Abstract
An intense inflammatory process is associated with Trypanosoma cruzi infection. We investigated the mediators that trigger leukocyte activation and migration to the heart of infected mice. It is known that nitric oxide (NO) modulates the inflammatory response. During T. cruzi infection increased concentrations of NO are produced by cardiac myocytes (CMs) in response to IFN-γ and TNF. Here, we investigated whether NO, IFN-γ and TNF regulate chemokine production by T. cruzi-infected CMs. In addition, we examined the effects of the NOS2 deficiency on chemokine expression both in cultured CMs and in hearts obtained from infected mice. After infection of cultured WT CMs with T. cruzi, the addition of IFN-γ and TNF increased both mRNA and protein levels of the chemokines CXCL1, CXCL2, CCL2, CCL3, CCL4 and CCL5. Interestingly, T. cruzi-infected NOS2-deficient CMs produced significantly higher levels of CCL2, CCL4, CCL5 and CXL2 in the presence of IFN-γ and TNF. Infection of NOS2-null mice resulted in a significant increase in the expression of both chemokine mRNA and protein levels in the heart of as compared with hearts obtained from infected WT mice. Our data indicate that NOS2 is a potent modulator of chemokine expression which is critical to trigger the generation of the inflammatory infiltrate in the heart during T. cruzi infection.
Keywords: Chemokines, nitric oxide, Trypanosoma cruzi, myocarditis
Introduction
Trypanosoma cruzi is the etiologic agent of Chagas disease, a major cause of heart disease in endemic areas of Latin America. Acute T. cruzi infection is accompanied by an intense inflammatory reaction in many tissues including the heart. The inflammatory reaction is critical for the control of the parasite proliferation. The inflammatory process in the heart may progress to fibrosis and remodeling resulting in a dilated cardiomyopathy accompanied by myocardial dysfunction [1].
The recruitment and migration of immune cells into the T. cruzi-infected myocardium is a multi-step process that depends on the activation of the inflammatory cells coordinated by receptors that recognize pro-inflammatory cytokines, adhesion molecules, extra-cellular matrix components and chemokines [2–4]. Recently, we and others have demonstrated that the chemokine receptor, CCR5 and its ligands (CCL3, CCL4 and CCL5) play a central role in the control of T-cell influx into myocardium of T. cruzi-infected mice [3, 5]. In the heart CD4+ and CD8+ lymphocytes generate IFN-γ which act together with TNF to activate cardiac myocytes (CMs) to synthesize nitric oxide (NO) which is important in the control of intracellular parasite multiplication [6]. The synthesis of NO is mediated by nitric oxide synthase (NOS). One of the NOS isoforms, inducible NOS (NOS2) is the principal NOS induced by proinflammatory stimuli [7]. The induction of NOS2 results in the production of elevated and sustained levels of NO [8]. NO production is a “double-edged sword” because while it is required for the intracellular killing of the parasite, its synthesis may also damage the myocardium [9]. NO produced by the actions of constitutively expressed NOS (eNOS) may also damage the heart and modulate the functions of immune and other cells.
The notion that elevated levels of NO contribute to tissue damage is not new since it has been implicated in other inflammatory disease states [10]. Interestingly, it was demonstrated that NOS2-deficient mice displayed a significant reduction in the inflammatory response when subjected to direct ozone[11] or endotoxin[12] challenge. These observations also implicate the involvement of NO in the inflammatory response. Moreover, previous reports described a novel function of NO; its ability to modulate chemokine production in several cell types [13, 14]. Therefore, the association between elevated NO levels and chemokines during T. cruzi infection suggests that NO may play important roles in the control of chemokines gene expression in the heart.
Previously, we demonstrated that IFN-γ, TNF, IL-1β and chemokines modulate the synthesis of NO by NOS2 in T. cruzi-infected cultured cardiac myocytes (CMs) and macrophages [6, 15, 16]. Notably, during acute T. cruzi infection the induction of myocardial IFN-γ and TNF influences the regulation of the chemokines expression (CCL2, CCL3, CCL4, CCL5 and CXCL9) and has been identified as a T-cell-activating factor and to induce T-lymphocyte recruitment [17]. In the myocardium of T. cruzi-infected mice cytokine induction of chemokine gene expression has been examined but the role of NO in the regulation of chemokine gene expression has not received attention.
The heart is a heterogeneous organ composed of many cell types including CMs, fibroblasts, endothelial cells, and vascular smooth muscle cells tissue. In addition, during infection there is an inflammatory infiltrate. Since the CMs have the capacity to produce high levels of NO after T. cruzi infection, it is important to investigate the contribution of NO in the regulation of chemokine production in the myocardium of infected mice as well as in primary cultures of CMs. We examined whether CMs are an important source of chemokines in vivo during T. cruzi infection and to determine the roles of IFN-γ, TNF and NO in the modulation of chemokine production. To address this question IFN-γ, TNF and NOS2-null mice were utilized. Here we demonstrate that CMs that are infected with T. cruzi or stimulated with IFN-γ and TNF produce high levels of chemokines. We also demonstrate that NOS2-deficiency affects the chemokine production in response to cytokines and T. cruzi in the myocardium of mice as well as in cultured CMs. Taken together, these data indicate that the release of NO by infected CMs may be essential for controlling parasite proliferation locally as well as the modulation of chemokine production and the regulation of leukocyte recruitment to the infected tissue.
Material and Methods
Infection of mice
The Y strain of T. cruzi was maintained in C57BL/6 mice (wild type-WT). Null mice [NOS2, IFN-γ and TNF receptor-1 (TNFR-1)] were obtained from Jackson Laboratories (Bar Harbor, ME). All mice were subsequently bred in our animal facility (School of Medicine, USP, Ribeirão Preto, Brazil). Seven to eight week old WT and the deficient mice were infected intraperitoneally with 1×103 trypomastigotes. All animal experiments were approved by the university Institutional Animal Care and Use Committee.
