Abstract
Endomorphin 1 (Endo-1 = Tyr-Pro-Trp-Phe-NH2), an endogenous opioid with high affinity and selectivity for μ-opioid receptors, mediates acute and neuropathic pain in rodents. To overcome metabolic instability and poor membrane permeability, the N- and C-termini of Endo-1 were modified by lipoamino acids (Laa) and/or sugars, and 2′,6′-dimethyltyrosine (Dmt) replacement of Tyr. Analogues were assessed for μ-opioid receptor affinity, inhibition of cAMP accumulation, enzymatic stability, and permeability across Caco-2 cell monolayers. C-terminus modification decreased receptor affinity, while N-terminus C8-Laa improved stability and permeability with slight change in receptor affinity. Dmt provided a promising lead compound: [C8Laa-Dmt1]-Endo-1 is 9 times more stable (t½ = 43.5 min), > 8-fold more permeable in Caco-2 cell monolayers, and exhibits 140-fold greater μ-opioid receptor affinity (Kiμ = 0.08 nM).
Keywords: Endomorphin 1, opioid peptides, lipoamino acids, liposaccharides, peptide delivery
Introduction
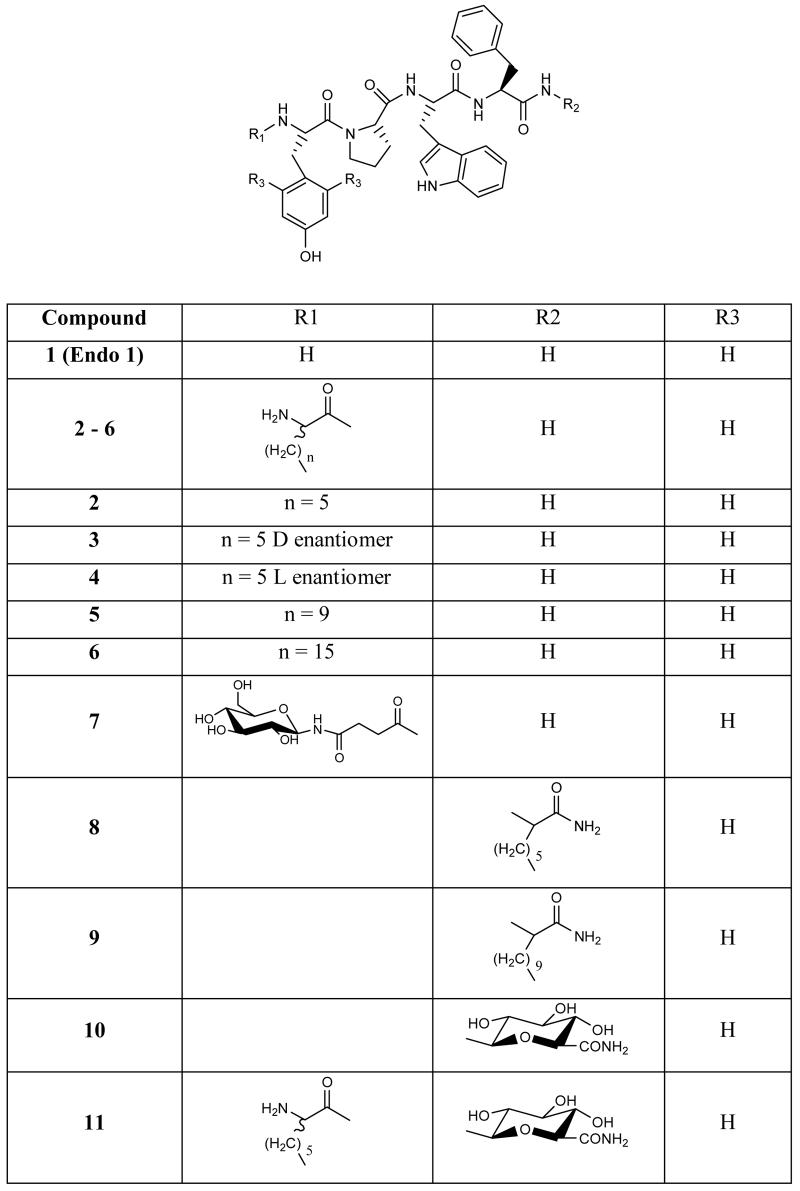
The opioid peptides are a family of endogenous neurochemical messengers that exhibit high affinity for one or more of the known opioid receptors. The opioid receptor system mediates an array of physiological processes including the respiratory, cardiovascular and gastrointestinal systems, immune responses, neuroendocrine function, reward, stress and pain perception and response.1 Two important members of the opioid peptide family are endomorphin 1 (Tyr-Pro-Trp-Phe-NH2) and endomorphin 2 (Tyr-Pro-Phe-Phe-NH2) which are unique among the opioid peptides as they exhibit high affinity and extraordinary selectivity for the μ-opioid receptor2, 3 – the receptor most associated with the alleviation of pain in the CNS.4 Endomorphin1 and 2 are being extensively investigated as potential analgesic agents,5-12 however, like all small peptides, the endomorphins suffer from very poor metabolic stability, with the half-live for both peptides in the order of minutes,.13,14 and low membrane permeability. In order for these peptides to fulfil their potential as pharmaceutically relevant pain modulating compounds, their pharmacokinetics must be improved. In this study we have chemically modified the termini of endomorphin 1 to investigate systematically how these changes can affect stability, permeability and biological activity. Structure activity studies on the endomorphins have revealed the importance of the Tyr1 and Trp3 residues to receptor binding15, 16 and Pro2 to the correct structural orientation of the peptide.17, 18 Our modifications, therefore, concentrated on the addition of lipidic and/or carbohydrate units to either termini while maintaining the native peptide sequence.
Increasing the lipophilicity of a compound is a well recognized strategy for increasing its passive diffusion across plasma membranes.19, 20 While the methodologies used to achieve the lipidation are many and varied,21, 22 the lipidic groups in this study were introduced as lipoamino acid (Laa) residues, containing either 8, 12 or 18 carbons. We have previously shown that the incorporation of Laas into short peptides can increase their metabolic stability and passive diffusion across biological barriers.23-26 For the majority of the analogues synthesized in this study, the lipoamino acids were introduced as a racemic mixture of both the l- and d-stereoisomers. There is evidence, however, that the diasteriomers of Laa conjugated peptides can behave differently in vivo, particularly in terms of their stability to enzymatic degradation.24, 27, 28 One of the more promising Laa conjugates in this study was therefore synthesized enantiomerically pure and both diastereomers (3 and 4) assessed individually.
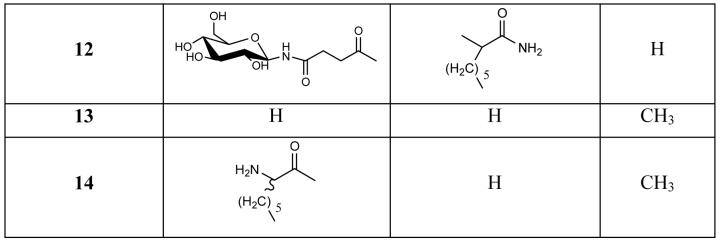
The benefits of glycosylation to the bioavailability of opioid and other neuroactive peptides has been well established in recent years.29-32 The integration of these polyhydroxylated, biologically compatible units can serve to alter water solubility and are useful in conjunction with lipidation as a method of customizing the physicochemical properties of a compound to optimize its bioavailability. The synthetic lipo- and glycol-endo-1 analogues assessed in this study are detailed in Figure 1.
Figure 1.
The lipo- glyco- and liposaccharide analogues of endomorphin 1 synthesised and evaluated in this study.
