Abstract
Equine herpesvirus 1 (EHV-1) causes rhinopneumonitis, abortion, and rarely, myeloencephalopathy. The neurovirulence of this virus is due to a point mutation in the DNA polymerase gene. Diagnosis by virus isolation has been replaced by real-time polymerase chain reaction (RT-PCR) assays that can detect strains, viral loads, and states; this may aid in control and management of the disease.
Résumé
L’herpèsvirus équin de type 1, pathotype non-neurogène, chez un cheval de race American Saddlebred âgé de 9 ans manifestant des signes neurologiques. L’herpèsvirus équin de type-1 (EHV-1) cause la rhinopneumonie, l’avortement et rarement la myéloencéphalopathie. La neurovirulence de ce virus est attribuable à une mutation ponctuelle à l’intérieur du gène de l’ADN polymérase. Le diagnostic par isolement viral a été remplacé par des essais d’amplification en chaîne par la polymérase en temps réel (ACP-TR) qui détectent les souches, les charges virales et les états et peuvent aider à contrôler et à gérer la maladie.
(Traduit par Isabelle Vallières)
On September 9, 2008 a 493-kg, 9-year-old, American Saddlebred mare of unknown vaccination status became acutely ataxic. The mare then began dog-sitting and collapsed while under saddle during a horse show at a local fair. Apparent grand mal seizure activity was observed for more than 30 min. Focal seizures were observed for the next 4 h by several witnesses, including the on-site veterinarian and a veterinarian visiting the show grounds. The mare was treated with xylazine, butorphanol, and DMSO at that time.
Case description
On September 11, 2008 the mare was transferred to the care of Dundas Veterinary Services, Winchester, Ontario. The mare had been purchased within the last 10 d, and had received rabies vaccination on the day of purchase. No previous vaccination history was available. Upon physical examination by the referring veterinarian, the mare appeared to be somnolent, ataxic upon ambulation, and had cold extremities. All vital parameters were within normal limits.
Several neurological deficits were observed, involving both cranial and peripheral nerves. Cranial nerve deficits consisted of miosis and absence of a pupillary light response in the left eye. The left side of the face was paretic with an asymmetry localized to the nasal planum and the nares. Quidding of food into the right cheek pouch and an inability to hold grain in the left side of the oral cavity were noted. Swallowing and tongue movements appeared normal. All other cranial nerve functions were normal. Bilateral proprioceptive deficits of the front limbs, hypermetria of all 4 limbs, and bilateral paresis of the hind limbs upon the sway test were present.
On September 12, 2008 the mare was transported to the Animal Health Laboratory, University of Guelph, Kemptville Campus, Kemptville, Ontario at the Kemptville College. Blood was collected prior to euthanasia by a barbiturate overdose and a necropsy was performed immediately after euthanasia.
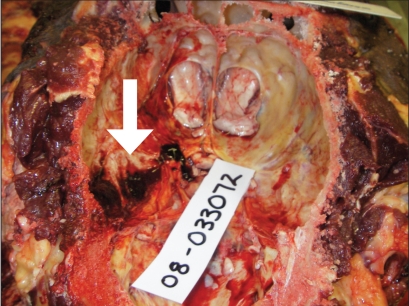
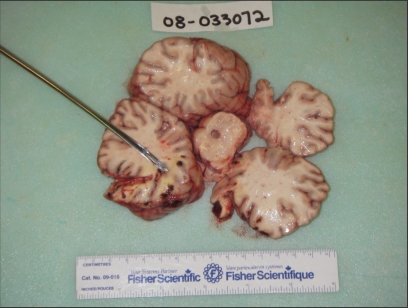
Samples of serum, brain, and spinal cord were collected for histopathology, rabies fluorescent antibody (FA) test, West Nile virus real time-polymerase chain reaction (RT-PCR), Eastern equine encephalitis virus RT-PCR, and Equine herpesvirus-1 RT-PCR. The necropsy revealed several gross lesions of significance within the central nervous system. There was a 2.8 × 2.0 × 0.1 cm solid supradural hematoma that adhered to the left caudoventral wall of the cranial vault (Figure 1). The hemorrhage abutted the ventrolateral aspect of the left occipital lobe and the caudoventral aspect of the left temporal lobe of the left cerebral cortex. In these lobes, at the level of the pons, there was an approximately 3.5 × 2.0 × 5 cm soft area of yellow discoloration in the white matter. Multiple 1–2 mm discrete, intense, foci of hemorrhage bordered this area at the junction of white and gray matter, and continued into the superficial gray matter and meninges (Figure 2). No other parts of the cerebellum, brainstem, or spinal cord were grossly affected.