Preparation and infection of embryonic cardiac myocyte (CM) cultures
Embryonic CM cultures were prepared as previously described [18]. Briefly, hearts from 19- to 20-day-old BALB/c mouse embryos were dissected, minced, and incubated for 5 minutes at 37°C in 0.05% trypsin (Invitrogen-GIBCO, Carlsbad, CA) and 0.01% collagenase type II (Worthington Biochemical Corp, Lakewood, NJ ) in a Ca2+- and Mg2+-free PBS solution. Trypsin activity was interrupted by adding 10% FBS (Hyclone, Logan, UT). Tissue clumps were incubated 8 or 9 times in a trypsin-collagenase solution. After trypsinization, the suspension was centrifuged, and the cell pellet was suspended in DMEM supplemented with 15% horse serum, 5% FBS, 2% chick embryo extract, 1 mmol/L glutamine, 1000 U/mL penicillin, and 50 mg/mL streptomycin (all from Sigma–Aldrich, ST. Louis, MO). For CM enrichment, the cell suspension was pre-plated in tissue culture flasks and incubated at 37°C for 45 minutes in a 5% CO2 atmosphere, after which time the cultures flasks were gently shaken, and the unattached myocytes were withdrawn with a pipette. The cells were then plated on a gelatin-treated 24-well tissue plate (Corning, Corning, NY) and the medium was replaced daily. The percentage of CD14+ and CD11b+ cells decreased significantly after cell pre-adhesion, reaching values close to zero (0.57% and 0.04%, respectively) when the cells were pre-incubated in uncoated plates for 45 minutes. CMs were infected with trypomastigotes that were obtained from infected cultured LLC-MK2 cells.
Chemokine Determinations
CCL2, CCL3, CCL4, CCL5, CXCL1 and CXCL2 levels were measured using commercial ELISA kits (R&D Systems, Minneapolis, MN). The isolated cardiac myocytes (CMs) were plated at 1 × 106 cells/well in 24-well plates and test stimuli were added as indicated for each experiment. After culture at 37 °C, the supernatants were harvested and chemokine levels were measured by ELISA in triplicate.
Total RNA extraction and cDNA preparation by reverse transcription (RT)
Total RNA was isolated from cultured CMs and from mouse cardiac tissue using the Trizol LS reagent according to manufacturer’s instructions. Briefly, the samples were homogenized and 0.2 ml of chloroform (Sigma Chemical Co., St. Louis, MO) and added to each 1 ml of Trizol reagent (Life Tech., Grand Island, NY). Samples were then centrifuged at 12,000 × g for 15 minutes at 4°C, and the aqueous phase was transferred to a clean tube. The same volume of isopropyl alcohol was added, and the samples were mixed in a vortex and incubated for 15 minutes at −20°C to precipitate the RNA from the aqueous phase. After a further centrifugation, the RNA pellet was washed in 75% ethanol, and samples were then suspended in water at 0.5μg of RNA/μl. Copy DNA was synthesized with Superscript II reverse transcriptase (GIBCO).
Chemokine mRNA detection
Chemokine (CCL3, CCL5 and CXCL1) and β-actin mRNA expression was analyzed by RT-PCR. RT-PCR assays were performed with Taq polymerase (GIBCO) in a PTC-100 thermal cycler (MJ Research, Watertown, MA). The reaction conditions were 35 cycles of 1 minute at 94°C, 1 minute at 54°C, and 2 min at 72°C, with a final extension step of 7 min at 72°C. For each set of primers, a negative sample (water) was run in parallel. The PCR products were separated by acrylamide gel electrophoresis and stained with silver nitrate. The PCR method for the chemokines tested was validated in the laboratory with plasmids containing the gene for each chemokine [6].
RNAse protection assay (RPA)
Total myocardial RNA was extracted from WT, IFN-γ null, TNFr1 null and NOS2 null mice, or from CM cultures, using Trizol reagent (Life Technologies) according to manufacturer’s instructions. Multi-probe template sets mCK-5b (containing DNA templates for Linphotactin/XCL1, RANTES/CCL5, Eotaxin/CCL11, MIP-1β/CCL4, MIP-1α/CCL3, MIP-2/CXCL2, MCP-1/CCL2, TCA-3/CCL1, L32 and glyceraldehydes-3-phosphate dehydrogenase (GAPDH), were purchased from Pharmingen (San Diego, CA). The DNA template was used to synthesize the α-[32P]UTP (3,000 Ci/mmol, 10 mCi/mL (Amersham Life Science, Buckinghamshire, UK)-labeled probes in the presence of a GACU pool using a T7 RNA polymerase. Hybridization with 10 μg of each target RNA was performed overnight followed by digestion with RNase A and T1 according to the standard Pharmingen protocol. The samples were treated by proteinase K-SDS mixture and then extracted with chloroform and precipitate in presence of ammonium acetate. The samples were loaded on an acrylamide-urea sequencing gel next to labeled DNA MW markers and labeled probes, and run at 50 watts with 0.5X Tris-borate/EDTA electrophoresis buffer (TBE). The gel was adsorbed to filter paper, dried under vacuum, and radioactivity of [α-32P]-labeled probes were measured by phosphorimaging.
Immunohistochemistry
Mouse hearts were removed 15 days post infection (PI), placed in compound-embedding medium (Sakura Finetek Inc., Torrance, CA) snap-frozen in liquid nitrogen, and stored at −70°C. Ten-micrometer sections were sectioned with a cryostat, collected on poly-lysine-coated slides, fixed with cold acetone and allowed to air–dry. The slides were placed in a humidified chamber, and endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 20 minutes and nonspecific binding was blocked with goat serum (diluted 1:200 in PBS) for 45 minutes. The slides were washed with PBS (pH 7.2) and incubated overnight with goat anti-mouse RANTES (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 100 times in PBS containing 1% bovine serum albumin. After extensive washes and incubation for 30 min with biotin-labeled rabbit anti-goat (Pharmingen), the reaction product was detected with avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, CA) and the color of the reaction was developed with diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich, Saint Louis, MO). The slides were then counterstained with Mayer’s hematoxylin. Controls were performed by incubating cells with non-immune goat immunoglobulin G and proceeding as described above.
