Summary
A 16-year old girl presented with rapid onset of muscular weakness and a history of severe dysphagia, dysphonia and significant wasting. On examination, she was dystrophic (BMI 15.7) and had signs of myopathy. Laboratory findings confirmed myopathy (CPK 106.4 µkat/L (6384 IU/L), AST 2.86 µkat/L (171.6 IU/L), myoglobin 1582 µg/L). There was profound hypokalaemia (S-K 1.8 mmol/L) suggesting hypokalaemic paralysis. Diagnosis of distal renal tubular acidosis (dRTA) was based on combination of hyperchloremic metabolic acidosis, severe hypokalaemia, high urinary pH and positive value of urinary anion gap. There was evidence of other signs of renal tubular impairment (urinary beta-2-microglobulin 213 mg/L, glomerulotubular proteinuria 1.01g/24h). Autoimmune tests (rheumatoid factor, antinuclear antibodies, autoantibodies to Ro/SSA and La/SSB) together with symptoms of xerostomia with swallowing difficulties and atrophic glossitis suggested primary Sjogren's syndrome (SS) as the underlying cause of dRTA. The renal biopsy confirmed chronic tubulo-interstitial nephritis compatible with this diagnosis. Full recovery of muscle weakness and hypokalaemia and acidosis followed after potassium and alkali replacement therapy. Corticosteroids were administered with subsequent addition of cyclosporine A because of disease activity. In conclusion, primary SS is a rare diagnosis in childhood and adolescence and should be considered in patients presenting with hypokalaemic paralysis, as this might be due to dRTA, even in the absence of apparent sicca syndrome.
Keywords: Hypokalaemia, paralysis, renal tubular acidosis, Sjogren's syndrome, potassium
Introduction
Potassium (K+) is predominantly an intracellular kation; only 2% of total body K+ is located in extracellular space. The serum potassium level (S-K+) provides useful information on total body K+ content. Hypokalemia indicates potassium depletion (renal wasting, gastrointestinal losses, decreased intake), but it may also reflect the transfer of extracellular K+ into cells (insulin administration, familial periodic paralysis, hypokalemic thyrotoxic periodic paralysis, acute alkalosis). Severe hypokalemia (S-K+ < 3.0 mmol/L) may produce muscular weakness with paralysis andrespiratory failure.1,2 The hypokalaemic paralysis represents a heterogenous group of disorders with a final pathway presenting as acute weakness. The approach to the patient with hypokalemic paralysis includes a vigorous search for underlying etiology and potassium replacement therapy.
Case Report
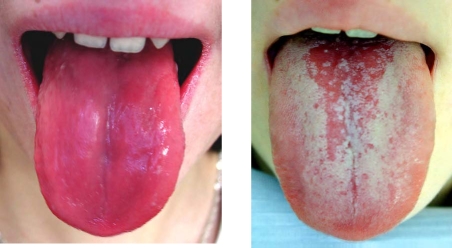
A 16-year old girl presented with rapid onset of muscular weakness within the last 2 days, one-week history of dysphagia and dysphonia, and significant wasting (−20% body weight) in the past 12 months. On physical examination, she was dystrophic, with body height of 169 cm (+0.5 SD), body weight 45 kg (−1.6 SD), and body mass index (BMI) 15.7 (−2.1 SD), respectively. She had signs of severe myopathy, especially manifested by inability to walk unassisted, together with pallor, dry skin and atrophic glossitis (Figure 1).
Figure 1.
Atrophic glossitis: Before and after treatment
The patient had accelerated erythrocyte sedimentation rate (ESR) 60/92 and high C-reactive protein, (C-RP 37 mg/L; normal value <5mg/L). Laboratory results confirmed myopathy with high serum creatine kinase (S-CK) activity of 40.7 and 106.4 µkat/L, respectively (normal value < 2.85µkat/L), (i.e. 2442 and 6484 IU/L), respectively (normal value < 2.85µkat/L, i.e. 171 IU/L), high serum aspartate-amino transferase activity (S-AST) 2.86 µkat/L (normal <0.66 µkat/L, i.e. 40 IU/L), and serum myoglobin 1582 µg/L (normal <80 µg/L). There was profound hypokalaemia (S-K+ 1.8 mmol/l; normal range 3.5–5.1 mmol/L) suggesting hypokalemic paralysis.