Figure 1.
The cranial vault showing a supradural hematoma adherent to the caudoventrolateral aspect of the left temporal lobe of the left cerebral cortex.
Figure 2.
Gross cerebral cortical lesions of the occipital and temporal lobes.
The laboratory results indicated that the mare was positive for equine herpesvirus-1 (EHV-1), non-neuropathogenic pathotype. All the other tests used to detect viruses were negative. Histopathology confirmed acute hemorrhage and necrosis of the brain and spinal cord. Associated with vasculitis and thrombosis, this was locally severe in the left occipital and temporal lobes of the cerebral cortex. The neuropil was affected most severely in the deep gray matter of the cortex, but all areas of the cortex had perivascular and multifocal hemorrhage. The areas of necrosis were defined by swollen axons with occasional digestion chambers, and intralesional infiltrates of foamy neutrophils and macrophages. While large areas of severe, acute hemorrhage were restricted to the cerebral cortex, all sections of the brain, trigeminal ganglion, and spinal cord had perivascular hemorrhage. In addition, there were endothelial and perivascular changes. Perivascular changes, similar to those found in the meninges and neuropil, entailed endothelial swelling, hyperplasia, pyknosis, rare mitosis, occasional intramural and marked perivascular edema, multinucleated endothelial cells, mild perivascular infiltrates of lymphocytes, macrophages, neutrophils, and very rarely, eosinophils. Perivascular macrophages contained lipofuscin and/or edema.
Discussion
The gross appearance, histological findings, and results of diagnostic testing were consistent with a diagnosis of EHV-1. Equine herpesvirus-1 has neuropathogenic and non-neuropathogenic strains that can exist in a lytic, nonreplicating, or latent state (1). This alpha-herpesvirus is transmitted via aerosols, primarily causing rhinopneumonitis and abortion, but rarely a paralyzing neurological condition (2). Equine herpesvirus-1 is ubiquitous worldwide, and most horses will have been exposed by 2 y of age (3). The ability of this virus to remain latent ensures that there is a reservoir for transmission (1). Clinical signs may be seen 1 to 10 d post-exposure and shedding may occur for up to 28 d. Recrudescence may occur at times of stress and manifest as clinical signs, or as silent shedders of the virus (4).
Recently, outbreaks of the neurological form have stricken equines globally with an apparent increased prevalence. Occurrence has been especially noted in closed herds such as racetracks, riding schools, and boarding farms (2). This has led to the hypothesis that EHV-1 is an emerging infectious disease due to its increased virulence and the associated behavioral changes (3).
The neurological signs induced by EHV-1 neuropathogenic infection are manifested as an acute paralytic crisis that may be mild or severe. Mild cases may include tail flaccidity, urinary incontinence and ataxia. More severe cases may also include hind end weakness, incoordination, proprioceptive deficits, dog-sitting, and eventually recumbency, which carries a poor prognosis (5). During the recumbent stage, the affected animal attempts to regain its righting abilities, resulting in an explosive thrashing episode that may be confused with seizuring (5). In the present case of the American Saddlebred mare, a true protracted seizure may have occurred due to the extensive cerebral cortical lesions, or the episode may have been misinterpreted as such. One must bear in mind that it is unlikely for an equine with status epilepticus to have had sufficient recovery time to stand and ambulate that same day.