Statistical analysis
The data were expressed as the mean ± SEM of the triplicate cultures or mice. Analysis was performed using ANOVA followed by the Student-Newman-Keuls test (INSTAT software, GraphPad, San Diego, CA). A P value <0.05 was considered to indicate significance.
Results
Chemokine production by T. cruzi-infected cardiac myocytes (CMs)
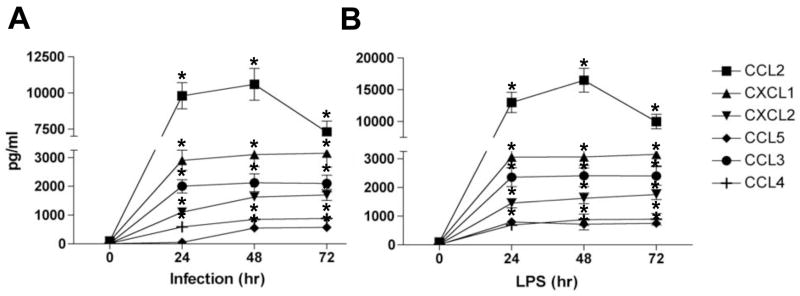
We first addressed the role of T. cruzi in modulating chemokine induction in CMs by evaluating the kinetics of chemokine release in culture supernatants. CMs of WT mice were cultured with trypomastigotes (culture-derived, Y strain) or LPS (used as a control), in the presence or absence of polymyxin-B, and the levels of chemokines were determined by ELISA. Trypomastigotes (Fig. 1A) and LPS (Fig. 1B) significantly increased the levels of CCL2, CCL3, CCL4, CCL5, CXCL1 and CXCL2 after 24 hours of incubation while CCL5 expression was significantly increased after 48hours (Fig. 1A). The addition of polymyxin-B abolished chemokine production by CMs stimulated with LPS, but not those infected with T. cruzi, excluding the possibility of LPS contamination (data not shown).
Figure 1. T. cruzi and LPS induce chemokines production by cultured cardiac myocytes (CMs).
Wild-type CMs were cultured in the presence of medium alone or in the presence, T. cruzi (parasite-to-cells ratio of 5:1) (A) or LPS (5 μg/ml) (B). At different time point the supernatants were harvested and chemokine production analyzed by ELISA. Data shown are representative of at least two independent experiments with similar results. The data are presented as mean ± standard deviation of triplicate samples. *P < 0.05, medium vs. stimulated cells.
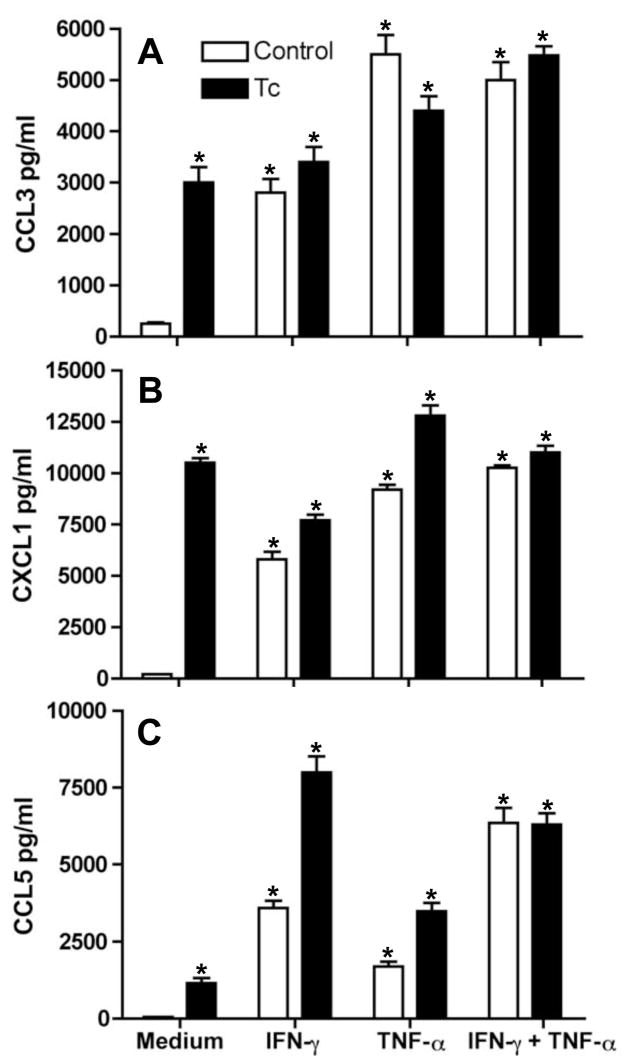
Next, we examined whether IFN-γ and TNF, cytokines previously observed in infected mouse heart tissue [19, 20] modulate T. cruzi-induced CMs chemokine production. CMs were cultured for 48 hours with IFN-γ and/or TNF and trypomastigotes. Uninfected CMs did not produce significant amounts of CCL3, CCL5 and CXCL1 in absence of cytokines (Figs. 2A–C However, figure 1 demonstrates significant levels of CCL3, CCL5 and CXCL1 after CM infection. The data displayed in figures 2A–C indicates that IFN-γ and/or TNF caused a significant increase of CCL3, CCL5 and CXCL1 production by CMs. The concomitant addition of parasites and IFN-γ or TNF enhanced CXCL1 and CCL5 production by CMs (Figs. 2B–C).3 Additionally, IFN-γ and TNF synergistically increased CCL3, CCL5 and CXCL1 production by CMs regardless of infection (Figs. 2A–C). These results indicate that IFN-γ and TNF modulate chemokine production by cultured CMs.
Figure 2. IFN-γ and TNF modulate chemokines production by CMs.
At 48 hr after incubation, CCL3 (A), CXCL1 (B) and CCL5 (C) levels were assayed by ELISA in supernatants of T. cruzi-infected (Tc) or uninfected (control) wild-type CMs stimulated with IFN-γ (100 U/ml) and TNF (100 U/ml) alone or simultaneously with both IFN-γ and TNF. Data shown are representative of three independent experiments with similar results. The Data are presented as the mean± SD of triplicate samples. *P < 0.05, medium vs. stimulated cells.