Results and Discussion
Chemistry
Solid phase peptide techniques were used to synthesise compounds 1-12. All peptides were purified to a single peak under two different analytical RP-HPLC conditions and provided the expected [M+H]+ peak in electrospray mass spectrometry. A summary of these data is provided the Supporting Information. All the compounds were eventually obtained as white or off white solids on lyophilisation from water with the glycosylated compounds exhibiting significant hydroscopic properties. The purified yields of the peptides varied from 51.8% for native Endo-1 (1) to a low 3% for the N-terminus glycosylated, C-terminus lipidated derivative 12. The yield of the N-glucose succinate derivative 7 was also low (9.8%) and this appeared to be the result of degradation of the sugar unit during TFA cleavage of the peptide from the resin. The C-terminus glycosylated peptides also showed some degradation but less than the N-terminus glyco-derivatives. The analogues bearing only lipoamino acids were observed to give much higher mass return on cleavage from the resin and provide a cleaner crude peptide but suffered from some loss during HPLC purification due to their poor water solubility and propensity to adsorb onto reverse-phase columns.
In the bulk of these studies, the lipoamino acids were incorporated into the peptides as racemates and gave rise to two diasteriomeric peptides that were not separated before the in vitro assays. Our previous studies have shown that peptides bearing the l-lipoamino acids can be more susceptible to degradation than the d-diastereomers.24, 27, 28 Enzymatic resolution of 2-amino-d,l-octanoic acid (C8Laa) using acylase I from Aspergillus melleus33 provided both enantiomers of this Laa in pure form and these building blocks were Boc-protected using a slightly modified procedure than that employed for the racemic form. It is imperative that the pH of the reaction mixture is carefully controlled between 4 and 8 in the case of the optically pure compounds to prevent racemization. A figure showing RP-HPLC traces of racemic C8Laa-Endo-1 (2) and each of the diastereomerically pure compounds 3 and 4 is supplied in the Supplementary Information and demonstrates that there is no evidence of racemisation of the Laa during the protection or coupling reactions.
Compounds 13 and 14 incorporated 2,6-dimethyltyrosine (Dmt) in place of the Tyr1 residue of endomorphin 1. The incorporation of Dmt into opioid peptides was first demonstrated to enhance their receptor binding and potency by Hansen et. al. in 1992.34 Recent studies have shown that replacement of the Tyr1 with Dmt can increase the in vitro receptor binding affinity and the in vivo analgesic activity of endo-135 and endo-236.37 Novel peptide mimetics bearing the Dmt moiety have also exhibited extremely high μ-opioid binding affinity.38, 39,40 Dmt was therefore incorporated into two of the peptides in our library, 13 and 14. Synthesis of N-Boc-2′,6′-dimethyl-l-tyrosine was achieved using a 5 step literature reported pathway from 3,5-dimethylphenol.41 Compounds 13 and 14 were synthesised using solution phase peptide synthetic techniques. Each coupling and purification gave approximately 65% yield until the Boc-C8Laa coupling. This reaction was observed to generate some impurities by TLC, and this compound was purified by repeated flash chromatography resulting in a reduced yield for 14 of 7.2%.
The analogues synthesised were initially examined with respect to their physicochemical properties. Compound 6, the C18Laa conjugate was found to be completely insoluble in water or buffered solutions, even with the addition of DMSO. This analogue was not pursued further in the study as it was clear that the use of long lipid chain auxiliary groups is not appropriate for short peptides. The C12Laa analogues 5 and 9 were also sparingly soluble in water but could be solubilised by the addition of DMSO; however, in both the stability and permeability assays evidence suggested that these compounds precipitated out of solution and were difficult to detect reliably using LC-MS due to these solubility issues. We were not able, therefore, to obtain reproducible stability data for compounds 5 or 9.
Stability and permeability
The in vitro stability and permeability of the endomorphin analogues were assessed using Caco-2 cells. The Caco-2 cell line, derived from human colorectal carcinoma cells, is now routinely used as an in vitro model of the GI tract and to obtain an initial prediction of oral bioavailability of a compound,42-45 and also as a preliminary blood-brain barrier (BBB) permeability screening tool.46-49
The stability of each of the analogues was assessed in a solution of digestive enzymes produced by homogenising fully differentiated Caco-2 cells and removing the cell debris by centrifugation. The half life of each analogue in the presence of the Caco-2 cell homogenate is presented in Table 1 and the degradation profiles are available graphically in the Supplementary Information.
Table 1.
The half life of each endo-1 analogue in an homogenate of Caco-2 cells and their apparent permeability (Papp cm/s) through Caco-2 cell monolayers. The data quantified by LC/MS and each point was expressed as a mean value ± S.D (n = 4). ND – not determined.
| Compound No. |
t½ (min) | Papp (× 10−7, cm/s) |
|---|---|---|
| 1 | 5 | 4.47 ± 0.989 |
| 2 | 23.22 | 35.4 ± 1.15 |
| 3 | 23.76 | ND |
| 4 | 23.76 | ND |
| 5 | ND | 12.4 ± 4.74 |
| 7 | 38 | 5.45 ± 1.02 |
| 8 | 3.5 | 8.23 ± 1.29 |
| 9 | N.D | 4.16 ± 0.399 |
| 10 | 4.3 | No permeability |
| 11 | 75 | 16.2 ± 1.89 |
| 12 | 33 | 11.4 ± 1.32 |
| Dmt analogues | ||
| 1 | 5 | 0.196 ± 0.017 |
| 2 | 47 | 2.47 ± 0.30 |
| 13 | 13.5 | 5.04 ± 0.704 |
| 14 | 43.5 | 5.96 ± 0.679 |
Endo-1 (1) degraded rapidly with a half-life of between 5-9 minutes and a similar degradation profile was observed for the C-terminus modified Endo-1 analogues (8,10). In contrast, modifications of both the N- and C-termini (compounds 11 and 12) significantly improved stability in Caco-2 cell homogenates. The half-lives of 11 and 12 were measured at 75 and 33 minutes, respectively, and these liposaccharide analogues were readily soluble in aqueous buffer. N-Terminus modified Endo-1 analogues (2 and 7) also exhibited improved enzymatic stability profiles with the half-lives of 23 and 38 minutes respectively. The enantiomerically pure analogues 3 and 4 were also examined and found to have identical degradation profiles, which were also identical to that of the racemic mixture 2. In the case of N-terminal modified endomorphin analogues, the stereochemistry of the Laa appears to have no effect on the rate of degradation of the compound. The substitution of Dmt for Tyr in Endo-1 (13) resulted in a slight improvement in enzymatic stability, with a half life of 13.5 minutes and this was again improved by conjugation of C8Laa to the N-terminus with analogue 14 exhibiting a t1/2 = 43.5 minutes.
As the modifications made to the endomorphin sequence are modular in nature, it is feasible that the Laa or sugar groups may be cleaved off in the process of metabolism, releasing the native peptide. Therefore, in addition to monitoring the enzyme treated solutions for each of the original peptides, the levels of Endo-1 were also measured. None of the analogues produced significant amounts of the parent peptide on enzymatic metabolism; therefore, none of the constructs produced acted as prodrug structures.
Caco-2 cell monolayers were used to examine the in vitro permeability of the endomorphin analogues. Native peptide, Endo-1 (1) displayed low permeability through Caco-2 cell monolayers with Papp 4.47 × 10−7 cm/s. The N-terminus modified analogue 2 exhibited significant permeability with Papp 3.54 × 10−6 cm/s, an ∼8-fold increase in permeability (Table 1). The C12Laa conjugate (5) was also expected to exhibit increased permeability; however, the increase in alkyl chain length of Laas decreased water solubility. While 5 appeared to be in solution at the start of the experiment, it could be observed precipitating out in the apical chamber adhering to the plastic assay plate. It is also possible that this lipophilic compound became associated with the cell membranes.50 As a result, 5 showed very low permeability with little detected in the apical solution following the assay. Analogue 7, with glucose succinate conjugated at the N-terminus, exhibited a similar Papp to Endo-1 (Table 1). Clearly, the hydrophilicity provided by glycosylation inhibited passive diffusion across the lipophilic membranes, and the compound was not transported by carbohydrate transporters across the monolayers.
