Abstract
Background
Most patients with major depressive disorder (MDD) experience a period of lengthy trial-and-error when trying to find optimal antidepressant treatment; identifying biomarkers that could predict response to antidepressant treatment would be of enormous benefit. We tested the hypothesis that pre-treatment anterior cingulate (ACC) activity could be a putative biomarker of rapid antidepressant response to ketamine, in line with previous findings that investigated the effects of conventional antidepressants. We also investigated patterns of ACC activity to rapid presentation of fearful faces compared to the normal habituation observed in healthy subjects.
Methods
We elicited ACC activity in drug-free patients with MDD (N=11) and healthy controls (N=11) by rapidly presenting fearful faces, a paradigm known to activate rostral regions of the ACC. Spatial-filtering analyses were performed on magnetoencephalographic (MEG) recordings, which offer the temporal precision necessary to estimate ACC activity elicited by the rapid presentation of stimuli. MEG recordings were obtained only once for both patients and controls. Patients were subsequently administered a single ketamine infusion followed by assessment of depressive symptoms four hours later.
Results
Although healthy subjects had decreased neuromagnetic activity in the rostral ACC across repeated exposures, patients with MDD showed robust increases in pretreatment ACC activity. Notably, this increase was positively correlated with subsequent rapid antidepressant response to ketamine. Exploratory analyses showed that pretreatment amygdala activity was negatively correlated with change in depressive symptoms.
Conclusion
Pretreatment rostral ACC activation may be a useful biomarker that identifies a subgroup of patients who will respond favorably to ketamine’s antidepressant effects.
Keywords: ketamine, anterior cingulate, major depressive disorder (MDD), magnetoencephalography (MEG), habituation, fearful faces, biomarker, predictor, antidepressant response
Introduction
Major depressive disorder (MDD) is a severe, recurrent, and disabling medical illness that is highly prevalent worldwide and often associated with a negative impact on quality of life and productivity. In recent years considerable effort has been spent trying to define more homogenous subgroups and identify putative endophenotypes associated with MDD. Identifying biomarkers to predict response to antidepressants would be enormously beneficial to patients with MDD, as it would minimize the lengthy trial-and-error process that currently occurs when trying to find the most optimal treatment.
Evidence from in vivo functional imaging suggests that enhanced activity in the anterior cingulate cortex (ACC) may be a biomarker for a subset of patients who subsequently respond to antidepressant treatment (1–3). Most of these studies have demonstrated this putative link with conventional antidepressants (i.e., SSRIs), which normally take several weeks to reach their full therapeutic effect and, despite long-term treatment, are only effective for a minority of patients (4).
In the search for rapid-acting therapeutics for MDD, recent studies have shown that ketamine, a non-selective NMDA antagonist, results in rapid and sustained (one to two weeks) antidepressant effects in patients with treatment-resistant MDD (5, 6). We therefore investigated whether pre-treatment ACC activity would be a putative biomarker of rapid antidepressant response to ketamine. We elicited ACC activity in drug-free MDD patients and healthy control subjects by rapidly presenting fearful faces, a paradigm known to activate rostral regions of the ACC, among other structures (7). There is also evidence that ACC activity shows habituation to repeated exposure to aversive stimuli in healthy subjects (8).
To measure ACC response to rapid fearful face presentation, spatial-filtering analyses were performed on magnetoencephalographic (MEG) recordings (9). Spatial-filtering algorithms have been shown to yield source distributions highly consistent with fMRI data (10, 11); with the current procedure, they offer the temporal precision necessary to estimate ACC activity elicited by the rapid presentation of fearful faces. We hypothesized that patients with MDD would display an aberrant pattern of ACC activity to rapidly presented fearful faces compared to the normal habituation observed in healthy subjects. We also hypothesized that ACC activity elicited by fearful faces in MDD patients would predict rapid antidepressant response to ketamine. Finally, given the emerging evidence that amygdala reactivity might predict antidepressant response in MDD patients, we conducted exploratory analyses of amygdala activity. Given the links between both hypo- and hyperactivation and antidepressant response (3, 12, 13), we had no specific a priori hypotheses regarding how pretreatment amygdala activity might be associated with antidepressant response in patients with MDD.