Urinary potassium and calcium excretion was increased (above 3.0 and 0.1 mmol/kg/24 hours, respectively). Other laboratory abnormalities included hyperchloremia (S-Cl− 120 mmol/L; normal 97–108 mmol/L), metabolic acidosis with plasma pH 7.315 (normal 7.35–7.45); base excess (BE) −10 ; plasma bicarbonate (HCO3−) 14.7 mmol/L, (normal 21–28 mmol/L), high urinary pH of 7.5 (normal 5.5), and a positive urinary anion gap [(U-Na 38 mmol/L + U-K 22 mmol/L) − Cl 39 mmol/L = + 21 ]. The value of transtubular gradient of potassium (TTKG) was calculated [(urine K/plasma K): (urine osmolality/plasma osmolality)] and exceeded 5.
Plasma anion gap [Na+ − (Cl− + HCO3−) ] was normal (9.7mmol/L). The combination of hyperchloremic metabolic acidosis, severe hypokalaemia, hyperkaliuria, normal plasma anion gap, high urinary pH, TTKG >5, and positive value of urinary anion gap suggested the diagnosis of renal potassium wasting due to distal renal tubular acidosis (dRTA).2,3 There was evidence of other signs of renal tubular impairment, such as high urinary beta-2-microglobulin 213 mg/L (normal <37mg/L) and glomerulo-tubular proteinuria 1.01g/24h, (normal <0.1g/24h). Renal biopsy was performed and the histologic evaluation of obtained tissue sample confirmed chronic tubulo-interstitial nephritis compatible with the diagnosis of dRTA. The high value of ESR, together with hyperimmunoglobulinemia (IgG 24.8g/L, normal 7.3–19.5 g/L), high circulating immunocomplexes (CIK) 106 U/L (normal range 0–50) and symptoms of xerostomia with swallowing difficulties and atrophic glossitis suggested dRTA of an autoimmune origin, most probably due to Sjogren's syndrome. The positive results of autoimmune tests, such as rheumatoid factor (RF), antinuclear antibodies (ANAs), antibodies to extractable nuclear antigens (ENA), including autoantibodies Ro/SSA and La/SSB, together with low values of sialometric measurements (7.5 ml/2×15 minutes) further confirmed primary Sjogren's syndrome as the underlying cause of dRTA.
Potassium replacement therapy (KCl administered intravenously and orally) and correction of metabolic acidosis (application of NaHCO3 and Shohl's solution) was started immediately after admission, later followed by pulses of metylprednisolone 1000 mg/day for 3 days replaced afterwards by oral methylprednisolone 36 mg/day. Cyclosporine A 3 mg/kg/day was also administered due to high disease activity. Full recovery of muscle weakness and normalisation of laboratory findings of hypokalaemia an acidosis followed after potassium and alkali replacement therapy within 14 days. Atrophic glossitis resolved after 20 days (Figure 1). Currently, the patient is doing well on therapy with cyclosporine A 4 mg/kg/den, Medrol (methylprednisolone) 4mg every other day, potassium chloride 2.5 g/day, Shohl's solution 9 ml twice daily.