The regulation of chemokine expression in CMs by NOS2
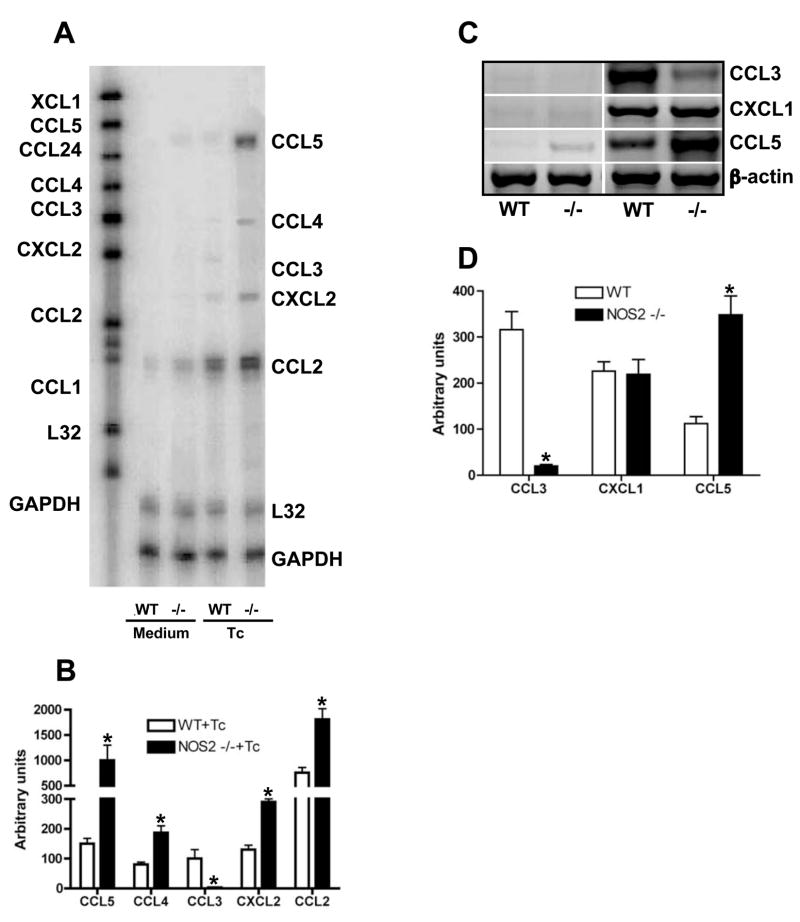
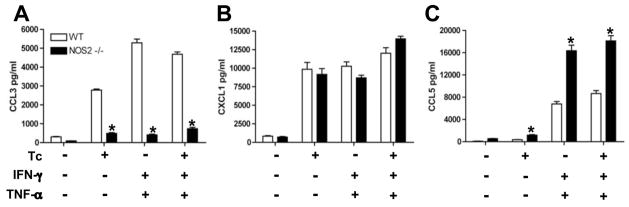
Previously we demonstrated that the pro-inflammatory cytokines IFN-γ, TNF and IL-1β as well as the chemokines CCL2, CCL4, CCL5, CXCL2, CXL9 and CXCL10 activate CMs to kill intracellular T. cruzi via NOS2-dependent NO production [6]. Parasite killing is associated with NOS2 activation [15]. It has also been observed that NO donors can modulate cytokine and chemokine production in mouse and human cells [14]. Therefore, we investigated the role of NO in the modulation of chemokine production by CMs. To evaluate the relative contribution of NO in the T. cruzi-triggered chemokine production, we analyzed the chemokine message expression after T. cruzi infection of cultured WT and NOS2-null CMs by RPA and RT-PCR assays. After infection, NOS2-null CMs displayed a significantly increased expression of CCL2, CCL4, CCL5 and CXCL2 as compared with those observed with WT CMs, while the expression of CCL3 was diminished in the absence of NOS2 (Figs. 3A–D). Furthermore, T. cruzi-induced CXCL1 expression was found to be comparable between WT and NOS2-null CMs (Figs. 3C–D). Therefore, it is likely that NOS2 activation (and consequent NO production) modulates T. cruzi-triggered chemokine secretion. To confirm this, cultured purified CMs from WT and NOS2-null mice were infected in the presence or absence of IFN-γ or TNF and the levels of chemokines determined by ELISA. TNF and IFN-γ significantly increased CCL5, but not CCL3, induction in T. cruzi-infected NOS2-null CMs. No effect was observed on CXCL1 production (Figs. 4A–C) when compared with the levels produced by infected WT CMs. In addition, we found higher levels of CCL2, CCL4 and CXCL2 in the supernatants harvested from infected NOS2-null CMs regardless of the presence of TNF or IFN-γ (data not show). Significant levels of CXCL9 or CXCL10 were not detected in the supernatants of WT or NOS2-null CMs with or without infection and/or stimulated with TNF and/or IFN-γ (data not show). These results indicate that NOS2 regulates chemokine production in cultured CMs in response to T. cruzi infection and TNF and IFN-γ stimulation.
Figure 3. NOS2 modulates T. cruzi-induced chemokine mRNA expression in CMs.
(A) Representative autoradiograph of RPA assay performed on 10μg of total RNA extracted from wild-type (WT) or NOS2-null (−/−) CMs cultured in presence of medium alone (medium) or medium containing T. cruzi (Tc) for 12 hr using one template set. (B) Bar represent normalized optical densities of RPA bands. (C) cDNA of total RNA from the same samples describe in A, was synthesized and PCR was performed by using specific primers. Equal amounts of cDNA were loaded in each lane. (D) Bar represent normalized optical densities of RT-PCR bands. Results shown are representative of three different experiments. *P < 0.05, NOS2-null vs. WT mice.
Figure 4. Modulation of chemokines production by NOS2 in CMs.