Methods and Materials
Subjects
The patient group comprised 11 right-handed patients (7 M, 4 F) with a diagnosis of MDD, currently depressed without psychotic features; diagnosis was confirmed by the Structured Clinical Interview for Axis I DSM-IV Disorders–Patient Version (14). All subjects were studied at the National Institute of Mental Health (NIMH) Clinical Research Center Mood Disorders Research Unit in Bethesda, Maryland, between January and December 2007. Inclusion criteria were a Montgomery-Asberg Depression Rating Scale (MADRS) (15) score of at least 22, a current or past history of lack of response to two adequate antidepressant trials, and a current major depressive episode of at least four weeks duration. Patients with a DSM-IV (16) diagnosis of drug or alcohol dependence or abuse, serious, unstable illness within the past three months, or uncorrected hypo- or hyperthyroidism were excluded. All subjects had been drug-free from any psychotropic drugs for at least two weeks (or five weeks for fluoxetine). The patients had a mean age of 43.8 ± 15.2 yrs, and mean baseline MADRS scores of 31.9 ± 3.3. Seventy three percent (8/11) had a current comorbid anxiety disorder.
Eleven healthy age- and gender-matched subjects (mean age = 35.9 ± 14.3 years, 7 M, 4 F) were recruited through advertisements on campus and in local newspapers. Healthy subjects underwent a screening visit at the NIMH that included a medical history and physical exam performed by a physician, and a Structured Clinical Interview for DSM-IV (17). Exclusion criteria included current medical illness or major psychiatric disorder, any lifetime history of mood disorders, psychosis, or substance use disorders, or first-degree relatives with a history of psychiatric disorders; no subject could be currently taking psychotropic medications. The study was approved by the NIMH Institutional Review Board. All subjects provided written informed consent.
Measures
Raters who trained together to establish reliability performed MADRS ratings before ketamine infusion and at 230 minutes post-infusion. MEG was obtained at the end of the drug-free period, one to two days before the ketamine infusion. Antidepressant response was expressed as percentage change score from baseline according to each individual’s MADRS score. Baseline and post-ketamine scores for anxiety symptoms and psychotic symptoms were obtained with the Hamilton Anxiety Scale (HAM-A) (18) and with the Brief Psychiatric Rating Scale (BPRS) positive symptoms subscale (19).
Ketamine administration
The ketamine infusion procedure was similar to a previously described procedure (6). Briefly, MDD patients openly received a single intravenous infusion of saline solution and 0.5 mg/kg of ketamine hydrochloride (Abbott Laboratories, Abbott Park, IL) over the course of 40 minutes. Depressive, anxiety, and psychotic symptoms were reassessed 230 minutes following ketamine infusion (6). Changes in psychiatric symptoms were expressed as percentage change from baseline, with positive percentages reflecting a reduction in symptoms. Post-assessment for anxiety symptoms was missing for one patient. We did not assess treatment response later because further analysis of previous data (6) showed that most patients with treatment-resistant MDD who demonstrate a significant treatment response to ketamine do so at the 230 minute post-infusion time point (15/17, 88.2%).
Stimuli and Procedure
The experiment involved presentation of four different stimuli: two fearful faces (one male, one female) and two geometric shapes (one blue circle, one red circle) (20). Participants received 130 consecutive exposures of each stimulus with a 15-second break in between each exposure set. Within each set, the stimulus appeared for 250 ms with a 650–850 msec interstimulus interval. Half the participants in each group were presented with a repeating fearful face first, and the other half with a repeating shape first. On 10 trials within each set, a plus sign was superimposed on the face or shape, and participants responded by button press. These trials ensured that participants maintained attention. There were no reaction time differences between groups (F < 1).
Data acquisition
Neuromagnetic data were recorded at 1200 Hz with a 0–300 Hz bandwidth using a CTF 275 MEG system (CTF Systems, Inc., Coquitlam, Canada) composed of a whole-head array of 275 radial first-order gradiometers housed in a magnetically shielded room (Vacuumschmelze, Hanau, Germany). Synthetic third-gradient balancing removed background noise on-line. Anatomical MRIs were obtained for coregistration with the MEG data in a separate session using a 1.5 T or a 3 T GE scanner.
Source analysis
We performed adaptive spatial-filtering to estimate source activity evoked by fearful faces compared to shapes (for similar analyses, see (21)). An adaptive spatial filter is derived from the sensor data covariance. It is optimized to suppress all activity except at the location of interest in source space (by minimizing the output of the filter with the constraint of unity pass gain at the location of interest) (reviewed in (9)). Volumetric images of power can be produced by constructing spatial filters at each point in a 3-dimensional grid encompassing the brain. Spatial-filtering analyses have successfully captured activity in structures involved in emotional face processing, including the amygdala, fusiform gyrus, and ACC (22, 23).