Discussion
Sjögren's syndrome (SS) is a chronic systemic autoimmune disease affecting predominantly the exocrine glands and usually presents as persistent dryeness of the mouth and eyes due to functional impairment of the salivary and lacrimal glands. This syndrome is named after the Swedish ophthalmologist who first described it over 70 years ago. The common histopathological feature of all organs affected is a potentially progressive lymphocytic infiltration.4 Extraglandular involvement in SS may include interstitial nephritis, isosthenuria, renal tubular acidosis, polyarthritis or arthralgias, vulvovaginitis, interstitial pneumonitis, thyroiditis, central nervous system involvement, and an increased incidence of lymphoma.4–10
Primary SS may occur as an isolated disorder, but secondary SS in association with another autoimmune disease is more common.5 The aetio-pathogenesis of primary SS is probably a sequential, multistep process that leads to selective damage of the exocrine glands, with consequent target organ dysfunction. The aethiopathogenic model of primary SS is based on the existence of an altered immune system with abnormal autoimmune response against self antigens expressed by the epithelium of the exocrine glands. This process may be initiated by a specific combination of intrinsic and extrinsic factors. These involve genetic background, including polymorphisms of cytokine genes, such as interleukin-10 (IL-10), tumor necrosis factor-alpha (TNFα) and interleukin-4 receptor alpha chain (IL4R); altered immune recognition probably triggered by some exogenous agents, such as hepatitis C virus or herpes and retroviruses; acquired abnormal immune responses, in particular T-cell dysfunction and B-cell hyperreactivity; altered apoptotic mechanisms; altered epithelial repair; and probably hormonal (oestrogen) and autonomic dysfunction.4
Clinical manifestations may consist of dryeness of the mouth and/or eyes; enlarged parotid glands, that are smooth, firm and tender; arthritis; desiccation of the skin and mucous membranes of nose, mouth, throat, bronchi, vulva and vagina; RTA; hepatobiliary disease; fibrinous pericarditis, and sensory neuropathy. Patients present with anemia, high ESR and C-RP. Patients with SS typically produce a variety of autoantibodies, such as RF, ANAs, and in particular autoantibodies to the nuclear antigens (ribonucleoprotein particles) Ro/SSA and La/SSB4–8. The Schirmer test measuring the quantity of tears secreted in 5 minutes in response to irritation from a filter paper strip placed under lower eyelid is also helpful in the diagnosis of SS, together with slit lamp examination of the eyes and salivary flow measurement. Primary SS is most prevalent in women in their fourth and fifth decades; the disease occurs nine times more often in women than in men, and very rarely occurs in children.4,5,7,8 A revised version of the European criteria proposed by the American-European Consensus Group (AECG) have been validated for adults and include 6 items: ocular symptoms, oral symptoms, evidence of keratoconjunctivitis sicca, focal sialoadenitis by minor salivary gland biopsy, instrumental evidence of salivary gland involvement, and presence of Ro/SSA or La/SSB autoantibodies. The presence of 4 of the criteria, with the exclusion of patients who have negative autoantibodies or minor salivary gland biopsy, was found to have a sensitivity of 89.5% and specificity of 95.2%.5,6 The clinical presentation of primary SS in childhood and adolescence may differ from the clinical presentation in adults, in spite of the fact that pathological and laboratory findings in children with SS are similar to those found in adults, with characteristic lymphocytic infiltration of exocrine glands and the presence of hypergammaglobulinemia, high ESR, positive ANAs and Ro/SSA and La/SSB autoantibodies.5
Recurrent parotid swelling is the most common feature of paediatric SS and is much more frequent than sicca symptoms.5,8 Therefore, the AECG adult criteria for SS should not be applied to children as the sensitivity is unacceptably low5, and pediatric criteria have been already suggested.7 These differ from the AECG criteria by inclusion of parotid enlargement or recurrent parotitis as additional oral symptoms, and recurrent conjunctivitis as an additional ocular symptom. They also include elevated amylase, dRTA, leukopenia, elevated ESR, the presence of ANAs and RF, and hypergammaglobulinemia as additional biochemical and serological criteria5,7,8 (Table 1).
Table 1.
Diagnostic criteria for Sjogren's syndrome in children and adolescents (Adapted from 5,7)
| I. Clinical symptoms |
| 1. Oral (dry mouth, recurrent parotitis or enlargement of parotid glands) |
| 2. Ocular (recurrent conjunctivitis, keratoconjuncitivis sicca) |
| 3. Other mucosal (recurrent vulvovaginitis) |
| 4. Systemic (fever of unknown origin, noninflammatory arthralgias, abdominal pain, hypokalaemic paralysis) |
|
II. Immunological abnormalities (presence of at least 1 of: anti-SSA, anti-SSB, high titer ANF, RF) |
| III. Other laboratory abnormalities |
| 1. Biochemical (elevated serum amylase) |
| 2. Hematologic (high ESR, leukopenia) |
| 3. Immunologic (polyclonal hyperimmunoglobulinemia) |
| 4. Nephrological (RTA) |
| 5. Histological proof of lymphocytic infiltration of salivary glands or other organs |
| 6. Objective documentation of ocular dryness (Bengal red staining, Schirmer test) |
| 7. Objective documentation of parotid gland ivolvement(sialography) |
| IV. Exclusion of all other autoimmune disease |
The applicability of paediatric criteria was confirmed by a multicentre survey, where signs and symptoms at disease onset were mainly recurrent parotid swelling (72.5%) followed by sicca symptoms. Abnormal laboratory tests were found in the majority of cases.8
RTA is an under-recognized complication of SS, as it occurs in 27% of adult patients10, and in 8.6% children with SS8. RTA in SS may be proximal (pRTA) or distal (dRTA). dRTA is characterized by decreased proton excretion due to a proton pump defect or back diffusion of protons, while in pRTA the capacity of tubules to reabsorb bicarbonate is impaired. The clinical spectrum of RTA in SS may be silent to life-threatening in the acute phase but generally has a good long-term renal outcome.3,9,10 Hypokalaemia was observed in 11/12 (92%) of paediatric patients with RTA due to SS9. However, the renal potassium wasting with hypokalaemic paralysis is still considered as uncommon in SS10–19, and has been documented in several adult cases10–12,14,16,19,20, and very rarely in paediatric patients, as only 7 published cases exist.9,13,18 The treatment of patients with SS is directed toward the particular areas of the body that are involved, in particular moisturizing mouth and eyes, and therapy of complications, such as infection.