At 48 hr, CCL3(A), CXCL1 (B) and CCL5 (C) levels were assayed by ELISA in supernatants of T. cruzi-infected (Tc) or uninfected WT and NOS2 null (−/− ) CMs stimulated with IFN-γ (100 U/ml) and TNF(100 U/ml) alone or simultaneously. Data shown are representative of three independent experiments with similar results. The data are the mean± SD of triplicate samples. *P < 0.05, NOS2 null vs. WT mice.
The modulation of chemokine production by NOS2 in vivo
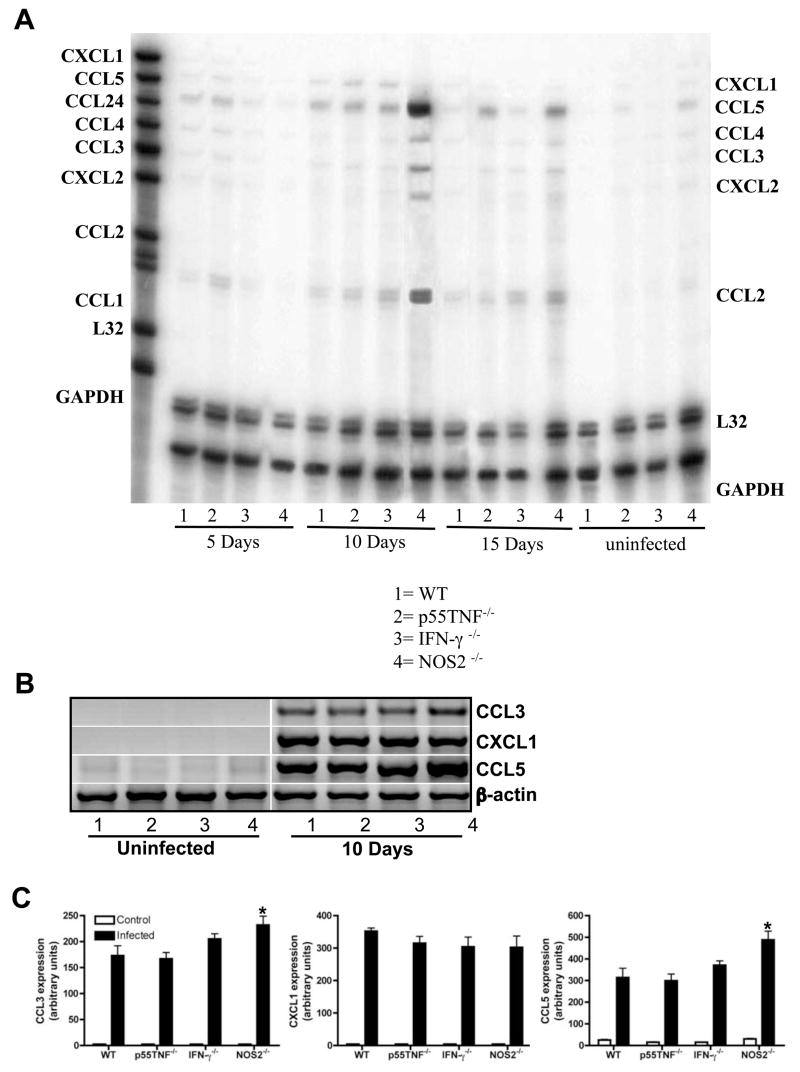
Next we examined whether in vivo T. cruzi-triggered chemokines production by CMs was also modulated by TNF, IFN-γ and NOS2. Hearts were obtained from T. cruzi-infected WT and null mice (IFN-γ, p55TNFR or NOS2) 5, 10 and 15 days post infection (PI), and the levels of chemokine mRNA expression were examined by RPA (Fig. 5A) and RT-PCR (Figs. 5B–C). Figures 5A–C demonstrates that all infected mice exhibited increased chemokine expression after infection when compared to uninfected mice. Interestingly, there was a dramatic increase in the expression of chemokines in the myocardium of infected NOS2-null mice on day 10 PI when compared with all other groups of infected mice but CCL5 was more significantly induced. Unexpectedly, myocardial CCL3 expression in infected-NOS2-null mice was increased. Thus, these data suggest that in the heart obtained from T. cruzi-infected mice cells other than CMs can synthesize CCL3 and its modulation is achieved through NOS2-dependent pathways.
Figure 5. NOS2 modulates T. cruzi-induced chemokine mRNA expression in CMs.
(A) Representative autoradiograph of RPA assay performed on 10μg of total myocardium RNA extracted from WT (lane1), TNF-R1 null (line 2), IFN-γ null (lane 3) and NOS2 null (lane #4) uninfected and infected mice on days 5, 10 and 15 after infection using one template set. (B) cDNA of total RNA from the same uninfected and 10 days after infection samples describe in A, was synthesized and PCR was performed by using specific primers. Equal amounts of cDNA were loaded in each lane. (C) Bar represent normalized optical densities of RT-PCR bands. Results shown are representative of three different experiments. *P < 0.05, NOS2-null versus WT mice.