The neurovirulent potential of EHV-1 is due to a mutation of the gene for the DNA polymerase enzyme (ORF 30) (1). A single amino acid variation at N752D results from a single nucleotide polymorphism (SNP) (2). This mutation is found in 86% of neuropathogenic isolates from horses with Equine herpesvirus myeloencaphalopathy (EHM) due to EHV-1 infection (1). The wild type adenine nucleotide mutates to a guanine nucleotide, thereby changing the amino acid that is encoded (1). The mutation is associated with a more rapid viral replication, higher levels of viremia, longer persistence and a preferential infection of CD4+ T lymphocytes. The cell-associated viremia is believed to facilitate the transfer of virus to endothelial cells in the central nervous system, leading to destruction of the arterial vascular endothelial cells supplying nervous tissue, resulting in vasculitis, thrombosis, and ischemia (2,6). Histopathologically, these changes cause myelin sheet alterations, axonal swelling, and perivascular mononuclear cuffing affecting mostly the white matter of the brain and spinal cord (7). In the present case all of these changes were noted. The gold standard for diagnosis of EHV-1 has always been virus isolation from blood or nasopharyngeal swabs, but this had a poor specificity, and was time consuming and expensive. Serological testing followed and included determining titers in acute and convalescent samples or a rapid ELISA SNAP test. These methods have been replaced by a new gold standard, namely RT-PCR (4). With increased specificity and sensitivity, RT-PCR allows quantitation of viral load, characterization of strains and states, is less expensive, and offers a more rapid turnaround period (1,4). This test can be designed to detect the DNA polymerase ORF 30 gene or the transcription factor gB gene. It appears that the gB gene assay is diagnostically more sensitive, allowing for low viral load detection, whereas the ORF 30 assay is unable to reach this level of sensitivity, resulting in false negatives. However, the gB method shows a greater discrepancy in results, perhaps due to dual EHV-1 strain infections (1). Most routine diagnostic laboratories perform the ORF 30 assay on blood and nasopharyngeal samples, as was done in this case.
Equine herpesvirus-1 RT-PCR results must be interpreted carefully by the general veterinary practitioner with the following information in mind. The ORF 30 assays are not 100% specific for differentiation between neuropathogenic and non-neuropathogenic strains. One study determined that approximately 14% of EHV-1 isolates from horses demonstrating EHM possess the nonmutated, wild type nucleotide and therefore lack the neuropathogenic marker. Conversely, approximately 6% of isolates from equines with no EHM signs, but with EHV-1 infection, contained the mutated, neuropathogenic marker (1). In the present case the mare fell under the former category of exceptions.
A thorough understanding of the RT-PCR assays and the interpretation of the results is critical for the management of affected animals and their herd mates. In addition, comprehension of viral states is essential (1). For instance, it is assumed by many that once clinical signs of neurological disease have commenced, there is no longer shedding of the pathogen into the environment (1). This assumption may have grave consequences as equines with EHV-1-induced myeloencephalopathy actually shed large amounts of the replicating virus in their nasal and pharyngeal secretions (1).
Equines afflicted by EHM due to EHV-1 infection are difficult to manage because there are no specific treatments available (5). In vitro studies have been used to investigate the efficacy of anti-viral medications such as acyclovir, ganciclovir, cidofovir, adefovir, 9-(2-phosphonylmethoxyethyl)-2,6-diaminopurine (PMEDAP), and foscarnet. Ganciclovir was determined to be the most efficacious at reducing plaque size in vitro, and therefore has been hypothesized to possess some therapeutic potential in vivo (8). One study using acyclovir as a prophylactic and therapeutic agent during an outbreak showed that severely affected animals living 6 mo past the initial onset of disease had evidence of residual deficits. Horses that managed to live 1 y after the initial event were apparently able to return to a level of performance comparable to that before the affliction (9). Historically, supportive care is all that can be offered to affected animals. For instance, reducing inflammation through the use of nonsteroidal anti-inflammatory drugs (NSAIDs) or corticosteroids and the administration of free radical scavengers such as dimethyl sulfoxide (DMSO) are potentially useful (5). Typically, the animal will maintain the desire to eat well. Watery bran mashes with mineral oil and salts should be encouraged to promote hydration and to reduce the risk of impaction. Finally, the prevention of injury while recumbent and attempting to stand is important (5). It was during this phase of the disease that our American Saddlebred mare was believed to have acquired her severe supradural cranial vault hemorrhages that were observed on postmortem examination.
Inevitably, 1/3 of infected animals succumb to the disease within months of diagnosis, and a large proportion of animals are humanely euthanized due to extensive suffering (2).
Prevention of EHM via vaccination is highly controversial. Currently, vaccines are available to aid in protection against rhinopneumonitis and abortive strains of the virus (EHV-1 and EHV-4). It has been suggested that these vaccines may offer cross-protection against the neurological form of the disease (5). However, outbreaks of EHM have been well documented in thoroughly vaccinated animals (3).
