Abstract
Objective
Hypertonic saline resuscitation reduces tissue damage by inhibiting polymorphonuclear neutrophils. Hypertonic saline triggers polymorphonuclear neutrophils to release adenosine triphosphate that is converted to adenosine, inhibiting polymorphonuclear neutrophils through A2a adenosine receptors. polymorphonuclear neutrophils also express A3 adenosine receptors that enhance polymorphonuclear neutrophils functions. Here we investigated whether A3 receptors may diminish the efficacy of hypertonic saline in a mouse model of acute lung injury.
Design
Randomized animal study and laboratory investigation.
Setting
University research laboratory.
Interventions
The effect of A3 receptors on the efficacy of hypertonic saline resuscitation was assessed in A3 receptor knockout and wild-type mice. Animals were treated with hypertonic saline (7.5% NaCl, 4 mL/kg) before or after cecal ligation and puncture, and acute lung injury and mortality were determined. The effect of timing of hypertonic saline exposure on A3 receptor expression and degranulation was studied in vitro with isolated human polymorphonuclear neutrophils.
Measurements and main results
Treatment of human polymorphonuclear neutrophils with hypertonic saline before stimulation with formyl methionyl-leucyl-phenylalanine inhibited A3 receptor expression and degranulation, whereas hypertonic saline-treatment after formyl methionyl-leucyl-phenylalanine-stimulation augmented A3 receptor expression and degranulation. Acute lung injury in wild-type mice treated with hypertonic saline after cecal ligation and puncture was significantly greater than in wild-type mice pretreated with hypertonic saline. This aggravating effect of delayed hypertonic saline-treatment was absent in A3 receptor knockout mice. Similarly, mortality in wild-type mice with delayed hypertonic saline-treatment was significantly higher (88%) than in animals treated with hypertonic saline before cecal ligation and puncture (50%). Mortality in A3 receptor knockout mice remained only 50% regardless of timing of hypertonic saline administration.
Conclusions
Polymorphonuclear neutrophils A3 receptors expression determines whether hypertonic saline resuscitation inhibits or aggravates polymorphonuclear neutrophils-induced acute lung injury. These findings suggest that A3 antagonists could improve the efficacy of hypertonic saline resuscitation by reducing side effects in patients whose polymorphonuclear neutrophils are activated before hypertonic saline treatment.
Keywords: polymorphonuclear neutrophils, A3 adenosine receptor, sepsis, acute lung injury, hypertonic saline resuscitation
Polymorphonuclear neutrophils (PMN) play a vital role in the immune defense against invading microorganisms, for example, in patients after severe trauma or major surgical operations (1). However, PMN can harm patients by the excessive release of cytotoxic mediators that damage host tissues and result in major posttraumatic complications such as acute lung injury, acute respiratory distress syndrome, and multiple organ failure syndrome (2, 3).
Since the early 1980s, interest in the concept of small-volume resuscitation with hypertonic saline (HS), typically using 4 mL/kg body weight of 7.5% NaCl solutions, has been driven by logistic considerations as well as by the superior hemodynamic effects of HS compared with isotonic crystalloids (4–6). The interest in HS resuscitation was further advanced by our reports that hypertonic conditions can regulate leukocyte function and prevent PMN-induced lung tissue damage (7–10), as well as by the confirmation of these findings by several other research groups (11–13). Various animal models and in vitro experiments with isolated human PMN have shown that HS can significantly reduce the risk of posttraumatic complication by inhibiting excessive PMN activation (7, 14–16). In addition to these inhibitory effects of HS, we and others found that HS can also enhance PMN functions when HS-treatment occurred after stimulation of PMN, for example, with formyl methionyl-leucyl-phenylalanine (fMLP) (8, 13, 15, 17, 18). Using a mouse model of hemorrhagic shock, Murao et al. (15) reported that HS resuscitation prevents lung damage only when HS is administered for initial resuscitation fluid, while delayed HS resuscitation aggravated lung tissue damage. Hashiguchi et al. (18) studied the effects of timing of HS-treatment in vitro using whole blood samples from trauma patients and found that early treatment with HS is important for effective inhibition of PMN activation. However, in the clinical realm, the treatment of patients with HS before the activation of PMN is not always possible, primarily because it is usually unknown to the trauma team whether, when, and in which patient subpopulations PMN are activated. Furthermore, the generalized treatment of trauma patients with HS may carry the risk of aggravating tissue damage in certain trauma patient subpopulations. Thus, a better understanding of how HS can enhance PMN function could help with the design of improved hypertonic resuscitation regimens that minimize the risk of negative side effects.