The C-terminus modified analogues, 8, 9 and 10, exhibited low permeability with 10 being undetected in the basolateral chamber over the course of the experiment. These results were, in part, due to the susceptibility to enzymatic metabolism demonstrated in the previous experiments. Samples taken from the apical chamber after the assay showed particularly low concentrations of these analogues. Compounds with both termini conjugated with a combination of Laa and carbohydrate units (11 and 12), exhibited higher permeability with Apparent Permeability (Papp) values ∼3-fold higher than Endo-1, but still lower than the N-terminal Laa conjugate 2.
The actual numerical Papp values obtained in the Caco-2 cell permeability assay depended greatly on various factors, such as passage number, age of the cells, TEER values and the media and growth conditions used.51-53 Therefore, the technique is best used in a comparative manner with all the compounds assessed in the same assay under the same conditions.52, 53 The Dmt analogues were examined in a different experiment to the other compounds; the native peptide 1 and the most promising compound from the previous experiment, 2, were run with the Dmt compounds to allow direct comparison (Table 1). Replacing the Tyr residue of Endo-1 with Dmt (13 and 14) resulted in a slight increase in lipophilicity and a corresponding increase in permeability of the compounds compared to 1 and 2. The permeability results clearly showed that, as in the case of the stability assays, N-terminal lipidation was the most effective method of improving the bioavailability of endomorphin 1.
Receptor binding and activity
While improvement to the bioavailability of a peptide is essential in the development of useful peptide-based pharmaceutics, this cannot be achieved without consideration of the effect these changes have on the biological function of the peptide. In the case of the endomorphin 1 analogues, the opioid receptor binding affinity and their ability to inhibit the production of cAMP within a stimulated cell were assessed using the human neuroblastoma cell line SH-SY5Y, which endogenously express μ- and δ-opioid receptors.54, 55 The results of the assays using intact SH-SY5Y cells are summarised in Table 2.
Table 2.
Binding affinity values of endomorphin 1 analogues (± SD) determined by competitive displacement of [3H]DAMGO from whole SH-SY5Y cells. Kiμ values determined by 7 concentrations ranging from 10 pM to 1 μM performed in triplicate. Data is the mean ± S.E.M.
| Compound | Kiμ (nM) |
|---|---|
| 1 | 1.11 ± 0.79 |
| 2 | 2.15 ± 1.18 |
| 3 | 2.92 ± 0.12 |
| 4 | 3.02 ± 0.14 |
| 5 | 15.4 ± 7.20 |
| 7 | 115 ± 42.1 |
| 8 | 121 ± 52.1 |
| 10 | 151 ± 66.1 |
| 11 | 148 ± 67.2 |
| 12 | 266 ± 122 |
| 13 | 0.059 ± 0.01 |
| 14 | 0.081 ± 0.03 |
The opioid receptor binding affinity was also assessed for a small number of the analogues using a competitive displacement assay with prepared rat brain membranes and the data is presented in Table 3. This assay allows for the assessment of both the μ- and δ-opioid receptor binding affinity was performed to initially ensure that remarkable selectivity of endomorphin for the μ-receptor was maintained upon substitution of the termini, though this was not a major focus of this study: it was performed to validate the affinity results obtained using the SH-SY5Y assay and there was excellent agreement between the two results.
Table 3.
Binding affinity values of endo-1 analogues determined by competitive displacement of [3H]DAMGO from prepared rat brain membranes, values are expressed as mean ± SE n = 3-4
| Compound | Kiμ (nM)a | Kiδ (nM)a | δ/μ |
|---|---|---|---|
| 1 | 1.15 ± 0.16 | 1,280 ± 134 | 1110 |
| 2 | 2.86 ± 0.61 | 1,760 ± 215 | 614 |
| 5 | 14.8 ± 1.6 | 836 ± 129 | 56 |
| 7 | 115 ± 7.3 | 6 320 ± 900 | 55 |
| 13 | 0.067 ± 0.02 | 5.0 ± 0.6 | 75 |
The receptor affinity results show that compounds modified only at the C-terminus (8 and 10) showed a marked decrease in affinity, as did those modified at both termini (11 and 12). It is clear that additions to the C-terminus have a significant detrimental effect on receptor binding of this peptide suggesting that the free amide group may be an important contact point in the binding complex. Chaturvedi et. al. also showed that glycosylation of the C-terminus of endomorphin 1 caused a greater decrease in affinity than glycosylation of the N-terminus56 and a structure-activity study suggested that the C-terminal Phe conformation is essential for binding affinity for the μ-opioid receptor.57,58,59
The N-terminus modified compounds (2-7) exhibited binding affinities close to that of the native peptide. Glucose succinate conjugated analogue (7) exhibited a much lower receptor affinity than the Laa conjugates and increasing the length of the alkyl side chain of the Laas (2, 5) decreased opioid receptor affinity. The glucose moieties would be highly solvated in an aqueous solution and might represent a sterically demanding group at the terminus. These data therefore indicate that, while N-terminus is more amenable to functionalisation than the C-terminus, there are steric constraints to which groups can be accommodated without severely altering receptor affinity.
The most promising result was that the Dmt bearing analogues, 13 and 14, exhibited pM affinity for the μ-opioid receptor, 20-times higher than the native peptide. This is consistent with the reports by Okada et. al., which showed that incorporating Dmt into endomorphin 1 and 2 significantly increases μ-opioid receptor affinity and analgesic activity assays in vivo.35, 39, 60 It is clear that the presence of a C8Laa residue on the N-terminus had little effect on the tight binding of the Dmt-peptide.
It is widely known that treatment of SH-SY5Y cells with forskolin causes an accumulation of cAMP within the cell.61 One of the physiological consequences of agonist binding to the μ-opioid receptor inhibits cAMP production.61 The peptide analogues were initially treated at a single concentration (100 nM) to establish whether these compounds were agonists or antagonist: all of the analogues tested showed some agonist activity and the extent to which they inhibited the accumulation of cAMP is presented in Table 4.
Table 4.
The level of inhibition of cAMP production in stimulated SH-SY5Y cells was induced by treatment with a 100 nM solution of the test compound. SH-SY5Y cells were stimulated by treatment with 30 μM solution of forskolin in each assay except in blank. Data presented are mean values (n = 3) ± S.E.M. from 3 experiments in triplicate
| Compound | cAMP level (fmol) | Inhibition level of cAMP (%) |
|---|---|---|
| Forskolin alone | 1211 ± 111 | - |
| Nonstimulated cells | 24 ± 13.8 | 100 |
| 1 | 462 ± 75.1 | 59.8 ± 3.9 |
| 2 | 395 ± 64.6 | 65.4 ± 3.35 |
| 5 | 516 ± 83.5 | 55.41 ± 4.29 |
| 6 | 60.1 ± 93.2 | 48.39 ± 4.84 |
| 8 | 994 ± 145.8 | 15.94 ± 7.5 |
| 10 | 805 ± 206.4 | 31.54 ± 10.7 |
| 11 | 748 ± 183.6 | 36.25 ± 9.53 |
| 14 | 159 ± 58.6 | 84.29 ± 3.04 |
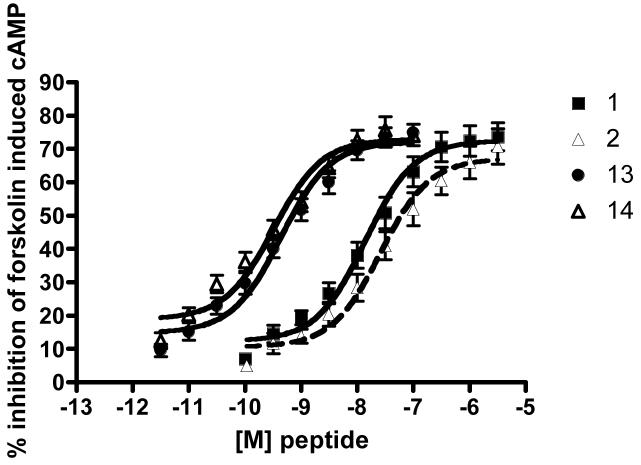
Analogues that showed the highest affinity (1, 2, 13, 14) were then further examined by means of a concentration-response curve (Figure 2). Inhibition of cAMP production was found to plateau between 70-75% for all four analogues (Figure 2). Lipoamino acid conjugated analogues and their parent peptides exhibited similar concentration response curves. The calculated IC50 values of cAMP levels for 1 and 2 were 14 and 26 nM, respectively, indicating a small drop in potency for the Laa conjugated analogue (Table 4). In comparison, the Dmt bearing analogues 13 and 14 both exhibited a leftward shift in dose response curve, and showed essentially equivalent subnanomolar IC50 values (Table 5). It is clear that the introduction of Dmt into the endomorphin sequence greatly enhanced not only the μ-opioid receptor affinity, but also the agonist activity of the peptide demonstrated by the inhibition of cAMP.
