Abstract
This paper reports the clinical findings, diagnostic imaging, surgical management, and necropsy of an unusual case of jejuno-jejunal intussusception in a calf that was diagnosed with the use of ultrasonography, and treated surgically by resection and end-to-end anastomosis. The calf fatally relapsed 8 d after laparotomy. Necropsy and histology revealed enteritis and myenteric ganglionitis.
Résumé
Occurrence répétée de l’intussusception jéjuno-jéjunale chez un veau. Cet article fait rapport sur les résultats cliniques, l’imagerie diagnostique, la gestion chirurgicale et la nécropsie d’un cas inusité d’intussusception jéjuno-jéjunale chez un veau, qui a été diagnostiqué par échographie et traité par chirurgie par une résection et une anastomose terminoterminale. Le veau a eu une rechute mortelle 8 jours après la laparotomie. La nécropsie et l’histologie ont révélé une entérite et une ganglionite myentérique.
(Traduit par Isabelle Vallières)
Intussusception occurs when a segment of the gastrointestinal tract telescopes into an adjacent segment, causing intestinal obstruction. The outer receiving segment and the inner inverting segment are called intussuscipiens and intussusceptum, respectively (1–3). The etiology of intussusception is referable to several disorders of intestinal motility. Essential factors are strong peristalsis in 1 bowel segment and distension of the segment immediately distal to it (3). Submucosal abscesses, fibroserous granulation (4), intestinal tumors such as mucosal papilloma or adenocarcinoma (5), and enteritis resulting in vigorous and uncoordinated intestinal motility or hypermotility and gas distension are considered to be predisposing factors (3). In a study of 336 cattle with intussusception, the small intestine was primarily involved in 281 (84%) cases. Other sites were: the ileocolic segment in 7 cases (2%), the cecocolic segment in 12 cases (4%) and the colon in 36 cases (11%) (2). Intussusception is frequently observed in adult animals (3,6) but appears to be most common in calves up to 2 months old (2).
This paper presents a case of a jejuno-jejunal intussusception in a calf that was diagnosed with the use of ultrasonography and treated surgically by resection and end-to-end anastomosis. The animal fatally relapsed 8 d later.
Case description
A 75-day-old female Brown Swiss calf was admitted to our clinic following 4 d of abdominal pain; the condition was unresponsive to medical treatment and was accompanied by anorexia and a complete lack of defecation.
On clinical examination, the physical condition of the animal was fair but it was reluctant to move. The calf had a rectal temperature of 39.4°C, breathing was costo-abdominal (32 breaths/ min), pulse was rhythmic but the rate had increased (120 bpm), the mucous membranes were slightly cyanotic, and the episcleral vessels were swollen. Severe abdominal distension was also observed. Abdominal auscultation revealed reduced peristaltic squishing sounds of the intestine. Ping sounds were not created by abdominal percussion with auscultation, but simultaneous ballottement and auscultation of the right side of the abdomen produced fluid-splashing sounds that indicated an abnormal accumulation of fluid in the gastrointestinal viscera. A small quantity of dark, firm faeces containing sand-like material was present in the rectum. Blood-gas analysis revealed a blood pH of 7.38 (reference range: 7.36 to 7.44) with base excess (BE) of 7.8 mmol/L (reference range: −3 to +3 mmol/L), hyponatraemia (131 mmol/L; reference range: 135 to 150 mmol/L), hypochloremia (88 mmol/L; reference range: 90 to 105 mmol/L), and a packed cell volume (PCV) of 0.38 L/L (reference range: 0.28 to 0.36 L/L).
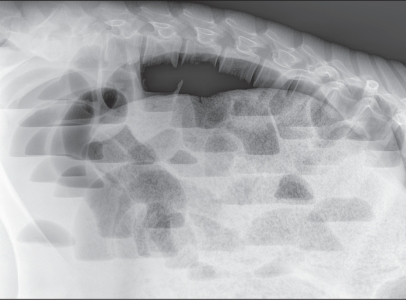
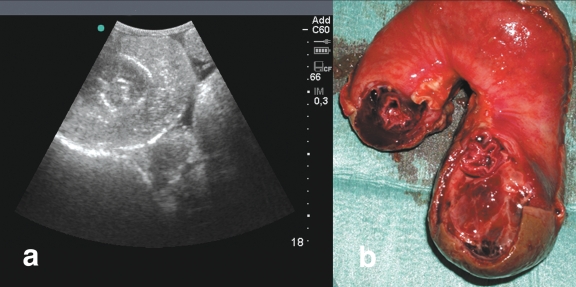
Standing lateral survey radiographs showed distended loops of small intestine throughout the abdomen, with a greater concentration in the dorsal portion. Horizontal gas-fluid interfaces were clearly visible (Figure 1). Transabdominal ultrasonography, performed with a 3.5 MHz curved transducer, identified mild peritoneal effusion and several loops of distended small intestine containing hypoecoic material, and weak peristaltic movements in the ventral abdomen. Ultrasonography of the lower region of the right flank showed an image of the classical bull’s eye or target sign (Figure 2a). The clinical, radiological, and ultrasonographic findings pointed strongly to a diagnosis of intussusception of the small intestine; laparotomy was recommended.
