Abstract
Current treatments for myasthenia gravis (MG) rely upon the administration of immunosuppressive agents which result in global, nonspecific attenuation of the immune response. An alternative approach would be to attempt to design therapies that specifically dampen autoreactivity without affecting general immunity. Recently, dendritic cells (DCs) have been shown to possess potent capabilities to tolerize T cells in an antigen-specific manner. We have observed that the selective activation of particular subsets of DCs utilizing granulocyte-macrophage colony-stimulating factor (GM-CSF) had profound effects on the induction of experimental autoimmune myasthenia gravis (EAMG). Specifically, treatment with GM-CSF effectively suppressed the induction of EAMG and down-modulated anti-AChR T cell and pathogenic antibody responses. These effects were associated with the activation of tolerogenic DCs, the enhanced production of suppressive cytokines, such as IL-10, and the mobilization of.CD4+ CD2S+ and FoxP3+ regulatory T cells (Tregs). We have further shown that GM-CSF effectively ameliorates clinical disease severity in mice with active, ongoing EAMG. Based on these observations, we hypothesize that the selective activation of particular DC subsets in vivo using pharmacologic agents, like GM-CSF, can suppress ongoing anti-AChR immune responses by mobilizing antigen-specific Tregs capable of suppressing autoimmune MG.
Keywords: myasthenia gravis, experimental autoimmune myasthenia gravis, dendritic cells, regulatory T cells, GM-CSF, acetylcholine receptor
Introduction
Autoimmune myasthenia gravis (MG) is a T cell-dependent, antibody-mediated disease in which the target antigen is well characterized. Autoantibodies directed against the skeletal muscle acetylcholine receptor (AChR) impair neuromuscular transmission resulting in a loss in the number of functional AChRs at the motor endplate and in muscle weakness.1
Despite being one of the better understood autoimmune diseases, current therapies for MG produce nonspecific, global immune suppression that usually must be continued lifelong to maintain disease control. These therapies, while often effective, are associated with significant chronic side effects and enhanced risk for infection and malignancy. The ideal therapy for MG would eliminate or suppress the autoimmune response to the AChR specifically without otherwise affecting the immune system. At least in theory, the design of an antigen-specific treatment for MG should be attainable since the autoantigen and immunopatho-genesis are relatively well characterized. Unfortunately, the best available evidence indicates that the autoimmune T cell and antibody responses in MG are highly heterogeneous.2–4 Thus, targeting a discreet population of critical T or B cells for therapeutic immunomodulation is, at best, a highly challenging proposition, particularly in light of the vast adaptability of the immune system. To ultimately achieve the goal of an antigen-specific therapy for MG, it is likely that the immune system itself will have to be harnessed and used as a tool to effectively recognize and suppress the heterogeneous repertoire of autoreactive immune cells.
DCs and Experimental Autoimmune Myasthenia Gravis
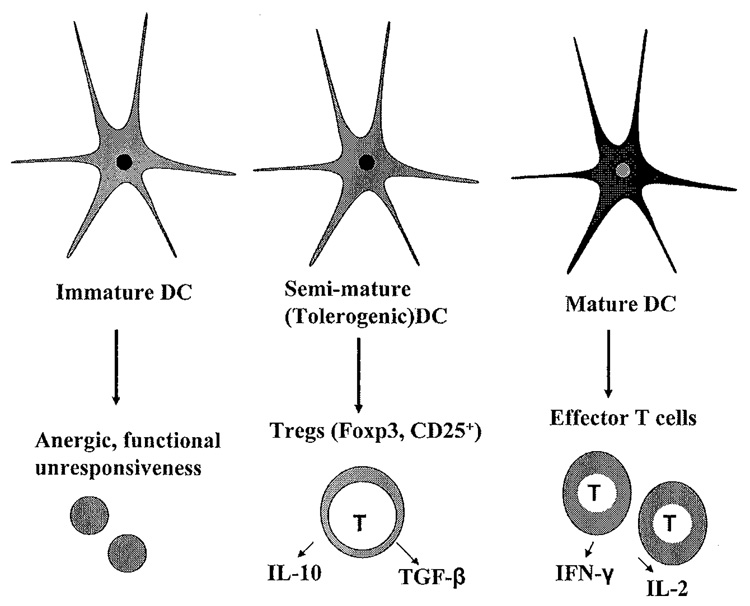
Dendritic cells (DCs) are the most potent antigen-presenting cells responsible for priming naïve T cells in the immune response.5–7 DCs not only activate lymphocytes, but also can educate T cells to tolerate self-antigens, thereby minimizing autoimmune reactions. Distinct subpopulations of DCs play a central role in immune regulation by preferentially inducing immunity or tolerance (FIG. I). Recent studies8–12 have shown that DCs may exert their tolerogenic functions through skewing of the Th1/Th2 balance, and through the generation of regulatory T cells (Tregs).10–12 The therapeutic manipulation of DCs and Tregs in autoimmune disease has recently generated a significant amount of interest. 13
FIGURE 1.