Because we expected that ACC responses to fearful faces would change across rapid, repeated exposures (eg, habituation), we divided the 120 exposures into four blocks of 30, and performed our source analyses on these blocks separately (trials for reaction time assessment were excluded). Covariance matrices were calculated over 30 unaveraged 500-ms epochs from the first face exposure set and the first shape exposure set (60 total epochs), from −250 to +250ms relative to stimulus onset, with a 2–30Hz bandwidth. Spatial-filter coefficients were calculated with a linearly-constrained minimum-variance beamformer algorithm (24) in 7mm steps across source space using a multi-sphere head model. At each voxel, spatial filter output was time-domain averaged to maximize signal-to-noise ratios (i.e., event-related spatial- filtering (25, 26)). Source activity was quantified as the log10 transformed ratio of power on face epochs over shape epochs in corresponding 50-ms time bins (e.g., 0–50ms post-face onset relative to 0–50ms post-shape onset). A sliding window analysis with 50% overlap captured source power from stimulus onset to offset (i.e., 0–250ms). The same procedures were carried out for the second face and second shape exposure sets.
AFNI software (27) was used to spatially coregister source images to participants’ MRIs. Source images were within-volume normalized to compensate for inter-subject variability in global power and spatially warped into Talairach space. Two-way ANOVAs, with Group (two levels) and Block (four levels) as factors, were conducted on each time window to identify regions that showed different patterns of change to repeating faces between groups. This was done for volumetric source power averaged across both repeating faces, and for each face individually. Significant interactions were followed up with linear trend analyses within groups. Statistical thresholds were set at p < .001, uncorrected for multiple comparisons, given our a priori hypothesis of group differences in ACC activity. For the omnibus analysis, we did calculate the false discovery rate (FDR, (28)) using a region of interest approach encompassing the ACC/BA24/32 (i.e., 125 voxel-wise tests) that also incorporated multiple tests done across time windows. For exploratory analyses of amygdala activity, we used a less conservative criterion of p < .01 to establish statistical significance.
Correlation analyses were conducted to determine whether ACC and amygdala activity predicted change in depressive symptoms following ketamine administration. We used nonparametric Spearman correlations following inspection of the distribution of MADRS percentage change scores showing a non-normal distribution. Given the small sample, these analyses were likely to be underpowered, and thus we elected to use a less conservative criterion of p < .05.
Results
Treatment response
Significant improvement in depressive symptoms occurred 230 minutes after the infusion based on change in MADRS score (t(10)=3.74, p < .005 (mean MADRS pretreatment score 31.9 ± 3.3; MADRS mean score 230 minutes after ketamine 20.4 ± 12)). We also observed a significant decrease in anxiety symptoms (mean HAM-A pretreatment score 23.4 ± 6.5; HAM-A mean score after 230 minutes 14.3 ± 7.8; t(9) = 3.39, p < .01), and a marginally significant decrease in psychotic symptoms (Mean BPRS-positive subscale pretreatment score 10.2 ± 1.4; BPRS positive symptoms mean score after 230 minutes 9.1 ± 1.2: t(10) = 2.08, p < .10).