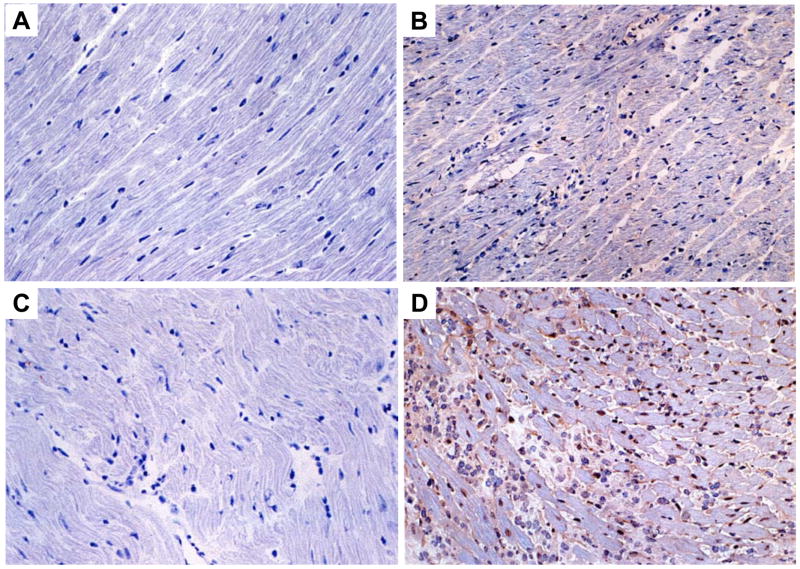
We then performed in situ analyses of CCL5 in myocardial sections obtained from WT and NOS2-null mice infected with T. cruzi. The data in figures 6A–D demonstrates a significant increase in the expression of CCL5 in the myocardium in the absence of NOS2 15 days PI (Fig. 6D) when compared with infected WT controls (Fig. 6B). The expression of CCL5 (Figs. 6B, 6D) as well as CCL2, CCL3 and CCL4 (data not show) was observed in the cardiac tissue obtained from infected mice, in close proximity to the inflammatory foci. Also, we found a CCL5+ mononuclear interstitial infiltrate in the myocardium of infected mice (Figs. 6B, 6D). Furthermore, in the myocardium of T. cruzi-infected NOS2-null mice there was a greater inflammatory infiltrate and more intense staining for CCL5 (Fig. 6D) as compared with WT sections 15 days PI (Fig. 6B). In myocardial sections obtained from uninfected WT and NOS2-null mice there was no inflammation and staining for chemokines was negative (Figs. 6A, 6C).
Figure 6. Higher RANTES expression in the myocardium of T. cruzi-infected NOS2 null mice.
The hearts of uninfected WT (A) and NOS2 null (C) and infected WT (B) and NOS2 null (D) mice 15 days PI (103 trypomastigotes) were analyzed by imunohistochemical studies and the presence of RANTES was evaluated by immunoperoxidase staining (see Material and Methods). The presence of the chemokines was demonstrated using DAB as the substratum for peroxidase, generating a brown coloration. The results shown are representative of three different experiments. Microphotographs are taken at X 200 magnification.
Discussion
Trypomastigotes of T. cruzi gain access to CMs by first invading the endothelium and the interstitial areas of the vascular wall and the myocardium. The parasite damages the extracellular matrix as a result of parasite enzymes and contributions from the host inflammatory response. When there is damage, such as that caused by ischemia and necrosis, the matrix is degraded and slippage of the ventricular layers with mural thinning and aneurysm formation ensues. Damage to this area of the heart and remodeling of the wall is frequently encountered in chagasic heart disease. Remodeling refers to the structural changes associated with inflammation, necrosis, hypertrophy, ventricular dilation and functional disturbances.
The parasite-induced inflammatory response in the myocardium is the result of many factors including cytokines, chemokines, fibronectin, kinins [21], endothelin [22], thromboxane [23] and parasites and their antigens. They may all contribute to the recruitment, migration and positioning of inflammatory cells into the heart tissue, leading to persistence of the myocarditis in infected mice [2]. Interestingly, the role of the CM in the inflammatory process has generally been regarded as either a passive bystanders or as a target of the parasite or activated leukocytes.
Previously, we demonstrated that the CM is a potential source of cytokines, chemokines and NO in vivo[6]. In the present study, we provide additional insights into the mechanisms by which T. cruzi, cytokines, chemokines, NO and CMs contribute to the establishment and regulation of the immune response in the infected heart. We demonstrated a reduction in inflammation in the heart of IFN-γ and in TNF-R1-null mice[17] and that IFN-γ acts synergistically with TNF to activate NOS2 expression in CMs [6]. In addition, as NO is believed to participate in the modulation of inflammatory reactions and the trafficking of leukocytes [24, 25]. We examined the possibility that NO actively participates in the modulation of chemokines production by CMs. In agreement with this idea, previous reports have described a novel function of NO is its ability to modulate chemokine production in other cell types, including endothelial cells, fibroblasts and keratinocytes [13, 14]. The addition of L-NMMA, an inhibitor of NO production, with IFN-γ and TNF in the T. cruzi-infected CM culture, significantly increased the production of CXCL2, CCL2, CCL4 and CCL5 (data not shown). During the acute T. cruzi infection in mice, chemokines and NOS2 are expressed in the heart [6, 26] and are detected simultaneously with the inflammatory cells composed of CD4+ and CD8+ cells, important sources of IFN-γ in the myocardium of T. cruzi-infected mice. We and others clearly demonstrated that CCL3, CCL4 and CCL5 and its receptor CCR5 play an important role in the control of T-cell influx into the heart of T. cruzi infected mice [3, 5]. The results of the present study taken together with our previous report demonstrating that T. cruzi-infected cultured CMs secrete TNF [6] supports the notion that in vivo 3 IFN-γ and TNF activates NOS2 in CMs and NO to modulate chemokine production by CMs in an autocrine and/or paracrine fashion. These events provide a critical signal for lymphocyte infiltration in the heart, commonly observed in Chagas disease.
Utilizing CMs obtained from NOS2-deficient mice we established a role for NOS2 in the modulation of chemokine production by these cells. We also demonstrated that NOS2 deficiency results in reduced CCL3 production by T. cruzi-infected CMs stimulated with IFN-γ and TNF. In contrast, CXCL1 production was not affected by NOS2 deficiency. Interestingly, cultured NOS2-null CMs activated with IFN-γ and TNF expressed significantly higher levels of CCL5 than WT CMs. These observations strongly suggest that NO released after CM activation by IFN-γ and TNF modulate the chemokine production by these cells. Infection of NOS2 null mice resulted in a marked increase in myocardial chemokine production. Although induction of chemokines was upregulated in the hearts obtained from T. cruzi-infected IFN-γ and TNFR1-null mice, the expression levels were not as high as those observed in hearts obtained from infected NOS2-null mice. These data suggest that NOS2 is required for control of chemokine expression in the myocardium of T. cruzi-infected mice. It is possible that NOS2 is still in part being activated in IFN-γ- and TNFR-null mice infected with T. cruzi via TNF or IFN-γ, respectively. The cytokines present in the myocardium of infected-null mice could also act together with chemokines thereby increasing NOS2 activation and further modulating chemokine production. It has been demonstrated that NO donors modulate chemokine production in various cell types [13, 14, 27]. These studies also demonstrated that NO can interfere with the activity of chemokines by several mechanisms such as through peroxynitrite-dependent tyrosine nitration [27], and it can function as an intracellular messenger in chemokine signaling pathways. NO may inhibit chemokine production by acting on the IκB-α levels and NF-κB DNA binding [13]. Although NO donors are thought to modulate chemokine production, the molecular targets of NO in CMs, as well as the mechanism by which NO does so in CMs remains to be elucidated.