DC subsets. Growing evidence suggests that DC function is largely dependent on differentiation status, which can be manipulated using various growth factors, such as GM-CSF. Fully mature DCs express high levels of MHC class II and costimulatory molecules and are potent inducers of immunity. Immature DCs are believed to lead to T cell unresponsiveness or anergy. DCs with a tolerogenic phenotype express relatively high levels of MHC class II and costimulatory molecules, but produce low levels of pro-inflammatory cytokines. Compared to fully mature DCs, these tolerogenic DCs induce the production of Tregs rather than effector T cells.
An experimental animal model of MG, experimental autoimmune myasthenia gravis (EAMG), is induced in mice by immunization with AChR purified from the electric organs of the electric ray, Torpedo californica (TAChR).14 In EAMG, anti-Torpedo AChR antibodies cross-react with mousc AChR and cause myasthenic symptoms.15 The immunopatho-genesis of EAMG involves the production of high-affnity anti-AChR antibodies whose synthesis is modulated by, and dependent upon, anti-AChR CD4+ T cells. 16. 17 The activation of anti-AChR T cells is, in turn, determined by their interactions with antigen-presenting cells (APCs). As the most potent APC of the immune system, DCs play an important role in MG by presenting self-antigens and promoting the priming and/or boosting of anti-AChR T cells.18–20 A number of studies have shown that autologous DCs modifed in a number of different ways ex vivo and administered to rodents with EAMG can have a protective effect on the development and progression of disease. 21–23 Thus, the manipulation of DCs may represent an effective therapeutic strategy to restore tolerance in an antibody-mediated autoimmune disease like EAMG.
DCs, GM-CSF, and Immune Tolerance
Growing evidence suggests that DC function is largely dependent on differentiation or maturation status, which can be manipulated using various growth factors, such as granulocyte-macrophage colony-stimulating factor (GM-CSF)24 DCs having a “semimatured” or “ tolerogenic” phenotype have been recently shown to have potential utility in organ transplantation and in the treatment of autoimmune diseases.25,26 DCs with a tolerogenic phenotype express relatively high levels of MHC class II and co-stimulatory molecules, and produce low levels of pro-inflammatory cytokines.27 Compared to fully mature DCs, these tolerogenic DCs induce the production of Tregs rather than effector T cells.12,28,29
In murine secondary lymphoid organs, two major DC subsets are characterized by their expression of the marker CD8α,30,31 and the administration of GM-CSF can induce differential activation of these subsets.24,26,32 GM-CSF is a potent growth factor for CD8α− DCs and has been shown to promote a Th2 response.33–36 Th2 cells can down-modulate immune responses, possibly by acting as growth and differentiation factors for Tregs,37,38 It has been demonstrated that the mobilization of tolerogenic DCs using GM-CSF can potently suppress induction and progression of disease in experimental autoimmune thyroiditis, a T cell-mediated disease, and that IL-10-producing Tregs are required for this effect. 39,40 Whether this approach would be effective in an antibody-mediated autoimmune disease like EAMG in which there is no significant T cell infiltration of the target tissue had not previously been examined.