There is no specific cure for SS. The treatment of SS consists of corticotherapy (methylprednisolone pulses or oral prednisone) with eventual addition of cyclosporine A in cases of highly active disease. Remission of SS and RTA has been observed after pulse high-dose corticosteroid infusion therapy.9,10,21 Potassium replacement therapy and alkalinisation is necessary in patients with RTA and renal potassium wasting10–20. Our patient fulfilled most of the paediatric criteria for primary SS, however parotid gland enlargement was not apparent. Hypokalaemia was the primary cause of weakness, paralysis and myopathy. The hypokalaemic paralysis revealing dRTA in paediatric SS should be considered a rare observation. Potassium replacement therapy, alkalinisation and application of glucocorticoids with cyclosporine A resulted in remarkable improvement, however a close follow-up is necessary. SS should be considered in the older child with otherwise unexplained RTA. Likewise, RTA should be excluded in children and adolescents with SS who develop weakness, fatigue or stunted growth. Early recognition would reduce long-term complications of RTA such as growth failure or rickets.9–11
In conclusion, primary SS is a rare diagnosis in childhood and adolescence, especially when predominantly manifested by hypokalaemia, but it should be considered in patients presenting with hypokalemic paralysis, as this might be due to renal involvement, even in the absence of apparent sicca syndrome.
References
- 1.Ahlawat Sushil K, Sachdev A. Classic diseases revisited: Hypokalemic paralysis. Postgrad Med J. 1999;75:193–197. doi: 10.1136/pgmj.75.882.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin SH, Lin YF, Halperin ML. Hypokalaemia nad paralysis. Q J Med. 2001;94:133–139. doi: 10.1093/qjmed/94.3.133. [DOI] [PubMed] [Google Scholar]
- 3.Bagga A, Bajpai A, Menon S. Approach to renal tubular disorders. Indian J Pediatr. 2005;72:771–776. doi: 10.1007/BF02734150. [DOI] [PubMed] [Google Scholar]
- 4.Ramos-Casals M, Font J. Primary Sjogren's syndrome: current and emergent aethiopathogenic concepts. Rheumatology. 2005;44:1354–1367. doi: 10.1093/rheumatology/keh714. [DOI] [PubMed] [Google Scholar]
- 5.Houghton K, Malleson P, Cabral D, Petty R, Tucker L. Primary Sjogren's syndrome in children and adolescents: Are proposed diagnostic criteria applicable? J Rheumatol. 2005;32:2225–2232. [PubMed] [Google Scholar]
- 6.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH. European Study Group on Classification Criteria for Sjögren's Syndrome. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartunkova J, Sedivá A, Vencovský J, Tesar V. Primary Sjögren's syndrome in children and adolescents: proposal for diagnostic criteria. Clin Exp Rheumatol. 1999;17:381–386. [PubMed] [Google Scholar]
- 8.Cimaz R, Casadei A, Rose C, Bartunkova J, Sediva A, Falcini F, Picco P, Taglietti M, Zulian F, Ten Cate R, Sztajnbok FR, Voulgari PV, Drosos AA. Primary Sjögren syndrome in the paediatric age: multicenter survey. Eur J Ped. 2003;162:661–665. doi: 10.1007/s00431-003-1277-9. [DOI] [PubMed] [Google Scholar]