Figure 2.
cAMP formation inhibition curve for analogues 1, 2, 13, 14. All data were expressed as the mean (n = 3) and S.E.M. of % maximum inhibition in triplicate. Data were plotted using the one-site competition functions (PRISM).
Table 5.
The IC50 (nM) from full dose response curve of cAMP formation inhibition. Data were from 3 experiments conducted in triplicate.
| Compound | IC50 (nM) |
|---|---|
| 1 | 14.0 ± 6.75 |
| 2 | 26.3 ±14.5 |
| 13 | 0.43 ± 0.19 |
| 14 | 0.32 ± 0.16 |
Conclusions
From this study on the effects of the functionalisation of endomorphin 1 we have gained insight into some viable strategies that could be employed to convert this simple opioid to a pharmaceutically relevant analgesic. Our results clearly indicated that attachment of auxiliary groups to the C-terminus of the peptide not only destroys much of the receptor affinity, but also offered little protection against degradation and therefore no improvement to the bioavailability of the peptide. Attachment of simple glucose units to either terminus did not provide significant improvement to epithelial permeability of the compound; furthermore, there is no evidence of sugar transporter involvement and the steric crowding associated with the sugar unit decreased receptor binding. The introduction of short chain lipoamino acids to the N-terminus of the peptide provided significant protection to metabolic enzymes and increased the passive diffusion of the peptide across Caco-2 cell monolayers, while having minimal effect on receptor affinity or agonist activity. The length of the lipid chain accommodated depended heavily on the water solubility of the resulting compound.
From this series of simple lipid or sugar modifications to endomorphin 1 and the replacement of Tyr1 by Dmt, we have generated a pentapeptide, [C8Laa-DMT1]-Endo1 (14), that exhibited t½= 43.5 minutes in a solution of digestive enzymes, which was >8 times that of the parent peptide as well as permeability of 30 times that of endomorphin 1. Remarkably, this stable, permeable peptide exhibits potent in vitro agonist affinity with a Kiμ = 0.08 nM, which is 140-fold greater than the parent peptide. and a subnanomolar inhibition of cAMP production. Compound 14, therefore, represents a potential new lead peptide in the journey towards endomorphin 1 based pharmaceutics.
Experimental
General
Dimethylformamide and trifluoroacetic acid (TFA) of peptide synthesis grade were purchased from Auspep (Parkville, Australia). HPLC grade acetonitrile was purchased from Labscan Asia Co. Ltd. (Bangkok, Thailand). Fmoc-protected amino acids and Rink amide MBHA resin (100-200 mesh, 0.78 mmol/g loading) were obtained from Novabiochem (Melbourne, Australia) and Reanal Finechem (Budapest, Hungary). Piperidine was purchased from Auspep, Melbourne Victoria. All other chemicals were purchased from the Aldrich Chemical company unless otherwise stated. Absorbance measurments were preformed on a Varian Cary 50 Bio UV-Vis Spectrophotometer. Electrospray ionization mass spectrometry was performed on a Perkin-Elmer Sciex API 3000 operating in positive ion mode and 1H and 13C NMR spectra were recorded on Bruker Ultra Shield 300, 400 or 500 MHz Topspin spectrometers using CDCl3 (Aldrich), CO(CD3)2 (Aldrich), DMSO-d6 (Cambridge Isotope laboratories, inc.) or D2O (Cambridge Isotope laboratories, inc.) as solvents. 13C signals were assigned with data from distortionless enhancement of polarization transfer spectra using a 135 degree decoupler pulse (DEPT 135). Melting points were determined using a Yanagimoto micro-melting point apparatus and thin-layer chromatography (TLC) performed on Kieselgel G (Merk). Optical rotations were measured using a Perkin-Elmer 241MC Polarimeter (wavelength λNa=589).
Lipoamino acid synthesis
2-Amino-d,l-octanoic acid, 2-amino-d,l-dodecanoic acid and 2-amino-d,l-octadecanoic acid were synthesised from diethyl acetamido malonate and the appropriate alkyl bromide using published procedures.26, 62, 63. The analogues bearing N-terminal Laas were synthesised using tert-butoxycarbonyl (Boc) protected Laas. Boc protection was achieved using published procedures and the spectral data for these compounds matched the reported data.26, 62, 63 For those analogues where the Laa residue was at the C-terminus or within the sequence, the amine group was protected using the 2-acetyldimedone (Dde) protecting group which was attached using literature procedures.64-66
Resolution of 2-amino-d,l-octanoic acid
d,l-2-Chloroacetamido-octanoic acid
The hydrochloride salt of 2-amino-d,l-octanoic acid (4.0 g, 25.2 mmol), chloroacetyl chloride (4.12 mL, 37.8 mmol) and pyridine (2.03 mL, 25.2 mmol) were dissolved in dioxane (2.32 mL, 27.8 mmol) and ethyl acetate (180 mL) and the solution was heated under refluxing conditions for 5 hours. After cooling to room temperature, the reaction mixture was washed with 10% citric acid in water (50 mL × 2). The organic solvent was dried (MgSO4), removed in vacuo, and the resulting residue lyophilized from 20% acetonitrile in water. The isolated white powder was identified as chloroacetamido-α-2-aminooctanoic acid.33
Yield; 2.0 g, 33.8%, ESMS (m/z); 200.2 [M-Cl]+ ([M-Cl]+ of C10H18NO3 requires 200.1), 1H NMR (400 MHz, CDCl3 ) δ8.65 (br, m, 1H, OH), 7.08 (d, 1H, J= 8.2 Hz NH), 4.58 (q, 1H α-CH), 4.09 (s, 2H, CH2Cl), 1.90 (m, 1H, β-CHH), 1.75 (m, 1H, β-CHH), 1.25 (br m, 8H, CH2), 0.85 (t, 3H, J=6.7 CH3). 13C NMR (100 MHz, CDCl3) δ176.02 (COOH ), 166.41 (NCOR), 52.46 (CH), 42.33 (CHCl), 31.86, 31.45, 28.72, 25.00, 22.45 (5 × CH2), 13.94 (CH3)
Hydrolysis of 2-chloroacetamido-octanoic acid33
d,l-2-Chloroacetamido-octanoic acid (1.5g, 6.3 mmol) was dissolved in distilled-water, and the pH of the solution was adjusted to 7.3 with 2 N lithium hydroxide (LiOH). Fresh acylase I enzyme (0.7 g) from Aspergillus melleus was added, and the solution was incubated at 37° C for 2 hours. The first white precipitant was isolated, and the solution was left to stand under the same conditions for a further 72 hours. A second white precipitant was isolated, and both precipitates were identified as 2-amino-l-octanoic acid. The remaining solution was acidified (pH 2) with 35% HCl and stirred overnight. The white precipitant was collected and identified as 2-amino-d-octanoic acid.
2-amino-l-octanoic acid: yield; 0.38 g, 75.8%, MS (m/z); 160.43 [M+H]+ ([M+H]+ of C8H17NO2 requires 160.13) [α]D; +21.4° in AcOH (c = 1g/mL) (Literature [α]D; +23° in 6N HCl (c = 1%))33.