GM-CSF Suppresses Induction of EAMG
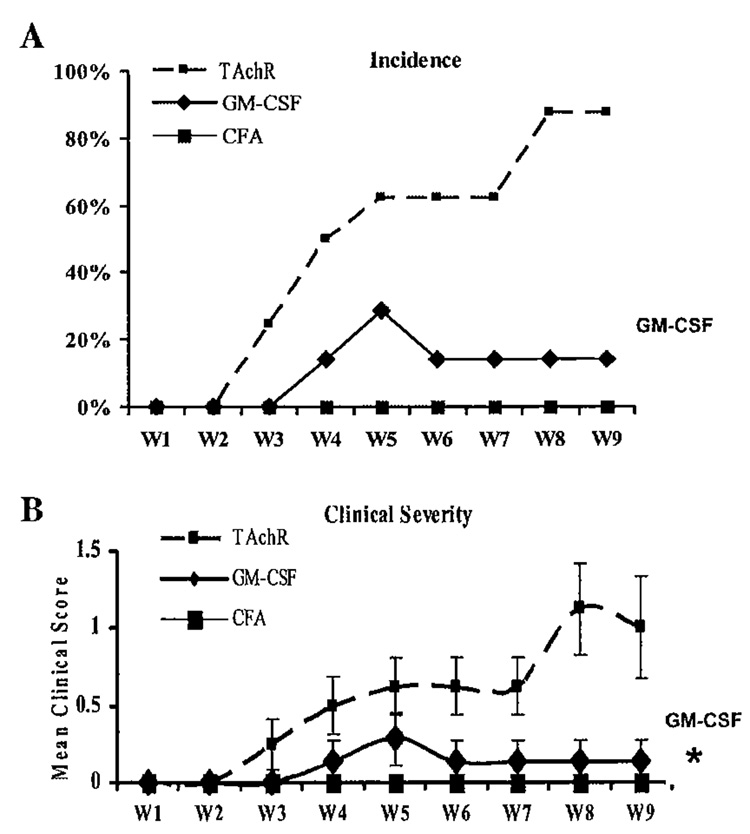
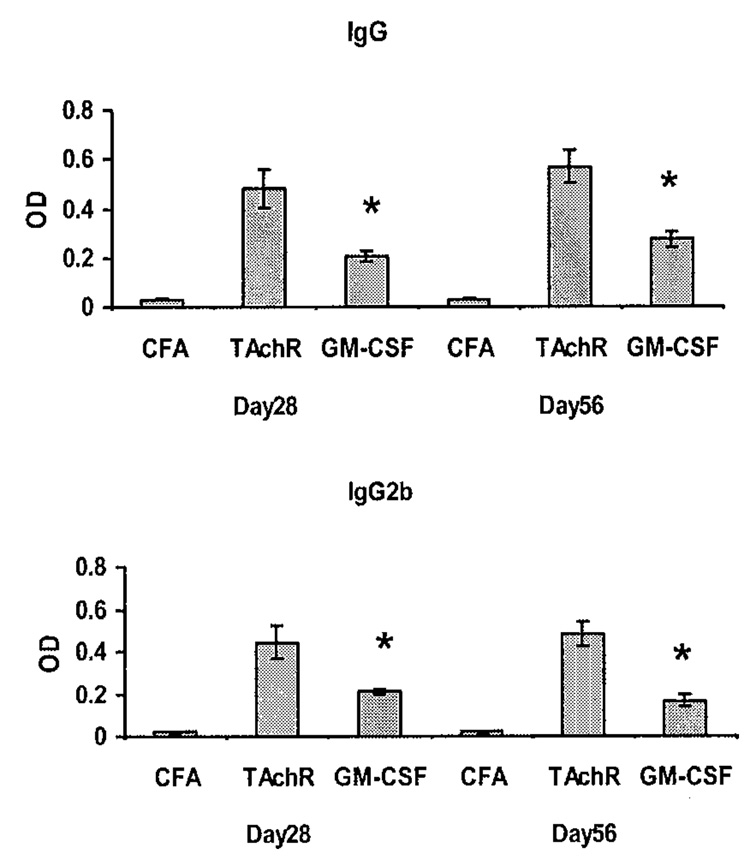
We administered GM-CSF to C57BL/6 mice prior to immunization with AChR and observed the effect on the frequency and severity of EAMG development.41 Compared to AChR-immunized controls, mice treated with GM-CSF prior to immunization, demonstrated a significantly reduced disease incidence and severity (FIG. 2). To determine if this relative protection from disease induction was associated with reduced production of pathogenic anti-AChR antibodies, we monitored serum anti-AChR antibody levels at different time points after TAChR immunization. Treatment with GM-CSF resulted in a decrease in total anti-mouse AChR IgG levels, and in particular, resulted in a prominent decrease in the murine complement-fixing IgG2b isotype, while IgG1 (non-complement-fixing) isotypes were relatively unaffected41 (FIG. 3). These results suggested that GM-CSF treatment significantly reduced circulating levels of IgG isotypes associated with complement-mediated lysis of the postsynaptic membrane.
FIGURE 2.
Frequency and severity of EAMG induction in GM-CSF-treated and untreated TAChR-immunized mice and negative control (CFA-immunized) mice. The percentage of animals exhibiting myasthenic weakness (A) and the mean clinical score (B) are plotted for each of the nine weeks of the observation period (Wl–W9 on the x-axis) (*P < 0.01).
FIGURE 3.
Serum concentration of anti-mouse AChR antibodies assayed by ELISA on day 28 and day 56 post-immunization. Treatment resulted in a decrease in total anti-mouse AChR IgG levels, with a prominent decrease in murine complement-fixing IgG2b isotypes, while IgG1 (non-complement fixing) isotypes were relatively unaffected (data not shown, see Ref. 41) (* P < 0.01).