Figure 1.
Standing lateral survey radiograph. The abdomen appears to be filled with numerous markedly gas-distended loops of small bowel with varying gas-fluid interfaces that suggest obstructive ileus.
Figure 2.
a — Ultrasonographic appearance of the jejuno-jejunal intussusception. The intussusception appears as 2 concentric rings, with a central circular core representing the inner lumen of the intussusceptum. The image is created by the difference in acoustic impedance between the thick-walled, congested, edematous outer intussuscipiens and the inner intussusceptum. b — Anatomical appearance of the intussusception after resection.
The calf was premedicated with xylazine, 0.02 mg/kg body weight (BW), IM and intubated with a 14-mm orotracheal tube after mask induction with isofluorane. General anesthesia was maintained with isofluorane in oxygen. The calf was positioned in left lateral recumbency on the surgery table. The right abdominal wall was prepared for aseptic surgery and a lateral incision of 25 cm was made in the right dorsal paracostal region for laparotomy. An intussusception that involved 50 cm of the jejunum was found in the distal jejunal area, where the intestinal wall was dark purple and severely thickened. The intussusceptum had adhered extensively to the intussuscipiens through a fibrinous reaction; therefore, a jejunal resection (Figure 2b) and end-to-end anastomosis were performed. Prior to closure, the abdominal cavity was washed with sterile saline solution.
Postoperative care consisted of procaine benzylpenicillin (Procacillina; Merial Italia SpA, 20090 Assago, MI, Italy), 40 000 IU/kg BW, IM, the nonsteroidal anti-inflammatory drug flunixin meglumine (Alivios; Fatro SpA, 40064 Ozzano Emilia, BO, Italy), 2.5 mg/kg BW, IV, and physiological saline solution, 1 L/h for 6 h, IV. The day after surgery, the animal had profuse green-brown diarrhea and auscultable physiological intestinal sounds. The appetite increased gradually and the consistency of the feces gradually normalized. Eight days after surgery the clinical condition deteriorated rapidly with depression, ruminal atony and tympany, dilated abdomen with fluid-splashing sounds on ballottement of the right flank, melena, metabolic alkalosis associated with hypochloremia (88 mmol/L), and a PCV of 0.32 L/L. Before further investigation could be completed, the animal died.
Necropsy revealed a new jejuno–jejunal intussusception involving a large, 30-cm tract of small intestine, located 1.5 m proximal to the anastomosis, and associated with mild fibrinous acute peritonitis. The portion of the invaginated jejunum revealed signs of transmural necrosis, infarction, large hemorrhagic areas, and fibrin deposits on the serous and mucosal surfaces. Intense hyperemia, acute catarrhal enteritis, and moderate-to-severe sero-fibrinous deposits affected the portion of small intestine preceding the intussusception. Although the mesenteric lymph nodes appeared slightly enlarged, neither lymph nodes nor mesenteric fat were involved in the invaginated intestinal portion. The healing of the surgical anastomosis appeared normal; at this site, the intestinal lumen was patent and there was no evidence of exudation or inflammation.
Samples for bacteriological examination were collected proximal and distal to the lesion and in the lumen of the intussusceptum. The samples were processed to isolate Enterobacteriaceae; the cultures revealed the presence of Escherichia coli.