We have previously found that HS induces the rapid release of cellular adenosine triphosphate (ATP) from PMN and that the released ATP is degraded to adenosine, which activates A2a adenosine receptors that block PMN activation through cAMP-mediated signal pathways (8, 17, 19–21). This inhibitory effect of HS was lost when previously stimulated PMN were treated with HS, possibly by the opposing actions of other adenosine receptor subtypes or of ATP receptors (8, 17, 20, 21).
Here, we investigated the roles of A3 adenosine receptors in the effects of HS on PMN in vitro and in neutrophil-mediated lung tissue damage in a mouse model of peritoneal sepsis.
MATERIALS AND METHODS
Isolation of Human Polymorphonuclear Neutrophils
The Institutional Review Board (IRB) of the Beth Israel Deaconess Medical Center approved all experiments described in this study. Peripheral venous blood was obtained from healthy human volunteers and PMN were isolated as described previously (21). Briefly, heparinized blood was mixed with 5% (w/v) Dextran-500 (Pharmacia, Piscataway, NJ) dissolved in a normal saline solution and allowed to sediment for 30 min at room temperature. The cells in the supernatant were separated by Percoll (Amersham Biosciences, Piscataway, NJ) gradient centrifugation and washed with Hanks’ balanced salt solution (HBSS; Irvine Scientific, Santa Ana, CA). PMN were suspended in fresh HBSS and used for experiments immediately. Cell isolation and all subsequent experiments were performed under sterile and pyrogen-free conditions.
Degranulation Assay
PMN (106) were preincubated for 10 min at 37°C with 5 μM cytochalasin B, as described previously (22) and the cells were then stimulated with 10 nM N-fMLP with or without HS. Cells were stimulated with HS by adding appropriate dilutions of 1M NaCl in HBSS, raising the tonicity of the cell cultures by 40 mM beyond isotonicity, a level of hypertonicity that can results from the infusion of hypertonic resuscitation fluids in vivo. This level of 40 mM hypertonicity was added either 5 min before fMLP-stimulation or 5 or 10 min after fMLP-stimulation and elastase release was determined as a measure of PMN degranulation. After stimulation under these conditions for 1 h at 37°C, cells were placed on ice for 10 min, centrifuged at 16,000 × g for 10 sec, and elastase activity released into the supernatant was measured as described previously (17, 23). Briefly, 40 μL of supernatant was mixed with 160 μL of a buffer consisting of 50 mM Tris/HCl and 100 mM NaCl, pH 7.4, containing 0.05% (v/v) Triton X-100. Enzymatic reactions were started by the addition of the elastase-specific chromogenic substrate N-methoxysuccinyl-(L-alanyl)2-L-prolyl-L-vaniline 4-nitroanilide (Sigma, St. Louis, MO) at a final concentration of 1 mM. After 30 min at room temperature, the change in optical density was measured at a wavelength of 405 nm.
A3 Adenosine Receptor Expression
Our previous work showed that treatment of PMN with HS causes the release of cellular ATP, which is rapidly converted to adenosine (19). We found that adenosine and autocrine feedback through A2a or A3 adenosine receptors expressed by PMN can down-regulate or up-regulate PMN degranulation, respectively (17). Thus, the availability of A3 receptors on the cell surface of PMN may play an important role in determining whether or not HS can enhance PMN degranulation.