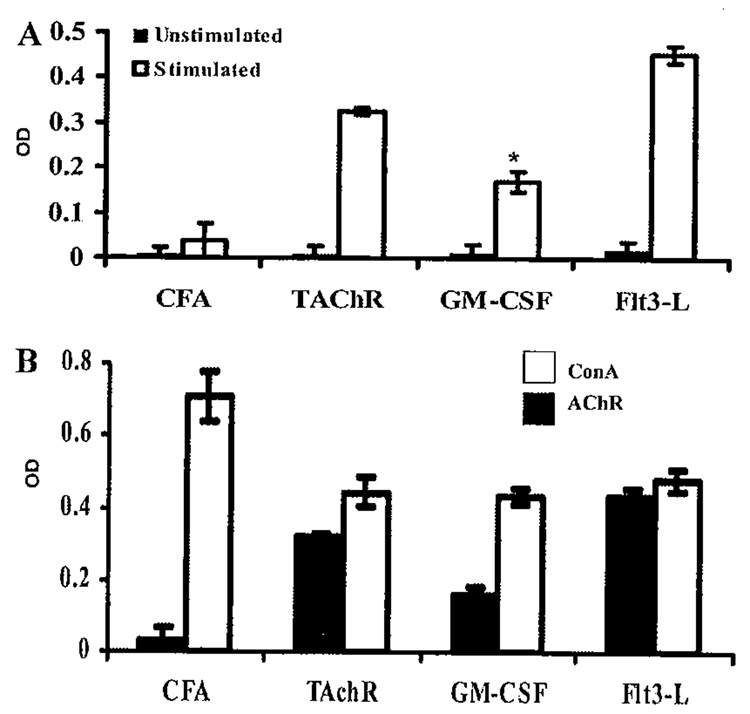
As expected, spleen cells from mice treated with GM-CSF had a significantly lower T cell proliferative response (P = 0.0025) to stimulation with TAChR, compared to immunized controls (FIG. 4). No differences were observed in the GM-CSF-treated, and untreated mice with nonspecific stimulation using the lectin concanavalin A.
FIGURE 4.
(A) Spleen cells from mice treated with fms-like tyrosine kinase receptor 3-ligand (Flt3-L) showed a significantly higher proliferative response to stimulation with TAChR, while spleen cells from mice treated with GM-CSF had a significantly lower (* P = 0.0025) response to TAChR compared to immunized controls41 (B) No differences were observed in the GM-CSF-treated, Flt3-L-treated, and untreated mice with non-specific stimulation using the lectin concanavalin A.
The effects of GM-CSF treatment on DC phenotye were ascertained by analyzing the expression of CD11c, MHC class II, and costimulatory molecules, as well as the production of pro-inflammatory cytokines from GM-CSF-treated and untreated mice after TAChR immunization. Spleens from mice treated with GM-CSF had relatively equal percentages of CD11c+ cells and comparable levels of expression of MHC class II and CD80 compared with untreated TAChR-immunized mice and control (Complete Freund’s Adjuvant [CFA]) mice. GM-CSF-treated mice, however, showed an increase in CD8α− CD11c+ cells: 3.55%, compared to 1.82 % in the untreated group. GM-CSF-treated mice also had relatively higher levels of expression of CD86. However, levels of pro-inflammatory cytokines, such as TNF-α, IL-12, and IL-Iβ, evaluated by RT-PCR, were low in GM-CSF-treated mice compared to untreated TAChR-immunized controls.41 These results suggested that DCs from GM-CSF-treated mice exhibit a tolerogenic phenotye.
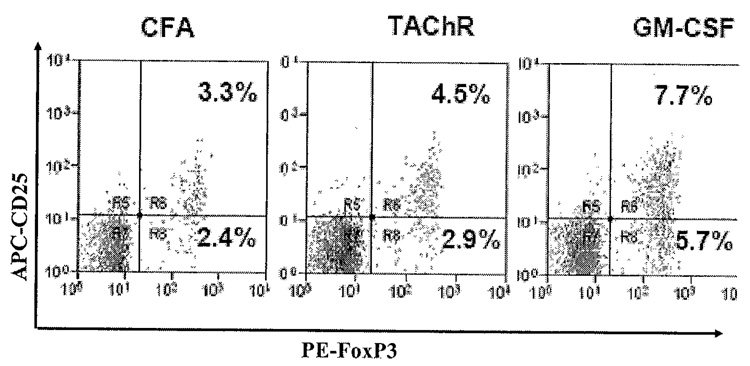
To determine if treatment with GM-CSF affects the relative numbers of T cells expressing a regulatory phenotye, we tested spleen cells for expression of known Treg cell surface markers by fluorescent activated cell sorter. We found significantly increased numbers of CD4+CD25+ cells in mice treated with GM-CSF compared to untreated TAChR-immunized control animals.41 We also found an expansion in the percentage of both CD25+ and CD25− cells expressing the transcription factor FoxP3 in GM-CSF-treated mice compared to untreated TAChR-immunized controls (FIG. 5).