The best method of protection against the spread of this disease is a sound biosecurity system in the area of concern, whether it is a farm, racetrack, or horse show. This entails quarantine and isolation of new additions for at least a month, preventing contamination of fomites, cleaning and disinfection of transportation equipment such as trailers, and minimizing stress (3,5).
Monitoring new additions to the herd for potential risk factors and signs of the disease are important. Risk factors include stress, fall and spring seasons, animals older than 5 y of age, having had a period of pyrexia equal to or greater than 103.5°F, and a peak temperature occurring on day 3 of the febrile period. These risk factors were predictive of neurovirulence and death (3,9). For obvious reasons, these generalized factors are easily confounded with the probability of other systemic diseases and are open to subjective interpretation. In the present case, at the time of physical examination the mare had already passed the febrile phase, which is common for most EHM-affected equines when presented to the general veterinary practitioner. Since there are no efficacious vaccines, the most useful method of prevention at this stage would be to test for carrier animals that are reservoirs for the virus and to manage them as such. This brings us back to the usefulness of RT-PCR and its ability to detect latent states of the virus (1). Should this method of testing become routine, it may be tailored into pre-purchase examinations and annual health checks. Most importantly, it may be instituted as mandatory testing for racetracks and equines being relocated or traveling to shows.
Acknowledgments
Dr. Jeff Sleethe was the referring veterinarian from Dundas Veterinary Services and performed the initial physical examination. Dr. Janet Shapiro performed the necropsy and contributed the pathology results for this case. CVJ
Footnotes
Tammy Heerkens will receive 50 copies of her article, courtesy of The Canadian Veterinary Journal.
References
- 1.Pusterla N, Wilson WD, Mapes S, et al. Characterization of viral loads, strain, and state of equine herpesvirus-1 using real time PCR in horses following natural exposure at a racetrack in California. Vet J. 2007 Nov 14; doi: 10.1016/j.tvjl.2007.09.018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Goodman LB, Loregian A, Perkins GA, et al. A point mutation in a herpesvirus polymerase determines neuropathogenicity. PLoS Pathog. 2007;3:e160. doi: 10.1371/journal.ppat.0030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Equine herpesvirus myeloencephalopathy: A potentially emerging disease. [Last accessed October 25, 2008];APHIS Info Sheet, Veterinary Services: Centers for Epidemiology and Animal Health. 2007 Jan; Available at: http://www.aphis.usda.gov/vs/ceah/cei/taf/emergingdiseasenotice_files/ehv.pdf.
- 4.Ella G, Decaro N, Martella V, et al. Detection of equine herpesvirus type 1 by real time PCR. J Virol Methods. 2005;133:70–75. doi: 10.1016/j.jviromet.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Pusterla N, Wilson WD, Madigan JE, Ferraro GL. Equine herpesvirus-1 myeloencephalopathy: A review of recent developments. Vet J. 2008 Sep 18; doi: 10.1016/j.tvjl.2008.08.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Wilson WD. Equine herpesvirus 1 myeloencephalopathy. Vet Clin North Am Equine Pract. 1997;13:53–72. doi: 10.1016/s0749-0739(17)30255-9. [DOI] [PubMed] [Google Scholar]
- 7.Stierstorfer B, Eichhorn W, Schmahl W, Brandmuller C, Kadden OR, Neubauer A. Equine herpesvirus type 1 (EHV-1) myeloencephalopathy: A case report. J Vet Med B Infect Dis Vet Public Health. 2002;49:37–41. doi: 10.1046/j.1439-0450.2002.00537.x. [DOI] [PubMed] [Google Scholar]
- 8.Garre B, vad der Meulen K, Nugent J, et al. In vitro susceptibility of six isolates of equine herpesvirus 1 to acyclovir, ganciclovir, cidofovir, adefovir, PMEDAP, and foscarnet. Vet Microbiol. 2007;122:43–51. doi: 10.1016/j.vetmic.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Henniger RW, Reed SM, Saville WJ, et al. Outbreak of neurologic disease caused by equine herpesvirus-1 at a university equestrian center. J Vet Intern Med. 2007;21:157–165. doi: 10.1892/0891-6640(2007)21[157:oondcb]2.0.co;2. [DOI] [PubMed] [Google Scholar]