Expression of the A3 adenosine receptors on the cell surface of human PMN was determined by immunofluorescence staining. PMN were allowed to adhere to glass coverslips. Then, the cells were treated with 40 mM HS either 10 min before or 10 min after stimulation with 10 nM fMLP, and A3 receptor expression on the cell surface was determined 20 min after the addition of fMLP. Cells were fixed by adding 3.7% paraformaldehyde in HBSS for 10 min at room temperature, followed by incubation with 10% human serum in HBSS for 60 min. Then, the cells were incubated for 60 min at room temperature with a rabbit anti-human A3 adenosine receptor antibody (20 μg/mL; Alpha Diagnostic, San Antonio, TX) that recognizes an extracellular receptor domain, followed by an additional 30 min with Alexa Fluor 488-labled goat anti-rabbit IgG antibody (1:1000 dilution; Invitrogen, Eugene, OR). Control cells were treated identically except that primary A3 receptor antibody was replaced with unrelated immunoglobulin G. Images were captured with a Leica DM-IRB inverted fluorescence microscope system equipped with an Openlab imaging system (Improvision, Waltham, MA) that permitted the semiquantitative analysis of receptor expression using maximum fluorescence intensity values of five different images with 20 to 25 cells each.
Mouse Sepsis Model
The use of laboratory animals was in accordance with National Institutes of Health guidelines and was approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center. Sepsis was induced by cecal ligation and puncture (CLP) as described by Baker et al. (24) using 8 to 10 wk old (20–25 g) male C57BL/6J wild-type (WT) mice (Jackson Laboratory, Bar Harbor, ME) or homozygous A3−/− mice A3 receptor knockout (A3KO) (generous gift from Dr. Francisco Villarreal, University of California San Diego, San Diego, CA) that have the same genetic background as the C57BL/6J mice.
WT or A3KO mice were anesthetized with isoflurane vapor. After or before sepsis was induced with CLP, resuscitation fluids were administered through a catheter placed into the right femoral artery. In brief, the right inguinal area was shaved, disinfected with povidone-iodine, and an incision of about 3 mm in length was made. Connective tissue was gently dissected to expose the femoral artery, which was ligated distally using a 5-0 suture. Holding the proximal side, the artery was cut with a No. 10 scalpel blade and a PE 10 catheter (Becton Dickinson, Sparks, MD) was inserted.
Mice were restrained in supine position and a 1-cm midline incision was performed. The cecum was exposed and ligated distal to the ileocecal valve with 4-0 suture, avoiding intestinal blockade. The cecum was punctured twice with a 22-G needle, returned into the abdomen, and the abdominal wall was closed in layers. After full recovery, the mice were returned to their cages and allowed access to standard diet and water ad libitum.
The WT animals were randomly divided into the following treatment groups with six animals in each group: 1) mice that did not receive any treatment except anesthesia before kill (control group); 2) sham animals that underwent cannulation and laparotomy without sepsis or resuscitation (Sham group); 3) untreated animals that underwent cannulation and CLP but did not receive resuscitation (CLP); 4) mice treated with HS before CLP received 4 mL/kg of 7.5% NaCl 5 min before CLP (HS→CLP group); 5) mice that received 4 mL/kg HS 15 min after CLP (CLP→HS 15 min); and 6) mice that received 4 mL/kg HS 60 min after CLP (CLP→HS). The dose of 4 mL/kg of 7.5% NaCl was used because this dose is most commonly administered in clinical trauma care. To determine how A3 receptors affect the effectiveness of HS-treatment in this mouse model of sepsis, we subjected the A3 receptor deficient (A3KO) mice to the same HS-treatments described above.
Neutrophil Elastase and Myeloperoxidase Activity Measurements
Mice were subjected to CLP before or after HS-treatment and PMN accumulation in the lungs was assessed 2 h after CLP by measuring activities of neutrophil elastase (NE) and myeloperoxidase (MPO) in lung tissue homogenates. Animals were anesthetized as described above and killed by inhalation of high doses of isoflurane. The pulmonary circulation was flushed with 10 mL cold HBSS through right ventricle and the whole lung was carefully removed, snap-frozen in liquid nitrogen, and stored at −80°C until analysis.