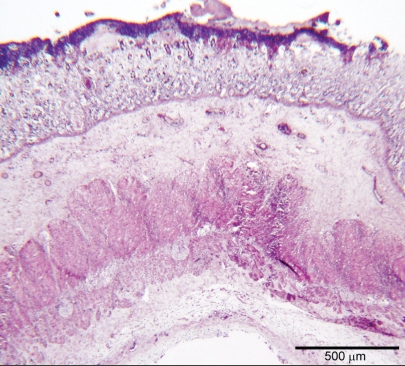
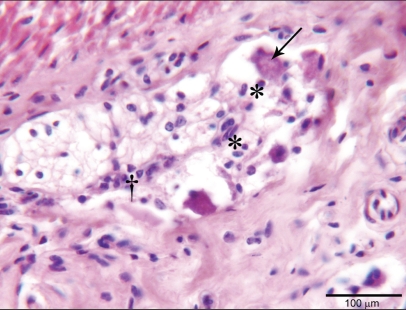
Histological examination of the invaginated jejunum showed extensive transmural coagulation necrosis with cell debris, karyorrhectic neutrophils, and hemorrhagic foci entrapped in a large amount of fibrin meshwork. Moreover, transmural lymphocytic and plasmacytic enteritis with severe edema affected tracts of intestine proximal and distal to the intussusception (Figure 3). In the intestinal myenteric plexi there were swollen neurons with a central clear cytoplasmic halo, peripheral nuclear displacement, and nuclear degeneration (central chromatolysis), surrounded by glial satellite cells with hyperchromatic chromatin and small amounts of mildly basophilic cytoplasm (satellitosis/activated satellite cells). Shrunken neuronal cells with hypereosinophilic cytoplasm and nondistinguishable nuclear borders (neuronal necrosis) were also visible, and were surrounded by glial cells (neuronophagia). Moderate numbers of lymphocytes and plasma cells were also detected. These histological findings were also visible in the intestinal tract affected to a lesser degree by the inflammatory changes (Figure 4) and were consistent with ganglia degeneration and necrosis associated with mild sub-acute to chronic ganglionitis.
Figure 3.
Photomicrograph of the jejunum proximal to the invaginated tract. There is extensive, severe, and erosive transmural enteritis. The mucosa is effaced by inflammatory changes consistent with severe leukocytic infiltrate extending to the subserosa, also showing abundant edema. Hematoxylin and eosin, bar = 500 μm.
Figure 4.
Intestinal Auerback plexus involved in degenerative and inflammatory changes of central neuronal chromatolysis with peripheral displacement of nuclei (arrow), satellitosis by glial cells (*) and a moderate number of infiltrating lymphocytes (†). Hematoxylin and eosin, bar = 100 μm.
Immunohistochemistry with polyclonal antibodies against E. coli was performed on histological sections from the intestine proximally, distally and in the intussusception. It revealed nonadhesive E. coli.
Discussion
Intussusception is a severe obstructive intestinal condition in which the intestinal lumen may be open and ingesta may pass through initially (7). In the present case, the symptoms observed referred to the ileus, but the animal’s clinical history indicated that the condition had started 4 d earlier.
In humans and many animal species, transabdominal ultrasonography is an excellent diagnostic procedure for abdominal disorders. Ultrasonographic descriptions of intussusception have been reported in humans (1), foals (8), adult cattle (9), cats (10), and dogs (11). On traverse scanning, the ultrasonographic image shows several concentric rings. Longitudinal sonography reveals a sandwich-like appearance of the alternating loops of bowel, with a loop-within-a loop appearance. Fluid distention of the more proximal small intestine was detected, usually with normal or nearly normal wall thickness and little or no peristaltic activity (8,9,12). In this case, the ultrasonographic appearance of the intussusception corresponds to that described using a 3.5 MHz transducer in a cow (9) and in horses (12). Although often inconclusive in adult cattle with small intestine ileus (9), because the site of intestinal obstruction is often at a greater distance from the abdominal wall than the penetration capacity of the transducer, transabdominal ultrasonography in calves is rapid, noninvasive, easy to perform, and allows identification of the type and localization of the intestinal lesion prior to laparotomy.
In this case, the most interesting findings were the repeated occurrence of intussusception and the widespread myenteric ganglionitis. The predisposition to jejuno-jejunal intussusception would appear to be high in calves because of the exposure to infectious agents causing diarrhea and peristalsis disorders (2). In animals (5) and in human beings (13,14), intestinal intussusception may be related to mesenteric neoplasia or severe mesenteric lymph node enlargement caused by mechanical stress. Finally, recurrent intussusception may be related to previous surgical anastomosis (15). In the present case, lesions consistent with lymphadenomegaly or mesenteric masses were not identified and this excluded any correlation with both intussusceptions. The physical distance between the initial and the subsequent intussusception, the absence of inflammatory signs at the site of the anastomosis associated with satisfactory healing, and the absence of intestinal stenosis and scarring, also suggest that the relapse was not related to surgery. Moreover, bacteriology and immunohistochemistry that identified nonadhesive E. coli, were not conclusive in demonstrating the role of a single bacterium in the pathogenesis of both intussusceptions. Recurrent intussusceptions have also been described in dogs and cats (16–18). Sivasankar (16) reported a case of a jejuno-jejunal intussusception in a dog that recurred 1 mo after surgical treatment, in a cranial position to the previous anastomosis. Histopathological examination of the resected tract revealed abnormalities of the inner muscular layer or in the neuromuscular control of the intestine. In dogs, frequency of recurrence was lower following surgical resection and anastomosis than after manual reduction. Recurrence tended to be proximal to the site of initial presentation. Although complications have been described, a surgical enteroplication has been suggested to prevent post-operative relapse (18).