2-amino-d-octanoic acid: yield; 0.086g, 17.0%, MS (m/z); 160.43 ([M+H]+ of C8H17NO2 requires 160.13) [α]D; −21.1° in AcOH (c =1 g/mL) (Literature [α]D; +23.5° in 6N HCl (c = 1%)).33
2-(tert-Butoxycarbonylamino)octanoic acid
Optically pure 2-aminooctanoic acid (0.1 g, 0.63 mmol) was dissolved in DIPEA (0.2 mL). Boc-carbonate (0.16 g, 0.75 mmol) was dissolved in dioxane:water (15 mL, 2:1), and added to the DIPEA solution. The mixture was stirred for 12 hours, and the pH maintained at 8 to 9 with DIPEA. The solvent was evaporated in vacuo, and the pH was adjusted to 4 with 10% citric acid in water. The crude product was extracted with EtOAc (30 mL × 2). The extracts were washed with saturated NaCl in water (20 mL × 5) until the pH of the aqueous washes was 6. The EtOAc layer was dried (MgSO4), and the solvent was removed in vacuo. The product was dissolved in 20% acetonitrile in water, and lyophilized to a white solid.
tert-Butoxycarbonyl - l-aminooctanoic acid
Yield 0.105g, 64.5%, M.S. [M + H]+ m/z: 260 ([M+H]+ of C13H25NO4 requires 260), [α]D25 = +18.9, (c =1,CHCl3), TLC Rf = 0.48 (CHCl3:MeOH:AcOH; 90:8:2 v/v, 0.02% Ninhydrin in ethanol dip), 1H NMR (400 MHz, CDCl3 ) δ5.06 (d, 1H, NH), 4.25 (q, 1H, αCH), 1.81 (m, 1H, β-CHH), 1.63 (m, 1H, β-CHH), 1.41 (s, 9H, C(CH3)3), 1.25 (m, 8H, CH2), 0.84 (t, 3H, J= 6.2 CH3). 13C NMR (100 MHz, CDCl3) 176.94 (COOH), 155.66 (CONH), 80.21 (C), 53.32 (CH), 32.21 (CH2), 31.48 (CH2), 28.76 (CH2), 28.23 (CH3), 25.17 (CH2), 22.45 (CH2), 13.96 (CH3)
tert-Butoxycarbonyl - d-aminooctanoic acid
Yield 0.08g, 49%, M.S. [M + H]+ m/z: 260 ([M+H]+ of C13H25NO4 requires 260), [α]D25 = −15.8, (c =1,CHCl3), TLC Rf = 0.48 (CHCl3:MeOH:AcOH; 90:8:2 v/v, 0.02% Ninhydrin in ethanol dip), 1H NMR (400 MHz, CDCl3 ) δ5.06 (d, 1H, NH), 4.25 (brq, 1H, αCH), 1.81 (m, 1H, β-CHH), 1.63 (m, 1H, β-CHH), 1.41 (s, 9H, C(CH3)3), 1.25 (m, 8H, CH2), 0.84 (t, 3H, J=6.2 CH3). 13C NMR (100 MHz, CDCl3) δ176.99 (COOH), 155.56 (CONH), 79.89 (C), 53.43 (CH), 32.43 (CH2), 31.50 (CH2), 28.79 (CH2), 28.24 (CH3), 25.14 (CH2), 22.45 (CH2), 13.96 (CH3)
N-(2,3,4,5-tetra-O-acetyl-β-d-glucopyranosyl)-succinate
This building block for N-terminal glycosylated peptides was synthesised from glucose pentaacetate via published procedures with the spectral data of the product being fully consistent with those of the literature reports.23, 63
Synthesis of 2,3,4-tri-O-acetyl-1-azido-1-deoxy-β-d-glucopyranuronic acid and immobilization onto Rink amide MBHA resin was achieved through a three step process from glucuronic acid via literature procedures.63, 67
Synthesis of N-(tert-Butoxycarbonyl)-2′,6′-dimethyl-l-tyrosine (DMT) was achieved using the 5 step literature reported pathway.41
Solid phase peptide synthesis
All peptides were assembled on Rink amide MBHA resin (100-200 mesh, 0.78 mmol/g loading) on a 0.5 mmol scale using HBTU/DIPEA activation and the in situ neutralisation protocol.68 The following protected amino acids were used: Fmoc-Phe, Fmoc-Trp(Boc), Fmoc-Pro, Fmoc-Tyr(tBu). The efficiency of each amino acid coupling was determined by the quantitative ninhydrin reaction69 and couplings repeated until an efficiency > 99.6% was achieved. The chloranil test70, 71 was performed to determine the coupling efficiency for amino acid coupled to a proline residue.
The N-Dde protected Laas were coupled to the growing peptides using standard coupling techniques, with the reaction allowed to proceed for 1 hour and repeated once. The, N-Dde protecting group was removed by treatment with 2% hydrazine hydrate in DMF (1 hour × 2) followed by efficient rinsing of the resin with DMF as usual. The removal of O-acetyl groups of glucose succinamide and glucuronic acid was carried out after removal of N-terminal protecting groups. The drained resin-peptide was suspended in 5 mL of 12.5 % (v/v) hydrazine hydrate in methanol and the suspension mixed for 18 hours at room temperature. The resin was then drained and washed well with DMF before preparing for cleavage as usual.
When construction of the peptide was complete, the resin was washed with DMF, DCM and MeOH and dried. The peptide was cleaved from the resin by treatment with TFA:water:triisopropylsilane (TIS) (95:2.5:2.5 v/v, 25 mL) for 6 hours. The resin was removed by filtration and washed with TFA. The solvent was removed from the peptide solution under a stream of nitrogen and the crude peptide precipitated with cold diethyl ether, collected and dissolved in 20% acetonitrile and lyophilized.
The purification of peptide analogues was achieved by preparative RP-HPLC on a Waters 600 controller and pump with a 490E programmable multi wavelength detector. The purity of peptide analogues was determined by analytical RP-HPLC (Shimazu; SCL-10AVP system controller, FCV-10ALVP pump, and SPD-6A UV detector), electrospray ionization MS (ESI-MS; Perkin-Elmer Sciex API 3000) and LC/MS (Shimadzu LC-10AT HPLC, Perkin-Elmer Sciex API 3000). A table detailing the yields, ESMS analysis and HPLC retention time for each analogue is provided in the Supporting information.
Solution phase synthesis of analogues 13 and 14
The DMT bearing analogues 13 and 14 were synthesised by segment condensation method in solution as described in recent literature reports.35, 60
Cell Culture
The Caco-2 and SH-SY5Y cell lines were obtained from the American Type Culture Collection (Rockville, USA). Caco-2 cells were maintained in T-75 flasks with Dulbecco's modified Eagle's medium (DMEM, Gibco life technology) supplemented with 10% foetal bovine serum (FBS), 1% l-glutamine and 1% nonessential amino acids, at 95% humidity at 37° C, in an atmosphere of 5% CO2. The medium was changed every second day. When the cells reached 80% of confluence, they were subcloned using 0.25% trypsin-EDTA. Passage number 55-75 was used in all of the assays. SH-SY5Y cells were maintained in DMEM:Hams F12 medium (Gibco life technology) supplemented with 10% FBS, 1% glutamine, 1% nonessential amino acids and 1000U/mL Steptomycin/Penecillin (Gibco life technology). Cells were subcloned in the same manner as Caco-2 cells. Passage numbers 10-20 were used in all assays.
Enzymatic stability assay
A suspension of Caco-2 cells in media was adjusted to ∼5 × 105 cells/mL and 100μL (5 × 104 cells) was added into each well in a 96-well cell culture plate. DMEM was supplemented with 10% FBS, 1% l-glutamine, 1% nonessential amino acids and 1% of 100 U/mL penicillin/streptomycin and this media was changed every second day for 21-28 days. To perform the assay, media was removed from the wells, each well washed with 0.2% EDTA (100 μL × 2) followed by washing three times with HBSS containing 25 mM Hepes (pH 7.4). Finally, 100 μL of this buffer was placed in each well and the plate cooled on ice. The cells were then disrupted by a two second pulse with a Sonics Vibracell ultrasonic processor set at 40% amplitude with 130w mode. The 96-well plate was centrifuged at 2,000 rpm for five minutes to precipitate the cell debris. Three of the wells' contents were assayed for total protein content using the Bio-Rad protein assay72 with BSA as a standard. The total protein content of the homogenate supernatant was adjusted with the buffer to 0.6-0.9 mg/mL in a clean 96-well plate.