Lungs were weighed and placed in 5 mL cold 50 mM potassium phosphate buffer, pH 6.0, containing 0.5% hexadecyl-trimethyl-ammonium bromide (Sigma, St. Louis, MO). Tissues were homogenized on ice using a tissue homogenizer. The homogenates were centrifuged at 20,000 ×g and 4°C for 30 min, and the supernatants were used for the measurement of enzyme activities.
Tissue homogenates (20 μL) were added in duplicates into a 96-well plate. For the elastase assay, 80 μL of a buffer consisting of 50 mM Tris/HCl and 100 mM NaCl, pH 7.4, containing 0.05% Triton X-100 (v/v) was added to each well, and enzymatic reactions were started by the addition of the elastase-specific chromogenic substrate N-methoxysuccinyl-(L-alanyl)2-L-prolyl-L-vaniline 4-nitroanilide (Sigma, St. Louis, MO) at a final concentration of 1 mM. After 30 min at room temperature, the change in optical density was measured at a wavelength of 405 nm using a plate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA). MPO activity was measured as a back-up. Lung tissue homogenates (20 μL) were incubated with 80 μL of a 50-mM phosphate buffer (pH 6.0) containing 0.0005% hydrogen peroxide (v/v) and 0.167 mg/mL of the MPO substrate o-dianisidine dihydrochloride (Sigma, St. Louis, MO) and changes in absorbance were measured at a wavelength of 450 nm. MPO activity was determined using a standard curve generated with known amounts of MPO (Sigma, St. Louis, MO). Protein concentrations of tissue homogenates were determined with the BCA protein assay kit (Pierce Biotechnology, Rockford, IL).
Lung Histology
Lungs from each treatment group were obtained 24 h after the CLP to study histologic features of acute lung injury. Animals were anesthetized and killed as described above. The trachea was exposed, cannulated with a dull 23-G needle (Monoject, Tyco Health Care Group, Mansfield, MA), and 0.5 mL of fixative (10% paraformaldehyde in HBSS) was instilled. The pulmonary circulation was flushed with 10 mL HBSS through the right ventricle and the whole lung was carefully removed. Lungs were immersed in fixative solution for 72 h, dehydrated with graded alcohol, and embedded in paraffin. A series of 4-μm thick tissue slices was obtained and stained with hematoxylin and eosin (H & E), and examined under the microscope.
Survival Study
A separate set of 8 WT or A3KO mice per group was used to evaluate how the different treatments affected survival after sepsis in WT and A3 receptor deficient animals. WT or A3KO mice were subjected to HS and CLP treatment as described above and differences in survival between mice treated with HS before CLP vs. mice treated with HS after CLP were evaluated. As control, a group of WT mice was included that received an equal volume of isotonic fluid (Ringers’ lactate, RL) instead of HS before CLP. The surgical procedures in these animals were identical to those described above, except that no tissue samples were harvested. Survival was monitored for 100 h and then any remaining mice were euthanized as described above.
Statistical Analyses
All values are expressed as mean ± standard deviation (SD). Statistical analyses were performed with ANOVA followed by Bonferroni test or Kaplan-Meier analysis. Differences between groups were considered statistically significant at p < 0.05.