Group differences in changes in ACC activity
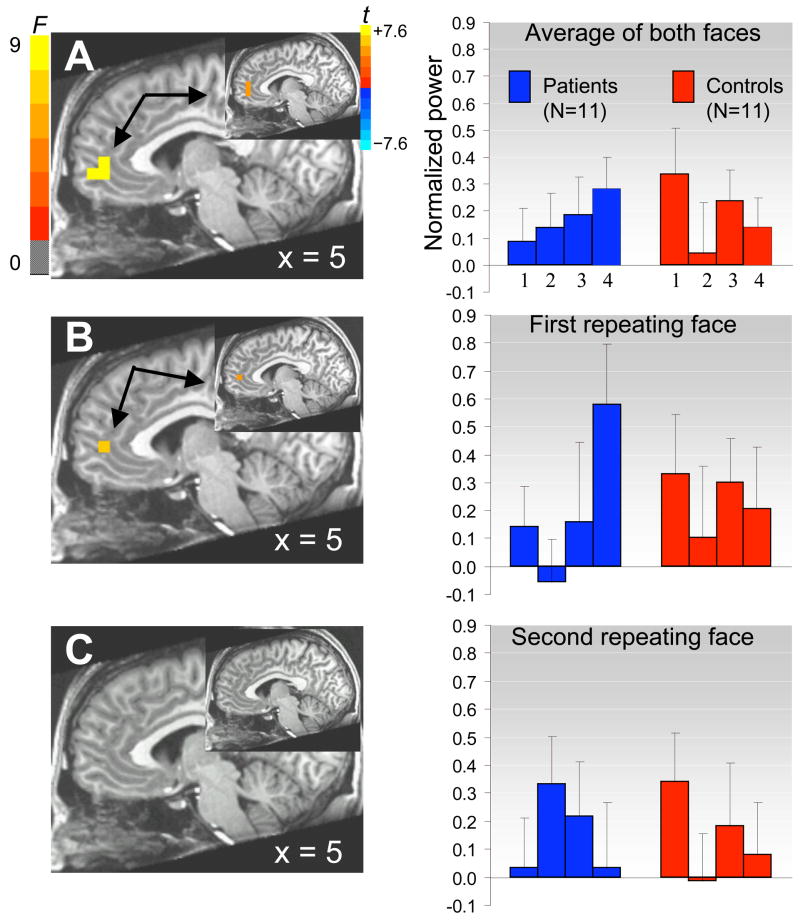
Source analyses revealed a significant Group by Block (2 × 4) interaction in the rostral ACC (BA 32) between 125–175ms post-stimulus onset for the average response to fearful faces (Figure 1A, peak: 5, 45, 9mm, F(3, 30) = 8.22, p < .001, FDR < 10%), suggesting group differences in evoked ACC response over repeated exposures to fearful faces. This interaction was driven by a strong linear increase in ACC activity over several repetitions in MDD patients (peak: 5, 46, 12mm, t(10) = 4.55, p < .002), together with a decrease over several repetitions in healthy controls (t(10) = −2.59, p < .05). Further analyses that considered each repeating fearful face separately indicated that this linear increase in ACC activity in MDD patients over several repetitions was observed for the first repeating face (Figure 1B; peak: 5, 46, 7mm, t(10) = 4.93, p < .001), but not the second (Figure 1C; p > .05).
Figure 1.
ACC shows contrasting changes in activity (i.e., increasing or decreasing for 120 repeated presentations) in patients with MDD versus healthy controls for the average of both faces (A), and separately for the first face (B) but not the second face (C). Sagittal images show the omnibus F statistics for the 2 × 4 interaction between group and block (blocks 1,2,3,4) between 125–175ms post-stimulus onset (p < .001); insets show the t statistics for the linear contrast across blocks in the patient group (p < .002). Bar graphs show average power increase averaged across 125 voxels within an ACC region of interest (BA 24,32) as a function of group and blocks. Errors bars are standard errors of the means.
Correlation between ACC activity and symptom change following ketamine
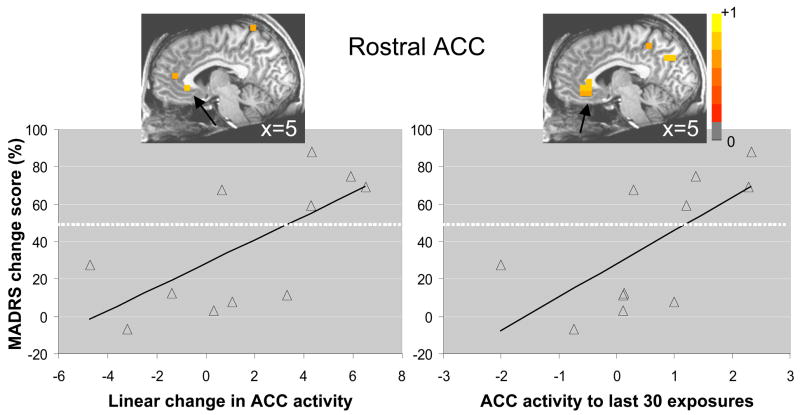
We calculated source images for each patient that reflected the linear contrast presented above to explore whether the increase in post-stimulus ACC activity from early to late presentations of the first repeating face correlated with change in depressive symptoms following ketamine administration. These analyses were performed for only the first fearful face because of the clear differential change in ACC activity between patients and healthy subjects for the first but not the second face (Figure 1). Correlation analyses revealed local maxima (p < .05) in ACC across several post-stimulus time windows. Collapsed across post-stimulus time windows, we observed that change in ACC activity following repeated exposure to the first fearful face was positively correlated with antidepressant response (peak: 5, 27, −3mm, rs(9) = .68, p < .05), suggesting greater increases in ACC activity to repeated exposures of a fearful face in those who showed a more positive change in depressive symptoms (i.e., greater reduction) (Figure 2). A similar positive correlation was observed for ACC activity during the last block of exposures to the first fearful face (peak: 5, 32, −3mm, rs(9) = .75, p < .01). Partial correlation analyses demonstrated little change in the positive associations between ACC peak activations and MADRS percentage change scores, when controlling for HAM-A percentage change scores (linear change, r (7) = .64, p < .07, and last block, r (7) = .60, p < .10).