The compounds to be tested were dissolved in HBSS-25 mM Hepes buffer to a final concentration of 200 μM stock solution including 1% by total volume DMSO. At the start of the assay, 100 μL of this solution was added to each well containing 100 μL cell homogenate. The concentration of test compound at the beginning of the experiment was 100 μM, and the assay was performed at 37° C shaking at 400 rpm. Samples (10 μL) were taken at selected time points (5, 10, 15, 20, 30, 40, 50, 60 and 120 minutes) and immediately added to 5 μL of TFA to stop digestion and then diluted with 85 μL of water/DMSO solution so that there was a final concentration of 10% by volume of DMSO. This addition of DMSO was shown to improve the resolution and reproducibility of the LC/MS analysis, particularly of the lipophilic peptides.
The concentration of the test compound in each sample was determined by LC/MS analysis using gradient elution (Shimadzu LC-10AT HPLC), coupled to a triple quadrupole mass spectrometer (Perkin-Elmer Sciex API 3000) and operating in selected ion monitoring (SIM) mode with positive ion electrospray. Each sample solution (10 μL) was injected into the system incorporating a 1:10 splitter and an elution gradient from 100% Solvent A (0.1% formic acid in water) to 90% Solvent B (0.1% formic acid in 90% acetonitrile/water) over 5.5 minutes at a flow rate of 0.3 mL/minute was used. A C18 column (50 × 2.0 mm, Phenomex®) or CN column (50 × 2.0 mm, Phenomex®) was used for HPLC. The concentrations were calculated by comparison with a standard curve generated and analysed on the same day as the samples.
Permeability assay
A suspension of Caco-2 cells in media was adjusted to ∼1 × 106 cells/mL and 100 μL (∼1 × 105 cells) of this suspension was added to the polycarbonate cell culture inserts (pore size 0.4 μm × 6.5 mm diameter, Transwell®). The media used consisted of DMEM supplemented with 10% FBS, 1% L-glutamine, 1% nonessential amino acids and 1% 100 U/mL penicillin/streptomycin. The media was changed every second day (0.6 mL in the basolateral chamber and 0.1 mL to the apical chamber) and the monolayers were used between 21-28 days after seeding. Permeability studies were carried out at pH 7.4 and at 37° C.. The tight junctions and integrity of the monolayers were monitored by the transepithelial electrical resistance (TEER, Ωcm2) across each monolayer, measured using the Millicell-ERS epithelial volt-ohmmeter system (Millipore Corporation). The TEER values of the assayed monolayers were measured at 1,500-4,280 Ωcm2 with minor TEER value differences of ±500 Ωcm2 detected after the assays. None of the Endo-1 analogues caused a significant drop in the TEER values during the assay indicating that they were not toxic to the cells. The Caco-2 cell monolayer integrity was also monitored by measuring the permeability of radiolabeled [14C]-D-mannitol (SIGMA; 55 mCi/mmol, 0.09 mCi/mL in 90% EtOH in water). A mannitol solution (1.80 μCi, 32.73 nmol/4mL) in HBSS-25 mM Hepes buffer was added to the donor chamber of three wells.
Compounds to be tested were dissolved in HBSS-25 mM Hepes buffer to a final concentration of 200 μM including 1% by total volume of DMSO. Prior to beginning the experiments, the monolayers were washed with pre-warmed HBSS-25 mM Hepes buffer, and incubated in the buffer for 30 minutes. At the start of the experiment, the apical chamber was emptied and 100 μL of test compound solution added.
The permeability study for each compound was performed in at least three wells at 37° C, with shaking at 400 rpm in a Heridolf Titramax. Samples (400 μL) were taken from the basolateral chamber at regular interval time points (30, 90, 120, 150 minutes), and replaced with the same volume of buffer. At the end of experiment (150 minutes), 50 μL was taken from the apical chamber. All the collected samples were stored at −20° C to protect against degradation.
The concentration of the peptides in each sample was quantified using the LC/MS method described above. The radioactivity of the [14C]-d-mannitol samples was quantified by liquid scintillation counting (Liquid Scintillation Systems, BECKMAN LS3801, USA). The Papp for each compound was calculated from the data using the following formula:
Papp (cm/s) = dC / dt × Vr / (A × C0)
dC/dt = steady-state rate of change in the chemical concentration (M/s) or radiochemical concentration (dpm/mL·s) in the receiver chamber.
Vr = volume of the receiver chamber (mL)
A = surface area of the cell monolayers
C0 = initial concentration in the donor chamber (M or dpm/mL)
Prepared rat brain membrane receptor binding assay
This assay relied on the method of competitive assays for μ- and δ-opioid receptor ligands using prepared rat brain P2 synaptosomes as described in detail previously.38
Intact SH-SY5Y cell receptor binding assay
SH-SY5Ycells were plated with binding buffer (50 mM Tris buffer, pH 7.4, 2% BSA) in 24 well plates and incubated overnight. Media was removed and cells were washed with PBS before pre-incubation in 300 μL binding buffer. Binding studies were performed with 100 μL of appropriate concentrations of [3H]DAMGO (a selective μ-opioid receptor agonist) and 100 μL competitor or blank in binding buffer at 37 °C for 60 min. Competition binding experiments were performed using 100 pM [3H]DAMGO in the absence or presence of increasing concentrations of unlabeled peptides. An excess of unlabelled DAMGO (1 μM) was used to determine non-specific binding. Following incubation, the cells were washed with PBS and recovered from culture plates using 500 μL 1 M NaOH before transfer to scintillation vials. Liquid scintillation cocktail (Ultima Gold, Packard, Meriden, USA) was added to these vials for counting in a liquid scintillation analyser. Data are expressed as the mean ± SEM of % specific binding of triplicate determinations performed on at least three independent plates of cells. Data were plotted using the one-site competition (PRISM, Graphpad Inc., San Diego, USA).
Inhibition of cAMP accumulation assay
SH-SY5Y cells endogenously expressing μ- and δ-opioid receptors were cultured (100 μL) in a 96 well-plate and incubated (usually overnight) until 80% confluent. Peptide analogues were diluted in DMEM culture medium containing 30 μM forskolin and applied to separate wells for agonist determination and co-incubated for antagonist studies. Cells were incubated with peptides at 37°C for 30 minutes, medium was aspirated and cells were lysed using lysis buffer from a cAMP Biotrak EIA kit (Amersham, Buckinghamshire, UK). An anti-cAMP antibody was added to the lysate, and the mixture incubated at 4°C for 2 hours followed by the addition of a cAMP peroxidase conjugate, and incubated at 4°C for a further hour. A 3,3′5,5′-tetramethylbenzidine (TMB) substrate provided by the Biotrak EIA kit was then incubated with cell lysate for 1 hour and finally 1 μM sulphuric acid was added to facilitate a colour change, which is proportional to the amount of cAMP captured. A cAMP standard curve from 0 to 3.2 pmol was also generated concurrently. Plates were then read in a plate reader at 450 nm and compared to the cAMP standard curve. All data are compared to the maximum cAMP response to forskolin and are represented as the mean ± standard error of three experiments performed in triplicate. All data are plotted using PRISM (GraphPad, San Diego, CA).
Acknowledgment
This work was supported in part by Neurotide Pty Ltd and a Biotechnology Innovation Fund grant, and in part by the Intramural Research Program of the NIH, and NIEHS.
Footnotes
Supplementary Information Available:
S1: Peptide Analogue Characterisation Data.
S2: Caco-2 cell homogenate stability assay results.
S3: Graph of apparent permeability values for compounds 10-12.