- 9.Pessler F, Emery H, Dai L, Wu Y-M, Monash B, Cron RQ, Pradhan M. The spectrum of renal tubular acidosis in paediatric Sjögren syndrome. Rheumatology. 2006;45:85. doi: 10.1093/rheumatology/kei110. [DOI] [PubMed] [Google Scholar]
- 10.Bossini N, Savoldi S, Franceschini F, Mombelloni S, Baronio M, et al. Clinical and morphological features of kidney involvement in primary Sjögren's syndrome. Nephrol Dial Transplant. 2001;16:2328–2336. doi: 10.1093/ndt/16.12.2328. [DOI] [PubMed] [Google Scholar]
- 11.Pun KK, Wong CK, Tsui EY, Tam SC, Kung AW, Wang CC. Hypokalemic periodic paralysis due to the Sjögren syndrome in Chinese patients. Ann Intern Med. 1989;110:405–406. doi: 10.7326/0003-4819-110-5-405. [DOI] [PubMed] [Google Scholar]
- 12.Hattori N, Hino M, Ishihara T, Moridera K, Ikekubo K, Kurahachi H. Hypokalemic paralysis associated with distal renal tubular acidosis. Intern Med. 1992;31:662–665. doi: 10.2169/internalmedicine.31.662. [DOI] [PubMed] [Google Scholar]
- 13.Chang YC, Huang CC, Chiou YY, Yu CY. Renal tubular acidosis complicated with hypokalemic periodic paralysis. Pediatr Neurol. 1995;13:52–54. doi: 10.1016/0887-8994(95)00080-y. [DOI] [PubMed] [Google Scholar]
- 14.al Jubouri MA, Jones S, Macmillan R, Harris C, Griffiths RD. Hypokalemic paralysis revealing Sjogren syndrome in an elderly man. J Clin Pathol. 1999;52:157–158. doi: 10.1136/jcp.52.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soy M, Pamuk ON, Gerenli M, Çelik Y. A primary Sjögren's syndrome patient with distal renal tubular acidosis, who presented with symptomes of hypokalemic periodic paralysis. Rheumatol Int. 2005;26:86–89. doi: 10.1007/s00296-005-0587-9. [DOI] [PubMed] [Google Scholar]
- 16.Cheng CJ, Chiu JS, Chen CC, Lin SH. Unusual cause of hypokalemic paralysis in aged men: Sjögren syndrome. South Med J. 2005;98:1212–1215. doi: 10.1097/01.smj.0000189906.32780.0c. [DOI] [PubMed] [Google Scholar]
- 17.Kawashima M, Amano T, Morita Y, Yamamura M, Makino H. Hypokalemic paralysis and osteomalacia secondary to renal tubular acidosis in a case with primary Sjogren's syndrome. Mod Rheumatol. 2006;16:48–51. doi: 10.1007/s10165-005-0446-2. [DOI] [PubMed] [Google Scholar]
- 18.Ohlsson V, Strike H, James-Ellison M, Tizard EJ, Ramanam AV. Renal tubular acidosis, arthritis and autoantibodies: primary Sjogren syndrome in childhood. Rheumatology. 2006;45:238–240. doi: 10.1093/rheumatology/kei175. [DOI] [PubMed] [Google Scholar]
- 19.Morovic-Vergles J, Galesic K, Vergles D. Primary Sjogren's syndrome presenting as hypokalemic paralysis. Ann Saudi Med. 2007;27:125–127. doi: 10.5144/0256-4947.2007.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor I, Parsons M. Hypokalemic paralysis revealing Sjögren's syndrome. J Clin Neurosci. 2004;11:319–321. doi: 10.1016/j.jocn.2003.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Saeki Y, Ohshima S, Ishida T, Umeshita-Sasai M, Nishioka K, Yamaguchi N, M Suemura M. Remission of the renal involvement in a patient with primary Sjögren's syndrome (SS) after pulse high-dose corticosteroid infusion therapy. Clin Rheumatol. 2001;20:225–228. doi: 10.1007/pl00011200. [DOI] [PubMed] [Google Scholar]