Figure 2.
(A) Nonparametric correlation between increased ACC activity (thresholded at p < .05) over repeated exposure to a fearful face and change in depressive symptoms 230 minutes after ketamine infusion; and (B) for ACC activity to the last 30 exposures only (i.e., block 4). Scatterplots with least squares lines display ACC activity values, averaged across all post-stimulus time windows, at the location of maximal correlations. Change in depressive symptoms is expressed by the percentage change in MADRS score, with positive percentages representing reductions in depressive symptoms. Dotted lines represent the 50% criterion for defining treatment-responders. Images are in radiological orientation (left=right, right=left).
Exploratory analyses of amygdala activity
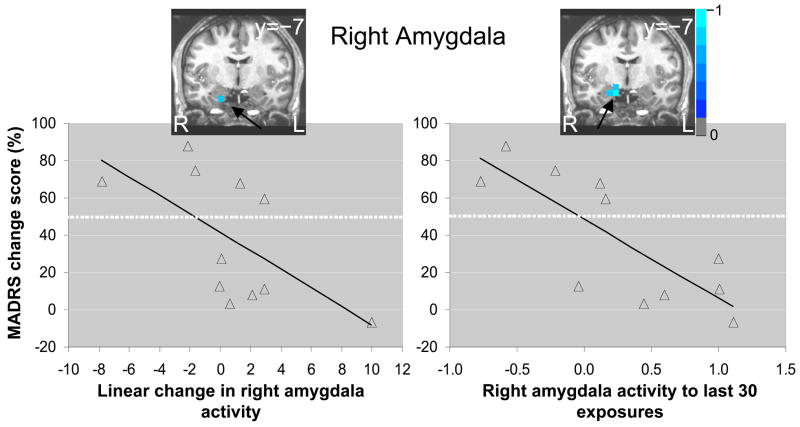
Source analyses failed to reveal group differential patterns of amygdala activity across repeated presentations. For both groups, there was an overall linear decrease in right amygdala activity between 125–175ms post-stimulus onset across repeated presentations for the average response to both faces (peak: 21, −12, −8mm, t (20) = −3.05, p < .01). Correlation analyses revealed local minima (p < .05) in right amygdala across multiple post-stimulus windows. Collapsed across post-stimulus time windows, we found that change in right amygdala activity following repeated exposure to the first fearful face was negatively correlated with antidepressant response (peak: 21, −7, −20mm, rs(9) = −.72, p < .05). Those who showed relatively larger decreases in right amygdala activity over repeated exposures also showed better improvement in depressive symptoms (Figure 3). We also observed a strong negative correlation between right amygdala activity during the last block of the exposures to the first fearful face and antidepressant response (peak: 16, −7, −14mm, rs(9) = −.82, p < .005). Similar to the ACC results, partial correlation analyses revealed that the magnitudes of negative association between right amygdala peak activations and MADRS percentage change scores remained high when controlling for HAM-A percentage change scores (linear change, r (7) = −.58, p < .10, and last block, r (7) = −.69, p < .05)
Figure 3.
Nonparametric correlation between decreased right amygdala activity (thresholded at p < .05) over repeated exposure to a fearful face and change in depressive symptoms 230 minutes after ketamine infusion (left), and for right amygdala activity to the last 30 exposures only (right). Scatterplots with least squares lines display right amygdala activity values, averaged across all post-stimulus time windows, at the location of maximal negative correlations. Images are in radiological orientation (left=right, right=left).
Discussion
Biological markers, or biomarkers, are quantitative measurements that provide information about biological processes, a disease state, or response to treatment (29). Biomarkers thus hold the potential to provide a better understanding of the etiology and pathophysiology of a complex and heterogeneous disorder like MDD; ultimately, particular target-based therapies could be matched to particular markers in subgroups of patients.