RESULTS
Treatment of PMN With HS After fMLP-Stimulation Increases Degranulation
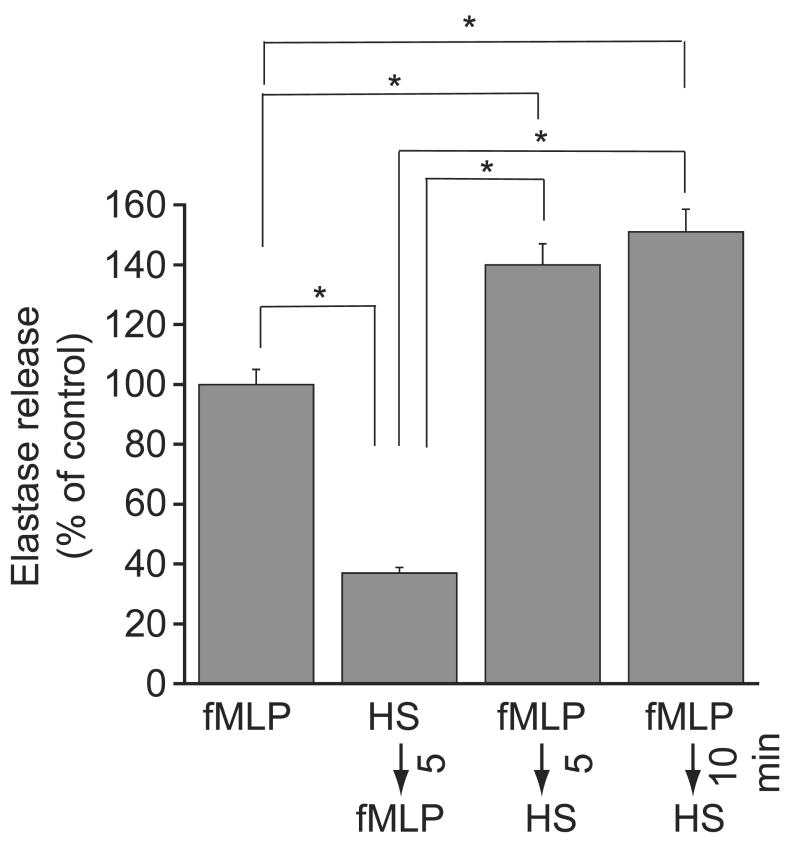
We examined how differences in the timing of HS-treatment influence degranulation of purified human PMN in vitro. Treatment of cells with HS before fMLP-stimulation significantly inhibited degranulation (Fig. 1). However, treatment with HS either 5 or 10 min after fMLP-stimulation significantly increased degranulation beyond fMLP-stimulated control values.
Figure 1.
Effect of timing of HS-treatment on PMN degranulation in vitro. Purified human PMN were stimulated with 10 nM fMLP and cells were treated with 40 mM HS either 5 min before fMLP-stimulation, or 5 or 10 min after fMLP-stimulation. Neutrophil degranulation was determined by measuring neutrophil elastase activity released into the supernatants after another 60 min. Data are expressed as the percentage of degranulation of control cells that were stimulated with fMLP alone. Each data point represents aggregate findings with PMN from four different healthy volunteers tested in triplicate. Values are expressed as mean ± SD. Statistical differences among groups are indicated by asterisks (ANOVA and Bonferroni test, *p < 0.0001).
Delayed HS-Treatment Increases Neutrophil Accumulation in the Lung After Sepsis
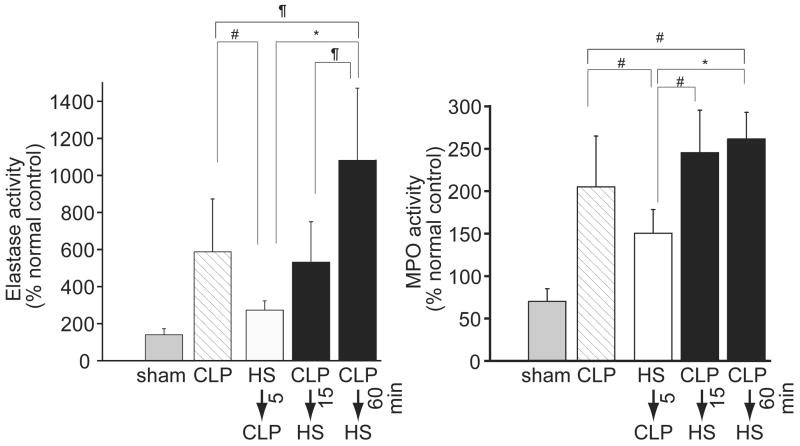
We used a mouse sepsis model to evaluate how differences in the timing of HS administration affect acute lung injury and inflammation. NE and MPO activities in the mice treated with HS before CLP were significantly lower than in untreated mice that received CLP (Fig. 2). Delayed administration of HS resulted in a time-dependent increase in lung inflammation as suggested by increased NE activity levels. NE activity levels were significantly higher in mice treated with HS 60 min after CLP compared with all other treatment groups. MPO activity was significantly increased even when HS was given as early as 15 min after CLP. These results suggest that treatment with HS before the onset of sepsis can attenuate inflammation and PMN accumulation in the lungs, whereas delayed treatment with HS exaggerates PMN influx into the lungs.