FIGURE 5.
TAChR-immunized mice were sacrificed at 14 days following treatment with GM-CSF and PBS. Splenocytes were isolated from animals and stained with FITC-labeled anti-mouse CD4, APC-labeled anti-mouse CD25, and PE-labeled anti-FoxP3. A statistically significant expansion in the percentage of FoxP3-expressing (both CD25+ and CD25−) cells was observed in GM-CSF-treated mice compared to untreated TAChR-immunized controls (P < 0.05).
Effects of GM-CSF on Progression of Ongoing EAMG
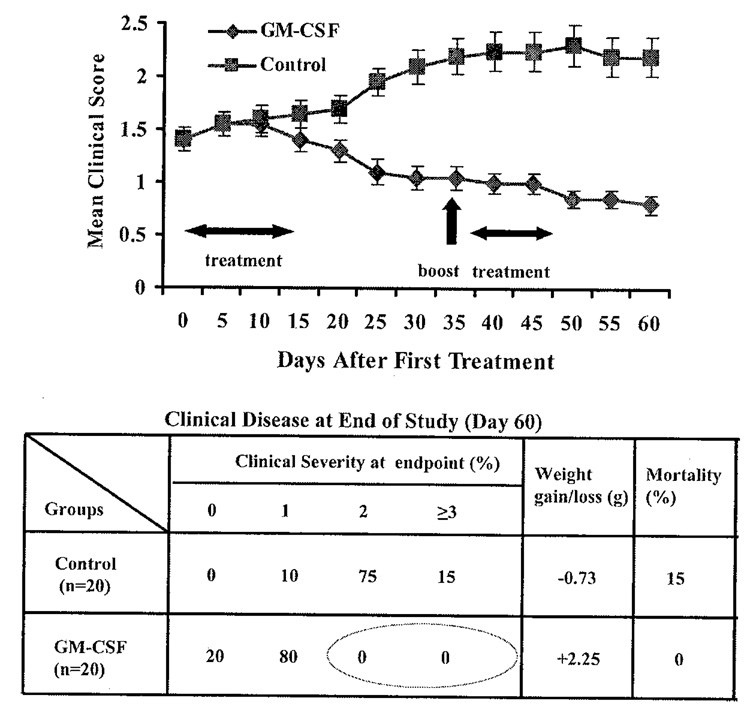
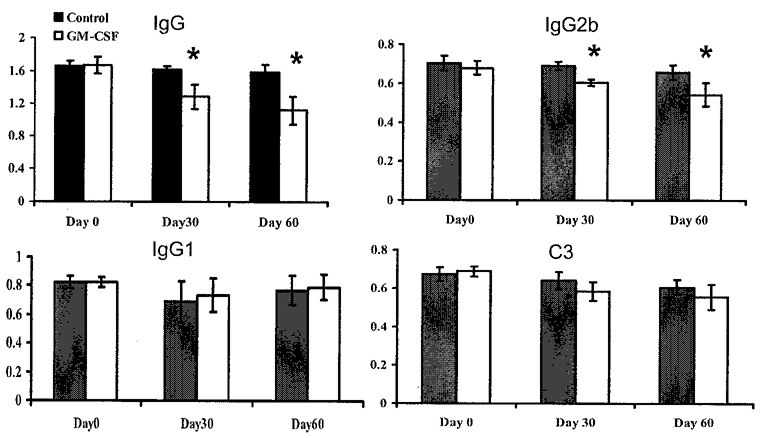
In more recent experiments, we have allowed mice to develop clinical EAMG before treating with GM-CSF We immunized mice with TAChR on days 0, 30, 60, and 90. At this point greater than 90% of the animals had clear clinical signs of EAMG. We then divided the animals into two groups with an equal distribution of animals with disease grades 0, 1, and 2, and then randomly treated one group with GM-CSF (2 µg × 10 days) while the other received phosphate-buffered saline (PBS). Both groups received a booster immunization 30 days after treatment initiation as shown in FIGURE 6, followed 7 days later by an additional 5-day course of GM-CSF The GM-CSF-treated animals exhibited significant improvement while, as expected, the untreated group worsened (FIG. 6). Significant differences in the mean clinical score between the two groups became apparent approximately 20 days after initiating therapy. As in our experiments with treatment prior to TAChR immunization, the beneficial changes in clinical manifestations were accompanied by suppression of ongoing anti-AChR antibody responscs (FIG. 7).