We tested the hypothesis that MEG could be used to provide a neurophysiologic biomarker associated with ketamine’s antidepressant effects. We measured ACC activity in response to rapid exposure to fearful faces in drug-free MDD patients and healthy control subjects. As expected, healthy subjects showed decreased neuromagnetic activity in the rostral ACC across repeated exposures, consistent with evidence that this region exhibits habituation to negative affective stimuli (8). MDD patients showed the opposite pattern, that is, robust increases in ACC activity over repeated exposures. Notably, we found that this increase in ACC activity in MDD patients was positively correlated with rapid antidepressant response to ketamine, an NMDA antagonist recently shown to have rapid and sustained antidepressant properties in treatment-resistant subpopulations of MDD patients (5, 6).
Mayberg and colleagues (1997) first reported that higher rostral ACC (BA 24a/b) metabolism differentiated eventual treatment responders from non-responders to conventional antidepressants at six weeks. Higher pre-treatment ACC metabolism was subsequently shown to predict antidepressant response to sleep deprivation (30) and paroxetine (3). Using fMRI, other researchers found that stronger ventral ACC response during negative emotional processing predicted antidepressant response to venlafaxine (1), while ACC activation during unsuccessful motor inhibitions predicted response to escitalopram in patients with MDD (13). Finally, theta band activity (i.e., 4–8Hz neural oscillations) in the rostral ACC, as measured by EEG, was found to predict response to the tricyclic antidepressant nortriptyline (31). The present study replicates previous findings and extends them to a novel, non-monoaminergic drug—ketamine—whose antidepressant effect occurs within hours.
The rapid antidepressant effects of ketamine have been postulated to occur via AMPA-mediated synaptic potentiation of critical neural circuits (32, 33). Increasing preclinical and clinical evidence demonstrates that synaptic plasticity, a fundamental mechanism of neuronal adaptation, is altered in mood disorders (34). A growing body of data also suggests that AMPA receptor trafficking (including receptor insertion, internalization, and delivery to synaptic sites) plays a critical role in regulating activity-dependent regulation of synaptic strength, as well as various forms of neural and behavioral plasticity (35). It is thus noteworthy that recent studies have shown that the chronic administration of antidepressants can increase synaptic AMPA GluR1 receptors (35, 36). Sleep deprivation is the only other known strategy that exerts an antidepressant effect as quickly (i.e., within hours or one day), and may share common cellular mechanisms with ketamine; Faraguna and colleagues (2008) demonstrated that sleep deprivation is also associated with enhanced AMPA-mediated synaptic plasticity (37). In toto, the data suggest that AMPA-mediated synaptic potentiation in critical circuits may play an important role in antidepressant action; ketamine and sleep deprivation bring about AMPA-mediated synaptic potentiation rapidly, whereas conventional antidepressants do so in a delayed manner, through a cascade of intracellular signaling changes (35).
In addition to ACC activity, pretreatment activity in the right amygdala may represent another useful biomarker of treatment response in patients with MDD. In the present study, amygdala response to fearful faces was negatively correlated with the antidepressant response observed 230 minutes after ketamine infusion. Lower amygdala activation was also found to predict treatment response to escitalopram and paroxetine in two previous studies of individuals with MDD (3, 13), however, the opposite association (i.e. greater pretreatment amygdala activation predicting antidepressant response) has been also observed (12, 38). Several factors could explain these divergent findings, including medication status, treatment response criteria, and duration between pretreatment measurement and symptom assessment.
Healthy subjects showed decreased neuromagnetic activity in the rostral ACC across repeated exposures to fearful faces, consistent with evidence that this region habituates to negative affective stimuli (8). The emotional salience of a fearful face may weaken upon repeated exposures, and may require less engagement of regulatory mechanisms supported by the rostral ACC, explaining the decrease in activity observed in healthy subjects. In contrast, MDD patients showed robust increases in ACC activity over repeated presentations. As the correlation analyses suggest, patients who showed significant treatment response were those driving this increased activity at the group level. These findings could reflect two key differences between MDD patients and healthy controls. First, the emotional salience of a stimulus may weaken more slowly over time in MDD patients; for instance, there is evidence that MDD patients exhibit sustained amygdala activity to negative words compared to healthy controls (39). Second, affective regulatory processes mediated by the rostral ACC may be delayed in MDD patients; that they become engaged eventually in some patients may reflect that the functional integrity of these processes is not entirely compromised. Antidepressants normalize the altered connectivity between the rostral ACC, limbic regions, and subcortical structures in individuals with MDD, suggesting indirectly that this circuit is not fully dysfunctional in treatment-responders (40, 41). Neurobiological mechanisms in patients might therefore display subtle quantitative abnormalities that are not necessarily qualitatively different from those in healthy individuals.