Figure 2.
Effect of timing of HS-treatment on PMN influx in the lungs after sepsis. Wild-type mice were subjected to sham operation or cecal ligation and puncture (CLP) and treated with HS at different time points relative to CLP. Neutrophil elastase activity (panel A) or myeloperoxidase activity (MPO, panel B) was measured in lung tissue homogenates 2 h after CLP. Data are expressed as percentage of normal healthy control animals that did not undergo surgery. Values represent mean ± SD of 6 animals in each group. Statistical differences were evaluated with ANOVA and Bonferroni test (*p < 0.0001, ¶p < 0.01, #p < 0.05).
Timing of HS-Treatment Modulates A3 Receptor Expression of PMN
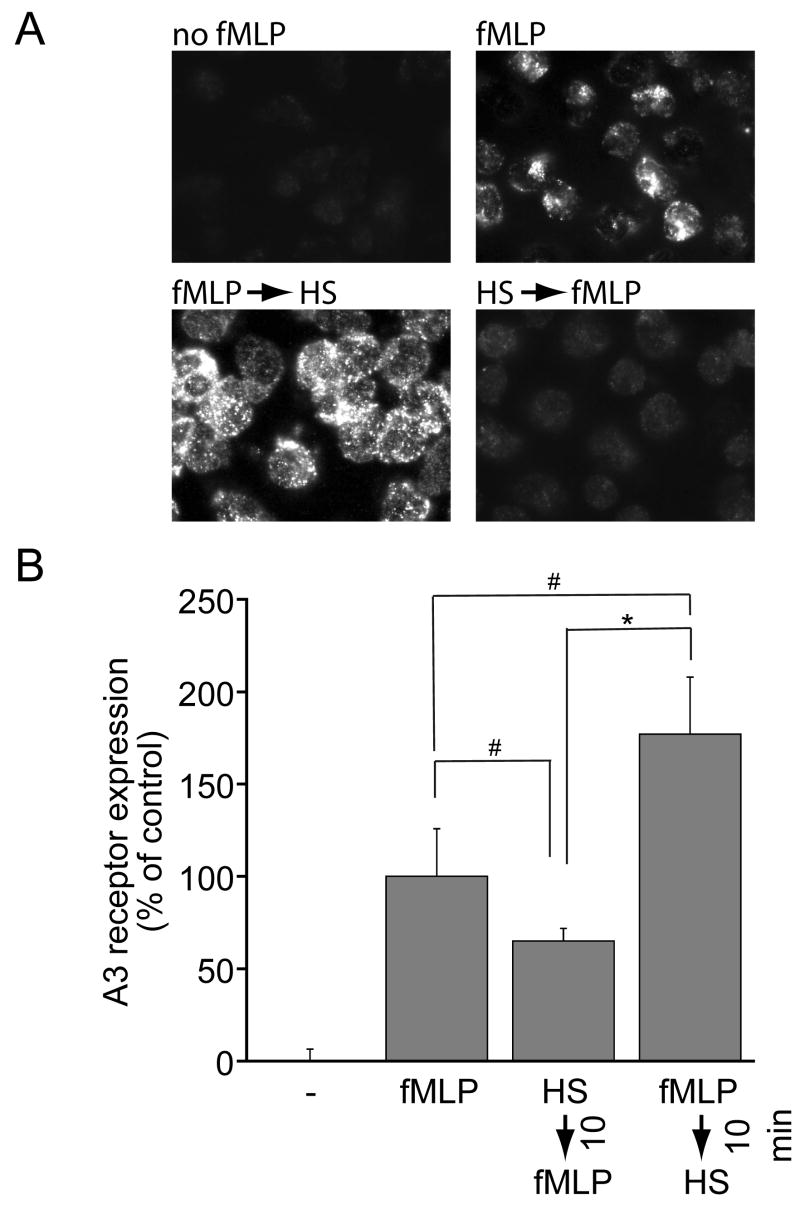
We studied A3 receptor expression of PMN that were treated with HS either before or after stimulation with fMLP. A3 receptor expression on the surface of PMN increased rapidly after stimulation with 10 nM fMLP (Fig. 3). We found that treatment with 40 mM HS 10 min before fMLP-stimulation partially inhibited A3 receptor expression. In contrast, delayed treatment with 40 mM HS 10 min after stimulation of PMN with fMLP markedly increased A3 receptor expression on the cell surface of PMN. These findings show that the opposing effects of HS correlate with differences in the cell surface expression of A3 adenosine receptors, suggesting that the enhancing effects of HS are a result of enhanced A3 receptor expression of previously stimulated PMN.
Figure 3.
