. [DOI] [PubMed] [Google Scholar]
- 18.Aikawa M, Rabkin E, Sugiyama S, Voglic SJ, Fukumoto Y, Furukawa Y, et al. An HMG-CoA reductase inhibitor, Cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 2001;103:276–283. doi: 10.1161/01.cir.103.2.276. [DOI] [PubMed] [Google Scholar]
- 19.Wolfrum S, Jensen KS, Liao JK, Faraci FM. Endothelium-dependent effects of statins. Arterioscler Thromb Vasc Biol. 2003;23:729–36. doi: 10.1161/01.ATV.0000063385.12476.A7. [DOI] [PubMed] [Google Scholar]
- 20.Christenson JT. Preoperative lipid control with simvastatin reduces the risk for graft failure already 1 year after myocardial revascularization. Cardiovascular Surg. 2001;9:33–43. doi: 10.1016/s0967-2109(00)00088-0. [DOI] [PubMed] [Google Scholar]
- 21.Donaldson MC, Mannick JA, Whittemore AD. Femoral-distal bypass with in situ greater saphenous vein. Long-term results with the Mills valvulotome. Ann Surg. 1991;213:457–63. doi: 10.1097/00000658-199105000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belkin M, Knox J, Donaldson MC, Mannick JA, Whittemore AD. Infrainguinal arterial reconstruction with nonreversed greater saphenous vein. J Vasc Surg. 1996;24:957–62. doi: 10.1016/s0741-5214(96)70041-1. [DOI] [PubMed] [Google Scholar]
- 23.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended-standards for reports dealing with lower extremity ischemia. Revised version J Vasc Surg. 1997;26:517–38. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 24.Shepard J, Cobbe SM, Ford I, Isles CG, Lorimer AR, Macfarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 25.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 26.Phen JF, Davis BR, Sacks FM, Rouleau JL, Pfeffer MA, Bernstein V, et al. Reduction of stroke incidence after myocardial infarction with pravastatin: the Cholesterol and Recurrent Events (CARE) study. Circulation. 1999;99:216–23. doi: 10.1161/01.cir.99.2.216. [DOI] [PubMed] [Google Scholar]
- 27.White HD, Simes J, Anderson NE, Hankey GJ, Watson JDG, Hunt D, et al. Pravastatin therapy and the risk of stroke. N Engl J Med. 2000;343:317–26. doi: 10.1056/NEJM200008033430502. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 29.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–42. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. Circulation. 1999;100:230–5. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, Rifai N, Lowenthal SP. Rapid reduction in C-reactive protein with cerivastatin among 785 patients with primary hypercholesterolemia. Circulation. 2001;103:1191–3. doi: 10.1161/01.cir.103.9.1191. [DOI] [PubMed] [Google Scholar]
- 33.Chester AH, Morrison KJ, Yacoub MH. Expression of vascular adhesion molecules in saphenous vein coronary bypass grafts. Ann Thorac Surg. 1998;65:1685–9. doi: 10.1016/s0003-4975(98)00274-4. [DOI] [PubMed] [Google Scholar]
- 34.Harley SL, Sturge J, Powell JT. Regulation by fibrinogen and its products of intercellular adhesion molecule-1 expression in human saphenous vein endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:652–8. doi: 10.1161/01.atv.20.3.652. [DOI] [PubMed] [Google Scholar]
- 35.Hoch JR, Stark VK, Hullett DA, Turnipseed WD. Vein graft intimal hyperplasia: leukocytes and cytokine gene expression. Surgery. 1994;116:463–71. [PubMed] [Google Scholar]
- 36.Stark VK, Hoch JR, Warner TF, Hullett DA. Monocyte chemotactic protein-1 expression is associated with the development of vein graft intimal hyperplasia. Arterioscler Thromb Vasc Biol. 1997;17:1614–21. doi: 10.1161/01.atv.17.8.1614. [DOI] [PubMed] [Google Scholar]
- 37.Eslami MH, Gangadharan SP, Belkin M, Donaldson MC, Whittemore AD, Conte MS. Monocyte adhesion to human vein grafts: a marker for occult intraoperative injury? J Vasc Surg. 2001;34:923–9. doi: 10.1067/mva.2001.118590. [DOI] [PubMed] [Google Scholar]