This study has several limitations. The lack of a placebo group might imply that the correlation between rostral ACC activation and antidepressant response is not directly related to ketamine administration; however, this is unlikely because we found a robust correlation with antidepressant response 230 minutes after ketamine infusion, a time point where we had previously showed that ketamine’s clinical effect robustly separates from placebo (6). In addition, we studied patients with very severe and treatment-resistant MDD, and these individuals had a high rate of comorbid anxiety disorders; this may limit the generalizability of our findings. The associations between rostral ACC and right amygdala activity and antidepressant response appeared independent of the concomitant anti-anxiety response observed following ketamine administration; however, the partial correlations analyses were clearly underpowered and would require a larger sample size to demonstrate the specificity of these relationships.
Finally, we did not obtain MEG measures after the administration of ketamine, so it cannot be determined here whether ketamine directly regulates rostral ACC and amygdala function in patients who display antidepressant response. However, Deakin and colleagues (2008) recently found that ketamine directly regulates orbitofrontal cortex and subgenual ACC BOLD activity in healthy individuals, suggesting a direct link with our data (42).
In conclusion, a growing number of studies suggest that targeting glutamatergically-mediated synaptic plasticity could be an effective strategy for treating MDD and other mood disorders (35). Indeed, several therapies targeting this system show substantial early promise for the treatment of mood disorders (35). Continued exploration of the antidepressant-like effects of glutamatergic drugs—like ketamine—may ultimately lead to the development of new treatments for MDD. The results presented here strongly implicate ACC dysfunction in the pathophysiology of MDD, and support the idea that pretreatment rostral ACC activation might be a useful biomarker for identifying a subgroup of patients who will respond favorably to ketamine within hours.
Acknowledgments
This study was supported by the Intramural Research Program at the National Institute of Mental Health (NIMH-NIH) and a NARSAD Award (CZ). Ioline Henter provided invaluable editorial assistance.
We would like to acknowledge the support of the Intramural Research Program of the National Institute of Mental Health. The author(s) declare that, except for income received from our primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. A patent application for the use of ketamine in depression has been submitted listing Drs. Manji and Zarate among the inventors. Drs. Manji and Zarate have assigned their rights on the patent to the US government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davidson RJ, Irwin W, Anderle MJ, Kalin NH. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- 2.Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 3.Saxena S, Brody AL, Ho ML, Zohrabi N, Maidment KM, Baxter LRJ. Differential brain metabolic predictors of response to paroxetine in obsessive-compulsive disorder versus major depression. Am J Psychiatry. 2003;160:522–532. doi: 10.1176/appi.ajp.160.3.522. [DOI] [PubMed] [Google Scholar]
- 4.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 5.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 6.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 7.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 8.Phan KL, Liberzon I, Welsh RC, Britton JC, Taylor SF. Habituation of rostral anterior cingulate cortex to repeated emotionally salient pictures. Neuropsychopharmacology. 2003;28:1344–1350. doi: 10.1038/sj.npp.1300186. [DOI] [PubMed] [Google Scholar]
- 9.Hillebrand A, Singh JB, Holliday IE, Furlong PL, Barnes GR. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapping. 2005;25:199–211. doi: 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulz M, Chau W, Graham SJ, McIntosh AR, Ross B, Ishii R, et al. An integrative MEG-fMRI study of the primary somatosensory cortex using cross-modal correspondence analysis. Neuroimage. 2004;22:120–133. doi: 10.1016/j.neuroimage.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 11.Singh KD, Barnes GR, Hillebrand A, Forde EM, Williams AL. Task-related changes in cortical synchronization are spatially coincident with the hemodynamic response. Neuroimage. 2002;16:103–114. doi: 10.1006/nimg.2001.1050. [DOI] [PubMed] [Google Scholar]
- 12.Canli T, Cooney RE, Goldin P, Shah M, Sivers H, Thomason ME, et al. Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport. 2005;16:1267–1270. doi: 10.1097/01.wnr.0000174407.09515.cc. [DOI] [PubMed] [Google Scholar]