References
- 1.Janecka A, Fichna J, Janecki T. Curr. Top. Med. Chem. 2004;4:1. doi: 10.2174/1568026043451618. [DOI] [PubMed] [Google Scholar]
- 2.Zadina JE, Hackler L, Ge L-J, Kastin A. Nature. 1997;386(3):499. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg IE, Rossi GC, Letchworth SR, Mathis JP, Ryan-Moro J, Leventhal L, Su W, Emmel D, Bolan EA, Pasternak GW. J. Pharmacol. Exp. Ther. 1998;286(2):1007. [PubMed] [Google Scholar]
- 4.Gentilucci L. Curr. Top. Med. Chem. 2004;4:19. doi: 10.2174/1568026043451663. [DOI] [PubMed] [Google Scholar]
- 5.Stone LS, Fairbanks CA, Laughlin TM, Nguyen HO, Bushy TM, Wessendorf MW, Wilcox GL. Neuroreport. 1997;8(14):3131. doi: 10.1097/00001756-199709290-00025. [DOI] [PubMed] [Google Scholar]
- 6.Csullog E, Joo G, Toth G, Dobos D, Benedek G, Horvath G. Pain. 2001;94(1):31. doi: 10.1016/S0304-3959(01)00338-4. [DOI] [PubMed] [Google Scholar]
- 7.Horvath G, Szikszay M, Tomboly C, Benedek G. Life Sci. 1999;65(24):2635. doi: 10.1016/s0024-3205(99)00532-9. [DOI] [PubMed] [Google Scholar]
- 8.Li Z-H, Shan L-D, Jiang Z-H, Guo S-Y, Yu G-D, Hisamitsu T, Yin DLQ-Z. Acta Pharmacol. Sin. 2001;22(11):976. [PubMed] [Google Scholar]
- 9.Obara I, Przewlocki R, Przewlocka B. Neurosci. Lett. 2004;360(12):85. doi: 10.1016/j.neulet.2004.01.056. [DOI] [PubMed] [Google Scholar]
- 10.Przewlocka B, Mika J, Labuz D, Toth G, Przewlocki R. Eur. J. Pharmacol. 1999;367(23):189. doi: 10.1016/s0014-2999(98)00956-x. [DOI] [PubMed] [Google Scholar]
- 11.Soignier RD, Vaccarino AL, Brennan AM, Kastin AJ, Zadina JE. Life Sci. 2000;67(8):907. doi: 10.1016/s0024-3205(00)00689-5. [DOI] [PubMed] [Google Scholar]
- 12.Zhao M, Wang J-Y, Jia H, Tang J-S. Brain Res. 2006;1076:68. doi: 10.1016/j.brainres.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Hau VS, Huber JD, Campos CR, Lipkowski AW, Misicka A, Davis TP. J. Pharm. Sci. 2002;91(10):2140. doi: 10.1002/jps.10202. [DOI] [PubMed] [Google Scholar]
- 14.Tomboly C, Peter A, Toth G. Peptides. 2002;23(9):1573. doi: 10.1016/s0196-9781(02)00100-6. [DOI] [PubMed] [Google Scholar]
- 15.Lengyel I, Orosz G, Biyashev D, Kocsis L, Al-Khrasani M, Ronai A, Tomboly C, Furst Z, Toth G, Borsodi A. Biochem. Biophys. Res. Comm. 2002;290(1):153. doi: 10.1006/bbrc.2001.6136. [DOI] [PubMed] [Google Scholar]
- 16.Yang YR, Chiu TH, Chen CL. Eu.r J. Pharmacol. 1999;372(3):229. doi: 10.1016/s0014-2999(99)00210-1. [DOI] [PubMed] [Google Scholar]
- 17.Paterlini MG, Avitabile F, Ostrowski BG, Ferguson DM, Portoghese PS. Biophys J. 2000;78(2):590. doi: 10.1016/S0006-3495(00)76619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Podlogar BL, Paterlini MG, Ferguson DM, Leo GC, Demeter DA, Brown FK, Reitz AB. FEBS Lett. 1998;439(12):13. doi: 10.1016/s0014-5793(98)01202-2. [DOI] [PubMed] [Google Scholar]
- 19.Adessi C, Soto C. Curr. Med. Chem. 2002;9(9):963. doi: 10.2174/0929867024606731. [DOI] [PubMed] [Google Scholar]
- 20.Blanchfield J, Toth I. Curr. Med. Chem. 2004;11(17):2375. doi: 10.2174/0929867043364621. [DOI] [PubMed] [Google Scholar]
- 21.Toth I, Malkinson JP, Flinn NS, Drouillat B, Horvath A, Erchegyi J, Idei M, Venetianer A, Artursson P, Lazorova L, Szende B, Keri G. J. Med. Chem. 1999;42(19):4010. doi: 10.1021/jm9910167. [DOI] [PubMed] [Google Scholar]
- 22.Ali M, Manolios N. Lett. Pept. Sci. 2001;8(35):289. [Google Scholar]
- 23.Kellam B, Drouillat B, Dekany G, Starr MS, Toth I. Int. J. Pharm. 1998;161(1):55. [Google Scholar]
- 24.Toth I, Flinn N, Hillery A, Gibbons WA, Artursson P. Int. J. Pharm. 1994;105(3):241. [Google Scholar]
- 25.Flinn N, Coppard S, Toth I. Int. J. Pharm. 1996;137(1):33. [Google Scholar]
- 26.Blanchfield JT, Dutton JL, Hogg RC, Gallagher OP, Craik DJ, Jones A, Adams DJ, Lewis RJ, Alewood PF, Toth I. J. Med. Chem. 2003;46(7):1266. doi: 10.1021/jm020426j. [DOI] [PubMed] [Google Scholar]
- 27.Caccetta R, Blanchfield JT, Harrison J, Toth I, Benson HAE. Int. J. Pep. Res. Ther. 2006;12(3):327. [Google Scholar]
- 28.Blanchfield JT, Lew RA, Smith AI, Toth I. Bioorg. Med. Chem. Lett. 2005;15(6):1609. doi: 10.1016/j.bmcl.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 29.Polt R, Dhanasekaran M, Keyari CM. Med. Res. Rev. 2005;25(5):557. doi: 10.1002/med.20039. [DOI] [PubMed] [Google Scholar]
- 30.Polt R, Palian MM. Drugs of the Future. 2001;26(6):561. [Google Scholar]
- 31.Egleton RD, Mitchell SA, Huber JD, Janders J, Stropova D, Polt R, Yamamura HI, Hruby VJ, Davis TP. Brain Res. 2000;881(1):37. doi: 10.1016/s0006-8993(00)02794-3. [DOI] [PubMed] [Google Scholar]
- 32.Egleton RD, Bilsky EJ, Tollin G, Dhanasekaran M, Lowery J, Alves I, Davis P, Porreca F, Yamamura HI, Yeomans L, Keyari CM, Polt R. Tetrahedron-Asymmetry. 2005;16(1):65. [Google Scholar]
- 33.Birnbaum SM, Fu S-CJ, Greenstein JP. J. Biol. Chem. 1953;203(1):333. [PubMed] [Google Scholar]
- 34.Hansen DW, Stapelfeld A, Savage MA, Reichman M, Hammond DL, Haaseth RC, Mosberg HI. J. Med. Chem. 1992;35(4):684. doi: 10.1021/jm00082a008. [DOI] [PubMed] [Google Scholar]
- 35.Jinsmaa Y, Marczak E, Fujita Y, Shiotani K, Miyazaki A, Li TY, Tsuda Y, Ambo A, Sasaki A, Bryant SD, Okada Y, Lazarus LH. Pharmacol., Biochem. Behav. 2006;84:252. doi: 10.1016/j.pbb.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki Y, Sasaki A, Niizuma H, Goto H, Ambo A. Bioorg. Med. Chem. 2003;11(5):675. doi: 10.1016/s0968-0896(02)00601-6. [DOI] [PubMed] [Google Scholar]
- 37.Bryant SD, Jinsmaa Y, Salvadori S, Okada Y, Lazarus LH. Biopolymers. 2003;71(2):86. doi: 10.1002/bip.10399. [DOI] [PubMed] [Google Scholar]