- 38.Rosenson RS, Tangney CC, Casey LC. Inhibition of proinflammatory cytokine production by pravastatin. Lancet. 1999;353:983–4. doi: 10.1016/S0140-6736(98)05917-0. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda U, Shimada K. Statins and monocytes [letter] Lancet. 1999;353:2070. doi: 10.1016/S0140-6736(05)77885-5. [DOI] [PubMed] [Google Scholar]
- 40.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 41.Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–92. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 42.de Sauvage N, Pernette RW, de Groot E, Zwinderman AH, Buirma RJA, Trip MD, et al. Regression of carotid and femoral artery intima-media thickness in familial hypercholesterolemia: treatment with simvastatin. Arch Intern Med. 2003;163:1837–41. doi: 10.1001/archinte.163.15.1837. [DOI] [PubMed] [Google Scholar]
- 43.Porter KE, Naik J, Turner NA, Dickinson T, Thompson MM, London NJM. Simvastatin inhibits human saphenous vein neointima formatin via inhibition of smooth muscle cell proliferation and migration. J Vasc Surg. 2002;36:150–7. doi: 10.1067/mva.2002.122029. [DOI] [PubMed] [Google Scholar]
- 44.Laufs U, Marra D, Node K, Liao JK. 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors attenuate vascular smooth muscle proliferation by preventing rho GTPase-induced down-regulation of p27(Kip1) J Biol Chem. 1999;274:21,926–21,931. doi: 10.1074/jbc.274.31.21926. [DOI] [PubMed] [Google Scholar]
- 45.Guijarro C, Blanco-Colio LM, Ortego M, Alonso C, Ortiz A, Plaza JJ, et al. 3-Hydroxy-3-methylglutaryl-CoA reductase and isoprenylation inhibitors induce apoptosis of vascular smooth muscle cells in culture. Circ Res. 1998;83:490–500. doi: 10.1161/01.res.83.5.490. [DOI] [PubMed] [Google Scholar]
- 46.Romano M, Diomede L, Sironi M, Massimiliano L, Sottocorno M, Polentarutti N, et al. Inhibition of monocyte chemotactic protein-1 synthesis by statins. Lab Invest. 2000;80:1095–1100. doi: 10.1038/labinvest.3780115. [DOI] [PubMed] [Google Scholar]
- 47.Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–35. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 48.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24,266–24,271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 49.Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, et al. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Nat Acad Sci USA. 1998;95:8880–5. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, et al. HMG-CoA reductase inhibitor mobilizes bone marrow-derived endothelial progenitor cells. J Clin Invest. 2001;108:399–405. doi: 10.1172/JCI13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–7. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–24. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 53.Dangas G, Smith DA, Unger AH, Shao JH, Meraj P, Fier C, et al. Pravastatin: an antithrombotic effect independent of the cholesterol-lowering effect. Thromb Haemost. 2000;83:688–92. [PubMed] [Google Scholar]
- 54.Bourcier T, Libby P. HMG-CoA reductase inhibitors reduce plasminogen activator inhibitor-1 expression by human vascular smooth muscle and endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:556–62. doi: 10.1161/01.atv.20.2.556. [DOI] [PubMed] [Google Scholar]
- 55.Essig M, Nguyen G, Prie D, Escoubet B, Sraer JD, Friedlander G. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors increase fibrinolytic activity in rat aortic endothelial cells: role of geranylgeranylation and Rho proteins. Circ Res. 1998;83:683–90. doi: 10.1161/01.res.83.7.683. [DOI] [PubMed] [Google Scholar]