- 13.Langenecker SA, Kennedy SE, Guidotti LM, Briceno EM, Own LS, Hooven T, et al. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biol Psychiatry. 2007;62:1272–1280. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.First MB, Spitzer RL, Gibbon M, Williams AR. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: New York State Psychiatric Institute, Biometrics Research; 2001. [Google Scholar]
- 15.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and statistical manual of mental disorders IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 17.First MB, Spitzer RL, Williams AL, Gibbon M. Structured Clinical Interview from DSM-IV (SCID) Washington, DC: American Psychiatric Association; 1995. [Google Scholar]
- 18.Hamilton MC, Schutte NS, Malouff JM. Hamilton Anxiety Scale [HAMA] In: Hamilton MC, editor. Source Book of Adult Assessment (Applied Clinical Psychology) New York: Plenum Press; 1959. pp. 154–157. [Google Scholar]
- 19.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:790–812. [Google Scholar]
- 20.Tottenham N, Tanaka J, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. doi: 10.1016/j.psychres.2008.05.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornwell BR, Baas JM, Johnson L, Holroyd T, Carver FW, Lissek S, et al. Neural responses to auditory stimulus deviance under threat of electric shock revealed by spatially-filtered magnetoencephalography. Neuroimage. 2007;37:282–289. doi: 10.1016/j.neuroimage.2007.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornwell BR, Carver FW, Coppola R, Johnson L, Alvarez R, Grillon C. Empirical evidence that amygdala activity can be resolved in magnetoencephalograms with event-related adaptive beamformers. Brain Res submitted. [Google Scholar]
- 23.Luo Q, Holroyd T, Jones M, Hendler T, Blair J. Neural dynamics for facial threat processing as revealed by gamma band synchronization using MEG. Neuroimage. 2007;34:839–847. doi: 10.1016/j.neuroimage.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekihara K, Nagarajan SS, Poeppel D, Marantz A, Miyashita Y. Reconstructing spatio-temporal activities of neural sources using an MEG vector beamformer technique. IEEE Trans Biomed Eng. 2001;48:760–771. doi: 10.1109/10.930901. [DOI] [PubMed] [Google Scholar]
- 25.Cheyne D, Bakhtazad L, Gaetz W. Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event-related beamforming approach. Hum Brain Mapping. 2006;27:213–229. doi: 10.1002/hbm.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson SE. Localization of event-related activity by SAM(erf) Neurol Clin Neurophysiol. 2004;2004:109. [PubMed] [Google Scholar]
- 27.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comp Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 28.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–78. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 29.Institute of Medicine. Workshop Summary. Washington, DC: Institute of Medicine; 2008. Neuroscience Biomarkers and Biosignatures: Converging Technologies, Emerging Partnerships. [PubMed] [Google Scholar]
- 30.Wu JC, Buchsbaum MS, Gillin JC, Tang C, Cadwell S, Wiegand M, et al. Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am J Psychiatry. 1999;156:1149–1158. doi: 10.1176/ajp.156.8.1149. [DOI] [PubMed] [Google Scholar]
- 31.Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, et al. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- 32.Maeng S, Zarate CA., Jr The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr Psychiatry Rep. 2007;9:467–474. doi: 10.1007/s11920-007-0063-1. [DOI] [PubMed] [Google Scholar]
- 33.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 34.Schloesser RJ, Huang J, Klein PS, Manji HK. Cellular plasticity cascades in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33:110–133. doi: 10.1038/sj.npp.1301575. [DOI] [PubMed] [Google Scholar]
- 35.Sanacora G, Zarate CA, Jr, Krystal JH, Manji HK. Targeting the glutamatergic dystem to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, et al. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology. 2007;32:793–802. doi: 10.1038/sj.npp.1301178. [DOI] [PubMed] [Google Scholar]
- 37.Faraguna U, Vyazovskiy VV, Nelson AB, Tononi G, Cirelli C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci. 2008;28:4088–4095. doi: 10.1523/JNEUROSCI.5510-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark CP, Brown GG, Archibald SL, Fennema-Notestine C, Braun DR, Thomas LS, et al. Does amygdalar perfusion correlate with antidepressant response to partial sleep deprivation in major depression? Psychiatry Res. 2006;146:43–51. doi: 10.1016/j.pscychresns.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- 40.Anand A, Li Y, Wang Y, Wu JC, Gao S, Bukhari L, et al. Antidepressant effect on connectivity of the mood-regulating circuit: an FMRI study. Neuropsychopharmacology. 2005;30:1334–1344. doi: 10.1038/sj.npp.1300725. [DOI] [PubMed] [Google Scholar]
- 41.Anand A, Li Y, Wang Y, Wu JC, Gao S, Bukhari L, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 42.Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry. 2008;65:154–164. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]