- 38.Okada Y, Tsuda Y, Fujita Y, Yokoi T, Sasaki Y, Ambo A, Konishi R, Nagata M, Salvadori S, Jinsmaa Y, Bryant SD, Lazarus LH. J. Med. Chem. 2003;46(15):3201. doi: 10.1021/jm020459z. [DOI] [PubMed] [Google Scholar]
- 39.Okada Y, Fujita Y, Motoyama T, Tsuda Y, Yokoi T, Li TY, Sasaki Y, Ambo A, Jinsmaa Y, Bryant SD, Lazarus LH. Bioorg. Med. Chem. 2003;11(9):1983. doi: 10.1016/s0968-0896(03)00068-3. [DOI] [PubMed] [Google Scholar]
- 40.Balboni G, Guerrini R, Salvadori S, Negri L, Giannini E, Bryant SD, Jinsmaa Y, Lazarus LH. J. Med. Chem. 2005;48:8112. doi: 10.1021/jm058259l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dygos JH, Yonan EE, Scaros MG, Goodmonson OJ, Getman DP, Periana RA, Beck GR. Synthesis (Stuttg) 1992;(8):741. [Google Scholar]
- 42.Artursson P, Karlsson J. Biochem. Biophys. Res. Comm. 1991;175(3):880. doi: 10.1016/0006-291x(91)91647-u. [DOI] [PubMed] [Google Scholar]
- 43.Wong AK, Ross BP, Chan YN, Artursson P, Lazorova L, Jones A, Toth I. Eur. J. Pharm. Sci. 2002;16(3):113. doi: 10.1016/s0928-0987(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 44.He X, Sugawara M, Kobayashi M, Takekuma Y, Miyazaki K. Int. J. Pharm. 2003;263(12):35. doi: 10.1016/s0378-5173(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 45.Zornoza T, Cano-Cebrian MJ, Nalda-Molina R, Guerri C, Granero L, Polache A. Eur. J. Pharm. Sci. 2004;22(5):347. doi: 10.1016/j.ejps.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Cruciani G, Pastor M, Guba W. Eur. J. Pharm. Sci. 2000;11:S29. doi: 10.1016/s0928-0987(00)00162-7. [DOI] [PubMed] [Google Scholar]
- 47.van der Sandt ICJ, Vos CMP, Nabulsi L, Blom-Roosemalen MCM, Voorwinden HH, de Boer AG, Breimer DD. Aids. 2001;15(4):483. doi: 10.1097/00002030-200103090-00007. [DOI] [PubMed] [Google Scholar]
- 48.Lohmann C, Huwel S, Galla HJ. J. Drug Target. 2002;10(4):263. doi: 10.1080/10611860290031903. [DOI] [PubMed] [Google Scholar]
- 49.Garberg P, Ball M, Borg N, Cecchelli R, Fenart L, Hurst RD, Lindmark T, Mabondzo A, Nilsson JE, Raub TJ, Stanimirovic D, Terasaki T, Oberg JO, Osterberg T. Toxicol in Vitro. 2005;19(3):299. doi: 10.1016/j.tiv.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 50.Lang VB, Langguth P, Ottiger C, WunderliAllenspach H, Rognan D, RothenRutishauser B, Perriard JC, Lang S, Biber J, Merkle HP. J. Pharm. Sci. 1997;86(7):846. doi: 10.1021/js960387x. [DOI] [PubMed] [Google Scholar]
- 51.Hosoya K, Kim KJ, Lee VHL. Pharm. Res. 1996;13(6):885. doi: 10.1023/a:1016005212640. [DOI] [PubMed] [Google Scholar]
- 52.Behrens I, Kamm W, Dantzig AH, Kissel T. J. Pharm. Sci. 2004;93(7):1743. doi: 10.1002/jps.20062. [DOI] [PubMed] [Google Scholar]
- 53.Sambuy Y, Angelis I, Ranaldi G, Scarino ML, Stammati A, Zucco F. Cell Biol. Toxicol. 2005;21(1):1. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- 54.Kazmi SMI, Mishra RK. Biochem Biophys Res. Comm. 1986;137(2):813. doi: 10.1016/0006-291x(86)91152-6. [DOI] [PubMed] [Google Scholar]
- 55.Kazmi SMI, Mishra RK. Mol. Pharmacol. 1987;32(1):109. [PubMed] [Google Scholar]
- 56.Chaturvedi K, Shahrestanifar M, Howells RD. Mol. Brain Res. 2000;76(1):64. doi: 10.1016/s0169-328x(99)00332-0. [DOI] [PubMed] [Google Scholar]
- 57.Tomboly C, Kover KE, Peter A, Tourwe D, Biyashev D, Benyhe S, Borsodi A, Al-Khrasani M, Ronai AZ, Toth G. J. Med. Chem. 2004;47(3):735. doi: 10.1021/jm0310028. [DOI] [PubMed] [Google Scholar]
- 58.Fujita Y, Tsuda Y, Li TY, Motoyama T, Takahashi M, Shimizu M. J. Med. Chem. 2004;47:3591. doi: 10.1021/jm030649p. [DOI] [PubMed] [Google Scholar]
- 59.In Y, Minoura K, Tomoo K, Sasaki Y, Lazarus LH, Okada Y, Ishida T. FEBS J. 2005;272(19):5079. doi: 10.1111/j.1742-4658.2005.04919.x. [DOI] [PubMed] [Google Scholar]
- 60.Li TY, Fujita Y, Tsuda Y, Miyazaki A, Ambo A, Sasaki Y, Jinsmaa Y, Bryant SD, Lazarus LH, Okada Y. J. Med. Chem. 2005;48(2):586. doi: 10.1021/jm049384k. [DOI] [PubMed] [Google Scholar]
- 61.Harrison C, McNulty S, Smart D, Rowbotham DJ, Grandy DK, Devi LA, Lambert DG. Br. J. Pharmacol. 1999;128(2):472. doi: 10.1038/sj.bjp.0702798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gibbons WA, Hughes RA, Charalambous M, Christodoulou M, Szeto A, Aulabaugh AE, Mascagni P, Toth I. Liebigs Ann. Chem. 1990;(12):1175. [Google Scholar]
- 63.Blanchfield JT, Toth I. Modification of peptides and other drugs using lipoamino acids and sugars. In: Howl J, editor. Peptide Synthesis and applications. Vol. 298. Humana Press; Totawa, New Jersey: 2005. p. 45. [DOI] [PubMed] [Google Scholar]
- 64.Nash IA, Bycroft BW, Chan WC. Tetrahedron Lett. 1996;37(15):2625. [Google Scholar]
- 65.Chhabra SR, Hothi B, Evans DJ, White PD, Bycroft BW, Chan WC. Tetrahedron Lett. 1998;39(12):1603. [Google Scholar]
- 66.Wang F, Manku S, Hall DG. Org. Lett. 2000;2(11):1581. doi: 10.1021/ol005817b. [DOI] [PubMed] [Google Scholar]
- 67.Malkinson JP, Falconer RA, Toth I. J. Org. Chem. 2000;65(17):5249. doi: 10.1021/jo000381z. [DOI] [PubMed] [Google Scholar]
- 68.Alewood P, Alewood D, Miranda L, Love S, Meutermans W, Wilson D. Method Enzymol. 1997;289:14. doi: 10.1016/s0076-6879(97)89041-6. [DOI] [PubMed] [Google Scholar]
- 69.Sarin VK, Kent SBH, Tam JP, Merrifield RB. Anal. Biochem. 1981;117:147. doi: 10.1016/0003-2697(81)90704-1. [DOI] [PubMed] [Google Scholar]
- 70.Vojkovsky T. Peptide Res. 1995;8(4):236. [PubMed] [Google Scholar]
- 71.Chan WC, White PD. Fmoc solid phase peptide synthesis: a practical approach. Oxford University Press; Oxford: 2000. [Google Scholar]
- 72.Bradford MM, Williams WL. Fed. Proc. 1976;35(3):274. [Google Scholar]