The results of several studies strongly suggest that in the heart, NO directly regulates the contractile properties of muscle cells and leads to depressed cardiac function and myocardial damage [26, 28]. Our results indicate that in the heart NO regulates diverse aspects of inflammation. NOS2 activation was shown to be a potent modulator of chemokine expression and correlated well with infection-associated inflammation in the heart. Collectively, the studies suggest that the release of IFN-γ and TNF-α by activated Th1 cells results in the development of infection-associated myocarditis and that the parasitic infection stimulates CMs to produce cytokines and chemokines which further amplify the inflammatory response. The contribution of chemokines by CMs further leads to the establishment of the inflammatory state in the myocardium. The chemokines CCL3, CCL4 and CCL5 are associated with CD4 and CD8 T cells in the myocardium. The NO production in the myocardium of T. cruzi-infected mice may play a role in the modulation of inflammation including by controlling parasite growth and modulating the synthesis of certain chemokines such as CCL2, CCL3, CCL4, CCL5 and CXCL2. Finally, NO may participate in the modulation of inflammatory reactions and the trafficking of leukocyte inhibiting the expression of adhesion molecules (e.g., ICAM-1, VCAM-1, E-selectin) on endothelial cells, and impeding the rolling, firm adherence and/or transmigration of monocytes and granulocytes to the myocardium. Thus, based on these observations, NO donors may be therapeutic adjunctive agents in the treatment of Chagas disease. However, many of these agents are regarded as too toxic at this point.
Acknowledgments
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and NIH grants AI-052739, AI-076218 and AI-076248 (HBT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Higuchi Mde L, Benvenuti LA, Martins Reis M, Metzger M. Pathophysiology of the heart in Chagas’ disease: current status and new developments. Cardiovasc Res. 2003;60:96–107. doi: 10.1016/s0008-6363(03)00361-4. [DOI] [PubMed] [Google Scholar]
- 2.dos Santos PV, Roffe E, Santiago HC, Torres RA, Marino AP, Paiva CN, Silva AA, Gazzinelli RT, Lannes-Vieira J. Prevalence of CD8(+)alpha beta T cells in Trypanosoma cruzi-elicited myocarditis is associated with acquisition of CD62L(Low)LFA-1(High)VLA-4(High) activation phenotype and expression of IFN-gamma-inducible adhesion and chemoattractant molecules. Microbes Infect. 2001;3:971–984. doi: 10.1016/s1286-4579(01)01461-7. [DOI] [PubMed] [Google Scholar]
- 3.Machado FS, Koyama NS, Carregaro V, Ferreira BR, Milanezi CM, Teixeira MM, Rossi MA, Silva JS. CCR5 plays a critical role in the development of myocarditis and host protection in mice infected with Trypanosoma cruzi. J Infect Dis. 2005;191:627–636. doi: 10.1086/427515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michailowsky V, Celes MR, Marino AP, Silva AA, Vieira LQ, Rossi MA, Gazzinelli RT, Lannes-Vieira J, Silva JS. Intercellular adhesion molecule 1 deficiency leads to impaired recruitment of T lymphocytes and enhanced host susceptibility to infection with Trypanosoma cruzi. J Immunol. 2004;173:463–470. doi: 10.4049/jimmunol.173.1.463. [DOI] [PubMed] [Google Scholar]
- 5.Marino AP, da Silva A, dos Santos P, Pinto LM, Gazzinelli RT, Teixeira MM, Lannes-Vieira J. Regulated on activation, normal T cell expressed and secreted (RANTES) antagonist (Met-RANTES) controls the early phase of Trypanosoma cruzielicited myocarditis. Circulation. 2004;110:1443–1449. doi: 10.1161/01.CIR.0000141561.15939.EC. [DOI] [PubMed] [Google Scholar]
- 6.Machado FS, Martins GA, Aliberti JC, Mestriner FL, Cunha FQ, Silva JS. Trypanosoma cruzi-infected cardiomyocytes produce chemokines and cytokines that trigger potent nitric oxide-dependent trypanocidal activity. Circulation. 2000;102:3003–3008. doi: 10.1161/01.cir.102.24.3003. [DOI] [PubMed] [Google Scholar]
- 7.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 8.Pfeilschifter J, Eberhardt W, Beck KF. Regulation of gene expression by nitric oxide. Pflugers Arch. 2001;442:479–486. doi: 10.1007/s004240100586. [DOI] [PubMed] [Google Scholar]
- 9.Silva JS, Machado FS, Martins GA. The role of nitric oxide in the pathogenesis of Chagas disease. Front Biosci. 2003;8:s314–325. doi: 10.2741/1012. [DOI] [PubMed] [Google Scholar]
- 10.Sittipunt C, Steinberg KP, Ruzinski JT, Myles C, Zhu S, Goodman RB, Hudson LD, Matalon S, Martin TR. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:503–510. doi: 10.1164/ajrccm.163.2.2004187. [DOI] [PubMed] [Google Scholar]
- 11.Fakhrzadeh L, Laskin JD, Laskin DL. Deficiency in inducible nitric oxide synthase protects mice from ozone-induced lung inflammation and tissue injury. Am J Respir Cell Mol Biol. 2002;26:413–419. doi: 10.1165/ajrcmb.26.4.4516. [DOI] [PubMed] [Google Scholar]