FIGURE 6.
Effects of GM-CSF on progression of established EAMG. Mean clinical score is shown for the two treatment groups, GM-CSF and control (PBS) (20 mice/group). Table shows number of mice in each clinical grade, weight/gain/loss, and mortality for the two treatment groups.
FIGURE 7.
Serum anti-TAChR IgG and IgG subclasses and circulating C3 for GM-CSF-treated and PBS-treated mice. Anti-mouse AChR IgG Ab and IgG isotypes were analyzed by ELISA (n = 20 per group) on day 0, day 30, and day 60, with day 0 corresponding to the date of treatment initiation. GM-CSF-treated mice showed significantly lower serum levels of anti-TAChR IgG and IgG2b at day 30 and day 60. Each column represents the mean ± SD of three individual experiments conducted in triplicate (*p < 0.05).
The strategy of assessing the effectiveness of treatment in very established disease is a relatively novel approach and represents a more appropriate model for treatment of MG in the clinic. While the precise mechanisms of GM-CSF’s therapeutic effects in established EAMG remain under investigation, we hypothesize that treatment with GM-CSF at the doses utilized in our studies selectively mobilizes tolerogenic DCs, which in turn induce the production of Tregs, which specifically down-modulate anti-AChR T cell and antibody responses.
Clinical Translation to Human MG
Since GM-CSF is an approved medication currently used in the fields of oncology and organ transplantation, the translation of the above findings would appear to be relatively straightforward. However, it must be stressed that the current use of GM-CSF in medicine is based on its hematopoietic and immune stimulatory effects. It is likely that a number of factors, including dosing, play a role in determining whether GM-CSF’s actions result in immune regulation or stimulation. Another important factor is likely clinical activity of disease, as active antigen presentation would presumably be an absolute requirement for subsequent induction of immune tolerance. Clearly the study of GM-CSF in EAMG will provide insight into the role of DCs and Treg cells in the regulation of an antibody-mediated autoimmune disease, the mechanisms underlying their effects on autoantibody production, and antibody-mediated destruction of the target tissue. This knowledge will then allow for clinical application of GM-CSF or related molecules to the treatment of autoimmune diseases like MG.
Acknowledgments
This work was supported in part by the Myasthenia Gravis Foundation of America, Osserman Postgraduate Fellowship Grant to J.R.S., and by grant (R01 AI 058190-01) to B.S.P from the National Institutes of Health, Bethesda, MD.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Vincent A, Palace J, Hilton-Jones D. Myasthenia gravis. Lancet. 2001;357:2122–2128. doi: 10.1016/S0140-6736(00)05186-2. [DOI] [PubMed] [Google Scholar]
- 2.Brocke S, Brautbar C, Steinman L, et al. In vitro proliferative responses and antibody titers specific to human acetylcholine receptor synthetic peptides in patients with myasthenia gravis and relation to HLA class II genes. J. Clin. Invest. 1988;82:1894–1900. doi: 10.1172/JCI113807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newsom-Davis J, Harcourt G, Sommer N, et al. T-cell reactivity in myasthenia gravis. J. Autoimmun. 1989;2 Suppl:101–108. doi: 10.1016/0896-8411(89)90121-2. [DOI] [PubMed] [Google Scholar]
- 4.Moiola L, Protti MP, Manfredi AA, et al. T-helper epitopes on human nicotinic acetylcholine receptor in myasthenia gravis. Ann. N.Y Acad. Sci. 1993;681:198–218. doi: 10.1111/j.1749-6632.1993.tb22887.x. [DOI] [PubMed] [Google Scholar]
- 5.Gad M, Claesson MH, Pedersen AE. Dendritic cells in peripheral tolerance and immunity. A.P.M.I.S. 2003;111:766–775. doi: 10.1034/j.1600-0463.2003.11107808.x. [DOI] [PubMed] [Google Scholar]