Effect of timing of HS-treatment on A3 adenosine receptor expression on the cell surface of PMN. Purified human PMN were treated with 40 mM HS either 10 min before or after stimulation with fMLP and A3 adenosine receptor expression on the cell surface was evaluated using fluorescence microscopy 20 min after fMLP addition (panel A). A3 receptor expression was semiquantitatively evaluated using Openlab image analysis software (panel B). Values represent mean ± SD of highest expression levels (gray values of pixels) of cells in five images acquired for each treatment group with >20 cells in each image. Statistical differences among groups were evaluated with ANOVA and Bonferroni test and are indicated by asterisks (*p < 0.0001, #p < 0.05).
Delayed HS-Treatment Does not Increase the Influx of PMN Into the Lungs of A3 Adenosine Receptor Knockout Mice
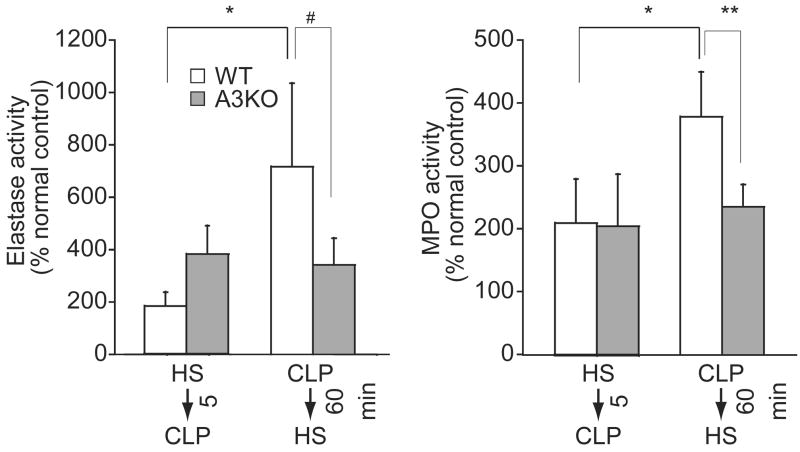
The above findings suggest that A3 adenosine receptors play an important role in the enhancing effects of HS on PMN functions. To evaluate the role of A3 receptors in the aggravating effects of delayed HS-treatment, we compared the responses of WT and A3KO mice to HS-treatment in a model of sepsis-induced acute lung injury. Delayed HS-treatment significantly increased PMN recruitment indicated by increased NE and MPO values in the lungs of WT mice but not of A3KO mice (Fig. 4). These findings suggest that A3 receptors are required for the aggravating effects of delayed HS-treatment on PMN accumulation and inflammation in the lungs of septic mice.
Figure 4.