Myenteric ganglionitis has been reported in humans (19) and in several animal species (20–22). This inflammatory neuropathy may be congenital or acquired. However, the etiology is often uncertain; bacteria-derived exotoxins and endotoxins, mycotoxins, viruses, and oxidative stresses are thought to play a role in the pathogenesis (23,24). Ganglionitis may also arise from inflammatory cytokine-mediated injury to autonomic neurons and this may cause alterations of intestinal motility characterized by pseudo-obstruction (25) or diarrhea (22) in humans (26), horses (21), dogs (22), pigs (27), and cows (20). Nitric oxide, which is abundant in inflammatory processes, is also a major inhibitory neurotransmitter in the enteric nervous system, recently considered to be involved in the pathogenesis of ileo-cecocolic intussusception in humans (28).
Peristalsis disorders have been suggested as possible causes of recurrent intussusception in humans affected by celiac disease in the absence of abdominal masses (13). In our case, the histological findings did not clearly point to primary ganglionitis within the myenteric ganglia but, most probably, it was a consequence of extensive erosive enteritis and transmural edema, probably caused by the intussusception. In this specific case, there may be a correlation between the simultaneous presence of myenteric ganglionitis and recurring intussusception but further investigation would be required to better understand the effect of enteritis-induced myenteric plexi damage on intestinal motility in calves.
Footnotes
Authors’ contributions
Drs. Pravettoni, Morandi, and Belloli described clinical findings, ultrasonographic examination, and post operative period. Dr. Zani described the radiological findings of the clinical case. Drs. Pravettoni and Scandella operated on the calf and described the surgery. Drs. Riccaboni and Rondena performed the necropsy and histology and wrote the pathological section. Dr. Pravettoni edited each section, and wrote the definitive version of the paper. CVJ
References
- 1.Byrne AT, Geoghegan T, Govender P, Lyburn ID, Colhoun E, Torreggiani WC. The imaging of intussusception. Clin Radiol. 2005;60:39–46. doi: 10.1016/j.crad.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Constable PD, St Jean G, Hull BL, Rings DM, Morin DE, Nelson DR. Intussusception in cattle: 336 cases (1964–1993) J Am Vet Med Assoc. 1997;210:531–536. [PubMed] [Google Scholar]
- 3.Pearson H. Intussusception in cattle. Vet Rec. 1971;89:426–437. doi: 10.1136/vr.89.16.426. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto M, Itoh H, Koiwa M, et al. Intussusception of the spiral colon associated with fibroserous granulation in a heifer. Vet Rec. 2007;160:376–378. doi: 10.1136/vr.160.11.376. [DOI] [PubMed] [Google Scholar]
- 5.Archer RM, Cooley AJ, Hinchcliff KW, Smith DF. Jejunojejunal intussusception associated with a transmural adenocarcinoma in an aged cow. J Am Vet Med Assoc. 1988;192:209–211. [PubMed] [Google Scholar]
- 6.Dziuk HE, Usenik EA. Intestinal intussusception in a cow. J Am Vet Med Assoc. 1964;145:348–351. [PubMed] [Google Scholar]
- 7.Anderson DE, Ewoldt JM. Intestinal surgery of adult cattle. Vet Clin North Am Food Anim Pract. 2005;21:133–154. doi: 10.1016/j.cvfa.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Bernard WV, Reef VB, Reimer JM, Humber KA, Orsini JA. Ultrasonographic diagnosis of small-intestinal intussusception in three foals. J Am Vet Med Assoc. 1989;194:395–397. [PubMed] [Google Scholar]
- 9.Braun U, Marmier O, Pusterla N. Ultrasonographic examination of the small intestine of cows with ileus of the duodenum, jejunum or ileum. Vet Rec. 1995;137:209–215. doi: 10.1136/vr.137.9.209. [DOI] [PubMed] [Google Scholar]