- 6.Rossi M, Young JW. Human dendritic cells: Potent antigen-presenting cells at the crossroads of Innate and adaptive immunity. J. Immunol. 2005;175:1373–1381. doi: 10.4049/jimmunol.175.3.1373. [DOI] [PubMed] [Google Scholar]
- 7.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and autoimmunity. Annu. Rev. Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 8.Macpherson G, Kushnir N, Wykes M. Dendritic cells, B cells and the regulation of antibody synthesis. Immunol. Rev. 1999;172:325–324. doi: 10.1111/j.1600-065x.1999.tb01376.x. [DOI] [PubMed] [Google Scholar]
- 9.Cerutti A, Qiao X, He B. Plasmacytoid dendritic cells and the regulation of immunoglobulin heavy chain class switching. Immunol. Cell. Biol. 2005;83:554–562. doi: 10.1111/j.1440-1711.2005.01389.x. [DOI] [PubMed] [Google Scholar]
- 10.Maldonado-Lopez R, Moser M. Dendritic cell subsets and the regulation of Th1/Th2 responses. Semin. Immunol. 2001;13:275–282. doi: 10.1006/smim.2001.0323. [DOI] [PubMed] [Google Scholar]
- 11.Min WP, Zhou D, Ichim De DE, et al. Inhibitory feedback loop between tolerogenic dendritic cells and regulatory T cells in transplant tolerance. J. Immunol. 2003;170:1304–1312. doi: 10.4049/jimmunol.170.3.1304. [DOI] [PubMed] [Google Scholar]
- 12.Kuwana M. Induction of anergic and regulatory T cells by plasmacytoid dendritic cells and other dendritic cell subsets. Hum. Immunol. 2002;63:1156–1163. doi: 10.1016/s0198-8859(02)00754-1. [DOI] [PubMed] [Google Scholar]
- 13.Raimondi G, Turner MS, Thomson AW, et al. Naturally occurring regulatory T cells: recent insights in health and disease. Crit. Rev. Immunol. 2007;27:61–95. doi: 10.1615/critrevimmunol.v27.i1.50. [DOI] [PubMed] [Google Scholar]
- 14.Christadoss P, Poussin M, Deng C. Animal models of myasthenia gravis. Clin. Immunol. 2000;94:75–87. doi: 10.1006/clim.1999.4807. [DOI] [PubMed] [Google Scholar]
- 15.Lindstrom JM. Experimental autoimmune myasthenia gravis: induction and treatment. In: Engel A, editor. Myasthenia Gravis and Myasthenic Disorders. New York: Oxford University Press; 1999. pp. 111–130. [Google Scholar]
- 16.Drachman DB. Myasthenia gravis. N. Engl. J. Med. 1994;330:1797–1810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- 17.Vincent A. Unraveling the pathogenesis of myasthenia gravis. Nat. Rev. Immunol. 2002;2:797–804. doi: 10.1038/nri916. [DOI] [PubMed] [Google Scholar]
- 18.Nagane Y, Utsugisawa K, Obara D, et al. Dendritic cells in hyperplastic thymuses from patients with myasthenia gravis. Muscle Nerve. 27:582–589. doi: 10.1002/mus.10362. [DOI] [PubMed] [Google Scholar]
- 19.Xiao BG, Duan RS, Link H, et al. Induction of peripheral tolerance to experimental autoimmune myasthenia gravis by acetylcholine receptor-pulsed dendritic cells. Cell Immunol. 2003;223:63–69. doi: 10.1016/s0008-8749(03)00118-7. [DOI] [PubMed] [Google Scholar]
- 20.Yang H, Kala M, Scott BG, et al. Cathepsin S is required for murine autoimmune myasthenia gravis pathogensis. J. Immunol. 2005;174:1729–1737. doi: 10.4049/jimmunol.174.3.1729. [DOI] [PubMed] [Google Scholar]
- 21.Duan RS, Adikari SB, Huang YM, et al. Protective potential of experimental autoimmune myasthenia gravis in Lewis rats by IL-10 modified dendritic cells. Neurobiol. Dis. 2004;16:461–467. doi: 10.1016/j.nbd.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Duan RS, Link H, Xiao BG. Long-term effects of IFN-γ, IL-10, and TGF-β-modulated dendritic cells on immune responses in Lewis rats. J. Clin. Immunol. 2005;25:50–56. doi: 10.1007/s10875-005-0357-4. [DOI] [PubMed] [Google Scholar]
- 23.Xiao BG, Duan RS, Link H, et al. Induction of peripheral tolerance to experimental autoimmune myasthenia gravis by acetylcholine receptor-pulsed dendritic cells. Cellular Immunol. 2003;223:63–69. doi: 10.1016/s0008-8749(03)00118-7. [DOI] [PubMed] [Google Scholar]
- 24.Pulendran B, Banchereau J, Burheholder S, et al. Flt3-Ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J. Immunol. 2000;165:566–572. doi: 10.4049/jimmunol.165.1.566. [DOI] [PubMed] [Google Scholar]