- 12.Shanley TP, Zhao B, Macariola DR, Denenberg A, Salzman AL, Ward PA. Role of nitric oxide in acute lung inflammation: lessons learned from the inducible nitric oxide synthase knockout mouse. Crit Care Med. 2002;30:1960–1968. doi: 10.1097/00003246-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Calkins CM, Bensard DD, Heimbach JK, Meng X, Shames BD, Pulido EJ, McIntyre RC., Jr L-arginine attenuates lipopolysaccharide-induced lung chemokine production. Am J Physiol Lung Cell Mol Physiol. 2001;280:L400–408. doi: 10.1152/ajplung.2001.280.3.L400. [DOI] [PubMed] [Google Scholar]
- 14.Giustizieri ML, Albanesi C, Scarponi C, De Pita O, Girolomoni G. Nitric oxide donors suppress chemokine production by keratinocytes in vitro and in vivo. Am J Pathol. 2002;161:1409–1418. doi: 10.1016/S0002-9440(10)64416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vespa GN, Cunha FQ, Silva JS. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect Immun. 1994;62:5177–5182. doi: 10.1128/iai.62.11.5177-5182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aliberti JC, Machado FS, Souto JT, Campanelli AP, Teixeira MM, Gazzinelli RT, Silva JS. beta-Chemokines enhance parasite uptake and promote nitric oxide-dependent microbiostatic activity in murine inflammatory macrophages infected with Trypanosoma cruzi. Infect Immun. 1999;67:4819–4826. doi: 10.1128/iai.67.9.4819-4826.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aliberti JC, Souto JT, Marino AP, Lannes-Vieira J, Teixeira MM, Farber J, Gazzinelli RT, Silva JS. Modulation of chemokine production and inflammatory responses in interferon-gamma- and tumor necrosis factor-R1-deficient mice during Trypanosoma cruzi infection. Am J Pathol. 2001;158:1433–1440. doi: 10.1016/s0002-9440(10)64094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meirelles MN, de Araujo-Jorge TC, Miranda CF, de Souza W, Barbosa HS. Interaction of Trypanosoma cruzi with heart muscle cells: ultrastructural and cytochemical analysis of endocytic vacuole formation and effect upon myogenesis in vitro. Eur J Cell Biol. 1986;41:198–206. [PubMed] [Google Scholar]
- 19.Reis MM, Higuchi Mde L, Benvenuti LA, Aiello VD, Gutierrez PS, Bellotti G, Pileggi F. An in situ quantitative immunohistochemical study of cytokines and IL-2R+ in chronic human chagasic myocarditis: correlation with the presence of myocardial Trypanosoma cruzi antigens. Clin Immunol Immunopathol. 1997;83:165–172. doi: 10.1006/clin.1997.4335. [DOI] [PubMed] [Google Scholar]
- 20.Reis DD, Jones EM, Tostes S, Jr, Lopes ER, Gazzinelli G, Colley DG, McCurley TL. Characterization of inflammatory infiltrates in chronic chagasic myocardial lesions: presence of tumor necrosis factor-alpha+ cells and dominance of granzyme A+, CD8+ lymphocytes. Am J Trop Med Hyg. 1993;48:637–644. doi: 10.4269/ajtmh.1993.48.637. [DOI] [PubMed] [Google Scholar]
- 21.Monteiro SVAC, Morrot A, Barros de Arruda L, Nagajyothi F, Granato A, Pesquero JB, Müller-Esterl W, Tanowitz HB, Scharfstein J. Bradykinin B2 Receptors of Dendritic Cells, Acting as Sensors of Kinins Proteolytically Released by Trypanosoma cruzi. Are Critical for the Development of Protective Type-1 Responses. PLOS Pathogens. 2007;3:1730–1744. doi: 10.1371/journal.ppat.0030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanowitz HB, Huang H, Jelicks LA, Chandra M, Loredo ML, Weiss LM, Factor SM, Shtutin V, Mukherjee S, Kitsis RN, Christ GJ, Wittner M, Shirani J, Kisanuki YY, Yanagisawa M. Role of endothelin 1 in the pathogenesis of chronic chagasic heart disease. Infect Immun. 2005;73:2496–2503. doi: 10.1128/IAI.73.4.2496-2503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashton AW, Mukherjee S, Nagajyothi FN, Huang H, Braunstein VL, Desruisseaux MS, Factor SM, Lopez L, Berman JW, Wittner M, Scherer PE, Capra V, Coffman TM, Serhan CN, Gotlinger K, Wu KK, Weiss LM, Tanowitz HB. Thromboxane A2 is a key regulator of pathogenesis during Trypanosoma cruzi infection. J Exp Med. 2007;204:929–940. doi: 10.1084/jem.20062432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 25.Laroux FS, Pavlick KP, Hines IN, Kawachi S, Harada H, Bharwani S, Hoffman JM, Grisham MB. Role of nitric oxide in inflammation. Acta Physiol Scand. 2001;173:113–118. doi: 10.1046/j.1365-201X.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- 26.Huang H, Chan J, Wittner M, Jelicks LA, Morris SA, Factor SM, Weiss LM, Braunstein VL, Bacchi CJ, Yarlett N, Chandra M, Shirani J, Tanowitz HB. Expression of cardiac cytokines and inducible form of nitric oxide synthase (NOS2) in Trypanosoma cruzi-infected mice. J Mol Cell Cardiol. 1999;31:75–88. doi: 10.1006/jmcc.1998.0848. [DOI] [PubMed] [Google Scholar]
- 27.Sato E, Simpson KL, Grisham MB, Koyama S, Robbins RA. Reactive nitrogen and oxygen species attenuate interleukin- 8-induced neutrophil chemotactic activity in vitro. J Biol Chem. 2000;275:10826–10830. doi: 10.1074/jbc.275.15.10826. [DOI] [PubMed] [Google Scholar]
- 28.Chandra M, Tanowitz HB, Petkova SB, Huang H, Weiss LM, Wittner M, Factor SM, Shtutin V, Jelicks LA, Chan J, Shirani J. Significance of inducible nitric oxide synthase in acute myocarditis caused by Trypanosoma cruzi (Tulahuen strain) Int J Parasitol. 2002;32:897–905. doi: 10.1016/s0020-7519(02)00028-0. [DOI] [PubMed] [Google Scholar]