- 10.Patsikas MN, Papazoglou LG, Papaioannou NG, Savvas I, Kazakos GM, Dessiris AK. Ultrasonographic findings of intestinal intussusception in seven cats. J Feline Med Surg. 2003;5:335–343. doi: 10.1016/S1098-612X(03)00066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamb CR, Mantis P. Ultrasonographic features of intestinal intussusception in 10 dogs. J Small Anim Pract. 1998;39:437–441. doi: 10.1111/j.1748-5827.1998.tb03752.x. [DOI] [PubMed] [Google Scholar]
- 12.Fontaine-Rodgerson G, Rodgerson DH. Diagnosis of small intestinal intussuception by transabdominal ultrasonography in 2 adult horses. Can Vet J. 2001;42:378–380. [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez G, Israel NR, White JJ. Celiac disease presenting as entero-enteral intussusception. Pediatr Surg Int. 2001;17:68–70. doi: 10.1007/s003830000395. [DOI] [PubMed] [Google Scholar]
- 14.Ricciardelli L, Rapicano G, Pinto A, et al. Invaginazione ileo-ileale da metastasi di carcinoma anaplastico tiroideo. Caso clinico e revisione della letteratura. Ann Ital Chir. 2006;77:63–67. [PubMed] [Google Scholar]
- 15.Gayer G, Zissin R, Apter S, Papa M, Hertz M. Adult intussusception — A CT diagnosis. Br J Radiol. 2002;75:185–190. doi: 10.1259/bjr.75.890.750185. [DOI] [PubMed] [Google Scholar]
- 16.Sivasankar M. Recurrent intussusception in a 14-month-old, spayed, female German shepherd cross. Can Vet J. 2000;41:407–408. [PMC free article] [PubMed] [Google Scholar]
- 17.Levitt L, Bauer MS. Intussusception in dogs and cats: A review of 36 cases. Can Vet J. 1992;33:660–664. [PMC free article] [PubMed] [Google Scholar]
- 18.Oakes MG, Lewis DD, Hosgood G, Beale BS. Enteroplication for the prevention of intussusception recurrence in dogs: 31 cases (1978–1992) J Am Vet Med Assoc. 1994;205:72–75. [PubMed] [Google Scholar]
- 19.De Giorgio R, Barbara G, Stanghellini V, et al. Clinical and morpho-functional features of idiopathic myenteric ganglionitis underlying severe intestinal motor dysfunction: A study of three cases. Am J Gastroenterol. 2002;97:2454–2459. doi: 10.1111/j.1572-0241.2002.06002.x. [DOI] [PubMed] [Google Scholar]
- 20.Baker JS, Hullinger RL, Appel G. Postpartum atony of the small and large intestine in a Holstein cow: A case of pseudo-obstruction. Cornell Vet. 1985;75:289–296. [PubMed] [Google Scholar]
- 21.Burns GA, Karcher LF, Cummings JF. Equine myenteric ganglionitis: A case of chronic intestinal pseudo-obstruction. Cornell Vet. 1990;80:53–63. [PubMed] [Google Scholar]
- 22.Willard MD, Mullaney T, Karasek S, Yamini B. Diarrhea associated with myenteric ganglionitis in a dog. J Am Vet Med Assoc. 1988;193:346–348. [PubMed] [Google Scholar]
- 23.Orandle MS, Veazey RS, Lackner AA. Enteric ganglionitis in Rhesus macaques infected with simian immunodeficiency virus. J Virol. 2007;81:6265–6275. doi: 10.1128/JVI.02671-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zachary JF. Peripheral nervous system. In: McGavin MD, Zachary JF, editors. Pathologic Basis of Veterinary Disease. St. Louis: Elsevier Saunders; 2007. pp. 953–964. [Google Scholar]
- 25.Schappi MG, Smith VV, Milla PJ, Lindley KJ. Eosinophilic myenteric ganglionitis is associated with functional intestinal obstruction. Gut. 2003;52:752–755. doi: 10.1136/gut.52.5.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffith JC, Jardine DL, Bailey W, Florkowski CM. Variegate porphyria presenting with acute autonomic dysfunction, intussusception and renal infarction. Scand J Gastroenterol. 2004;39:500–503. doi: 10.1080/00365520410008395. [DOI] [PubMed] [Google Scholar]
- 27.Balemba OB, Semuguruka WD, Hay-Schmidt A, Johansen MV, Dantzer V. Vasoactive intestinal peptide and substance P-like immunoreactivities in the enteric nervous system of the pig correlate with the severity of pathological changes induced by Schistosoma japonicum. Int J Parasitol. 2001;31:1503–1514. doi: 10.1016/s0020-7519(01)00273-9. [DOI] [PubMed] [Google Scholar]
- 28.Cserni T, Paran S, Puri P. New hypothesis on the pathogenesis of ileocecal intussusception. J Ped Surg. 2007;42:1515–1519. doi: 10.1016/j.jpedsurg.2007.04.025. [DOI] [PubMed] [Google Scholar]