- 25.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 26.Lu L, Thomson AW. Manipulation of dendritic cells for tolerance induction in transplantation and autoimmune disease. Transplantation. 2002;73:S19–S22. doi: 10.1097/00007890-200201151-00008. [DOI] [PubMed] [Google Scholar]
- 27.Wakkach A, Fournier N, Brun V, et al. Characterization of dendritic cells that induce tolerance and T regulatory I cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 28.Groux H, Fournier N, Cottrez F. Role of dendritic cells in the generation of regulatory T cells. Semin. Immunol. 2004;16:99–106. doi: 10.1016/j.smim.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Rutella S, Lemoli RM. Regulatory T cells and tolerogenic dendritic cells: from basic biology to clinical applications. Immunol. Lett. 2004;94:11–26. doi: 10.1016/j.imlet.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Martin P, Del Hoyo GM, Anjuere F, et al. Concept of lymphoid versus myeloid dendritic cell lineages revisited: both CD8alpha(−) and CD8alpha(+) dendritic cells are generated from CD4 (low) lymphoid-committed precursors. Blood. 2000;96:2511–2519. [PubMed] [Google Scholar]
- 31.Penna G, Vulcano M, Sozzani S, et al. Differential migration behavior and chemokine behavior by myeloid and plasmacytoid dendritic cells. Hum. Immunol. 2002;63:1164–1171. doi: 10.1016/s0198-8859(02)00755-3. [DOI] [PubMed] [Google Scholar]
- 32.O’keefe M, Hochrein H, Vremec D, et al. Effects of administration of progenipoietin 1, Flt3 ligand, granulocyte colony-stimulating, and pegylated granulocyte colony-stimulating factor on dendritic cell subsets in mice. Blood. 2002;99:2122–2130. doi: 10.1182/blood.v99.6.2122. [DOI] [PubMed] [Google Scholar]
- 33.Maldonado-Lopez R, De Smedt T, Pajak B, et al. Role ofCD8α+ and CD8α− dendritic cells in the induction of primary immune responses in vivo. J. Leukocyte Biol. 1999;66:242–246. doi: 10.1002/jlb.66.2.242. [DOI] [PubMed] [Google Scholar]
- 34.Daro E, Butz E, Smith J, et al. Comparison of the functional properties of murine dendritic cells generated in vivo with flt3 ligand, GM-CSF and flt3 ligand plus GM-CSF. Cytokine. 2002;17:119–130. doi: 10.1006/cyto.2001.0995. [DOI] [PubMed] [Google Scholar]
- 35.Daro E, Pulendran B, Brasel K, et al. Polyethylene glycol-modified GM-CSF expands CD11b(high)CD11c(high) but not CD11b(low) CD11c(high) murine dendritic cells in vivo: a comparative analysis with Flt3 ligand. J. Immunol. 2000;165:49–58. doi: 10.4049/jimmunol.165.1.49. [DOI] [PubMed] [Google Scholar]
- 36.Arpinati M, Green CL, Heimfeld S, et al. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood. 2000;95:2484–2490. [PubMed] [Google Scholar]
- 37.Crane IJ, Forrester JV. Th1 and Th2 lymphocytes in autoimmune disease. Crit. Rev. Immunol. 2005;25:75–102. doi: 10.1615/critrevimmunol.v25.i2.10. [DOI] [PubMed] [Google Scholar]
- 38.Kroemer G, Hirsch F, Gonzalez-Garcia A, et al. Differential involvement ofTh1 and Th2 cytokines in autoimmune disease. Autoimmunity. 1996;24:25–33. doi: 10.3109/08916939608995354. [DOI] [PubMed] [Google Scholar]
- 39.Vasu C, Dogan RE, Holterman MJ, et al. Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor-3 ligand, activates thyroglobulin-specific CD4+/CD25 + T cells and suppresses experimental autoimmune thyroiditis. J. Immunol. 2003;170:5511–5522. doi: 10.4049/jimmunol.170.11.5511. [DOI] [PubMed] [Google Scholar]
- 40.Gangi E, Vasu C, Cheatem D, et al. IL-10-producing CD4+CD25+ regulatory T cells playa critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis. J. Immunol. 2005;174:7006–7013. doi: 10.4049/jimmunol.174.11.7006. [DOI] [PubMed] [Google Scholar]
- 41.Sheng JR, Li LC, Ganesh BB, et al. Suppression of experimental autoimmune myasthenia gravis (EAMG) by granulocyte-macrophage colony-stimulating factor (GM-CSF) is associated with an expansion of FoxP3+ regulatory T cells. J. Immunol. 2006;177:5296–5306. doi: 10.4049/jimmunol.177.8.5296. [DOI] [PubMed] [Google Scholar]