Effect of timing of HS-treatment on inflammation in wild-type mice (WT) and A3 receptor knockout mice (A3KO) mice. WT or A3KO mice were treated with 4 mL/kg HS 5 min before or 60 min after CLP and elastase (left panel) and MPO activity (right panel) in lung homogenates was measured as a marker of lung inflammation and PMN accumulation. Data are expressed as percentage of values measured in untreated healthy control animals. Values represent mean ± SD of six animals in each group. Statistical analysis was performed with ANOVA and Bonferroni test (*p < 0.001, **p < 0.01, #p < 0.05).
Delayed HS-Treatment Increases Mortality in WT but not in A3 Knockout Mice
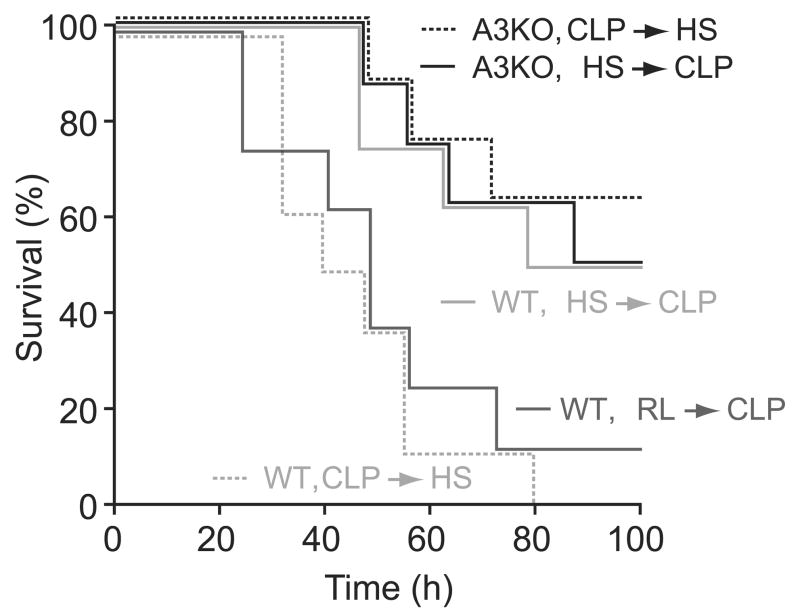
Next, we tested if delayed HS-treatment can increase mortality after sepsis. WT and A3KO mice were treated with HS before or after CLP as described above, and survival was monitored for 100 h (Fig. 5). Survival in WT mice treated with HS before CLP was significantly improved compared with controls treated with equal volumes of isotonic fluid (RL). On the other hand, survival in WT mice treated with HS after CLP was significantly lower than in WT mice treated with HS before CLP, none of which survived longer than 80 h (p < 0.05). Although just 12.5% of the isotonic controls (RL group) survived beyond 80 h, HS-treatment before CLP increased survival to 50%. These results indicate that HS-treatment can benefit animals when HS is administered before the onset of CLP; however, HS can have detrimental effects when it is administered after the onset of sepsis. Survival in A3KO mice that were treated with HS after CLP was significantly higher than that in their WT counterparts (p < 0.05). Therefore, the detrimental effects of delayed HS-treatment seem to be completely abolished in the absence of A3 adenosine receptors.
Figure 5.
Effect of timing of HS-treatment on survival in wild-type (WT) and A3 receptor knockout mice (A3KO) mice. WT mice or A3KO mice were treated with 4 mL/kg HS either 5 min before or 60 min after CLP and survival was evaluated for 100 h after CLP. WT mice treated with 4 mL/kg Ringer’s lactate (RL) 5 min before CLP served as controls. The survival curves in each group (n = 8) were analyzed using Kaplan-Meier statistics. At the 72-h time point, survival in WT mice that received delayed HS-treatment was significantly lower than in controls, WT mice treated with HS before CLP, and in A3KO mice, regardless of the timing of HS-treatment. Survival in all groups, except in WT mice with delayed HS-treatment, was significantly higher compared with RL controls (p < 0.05).
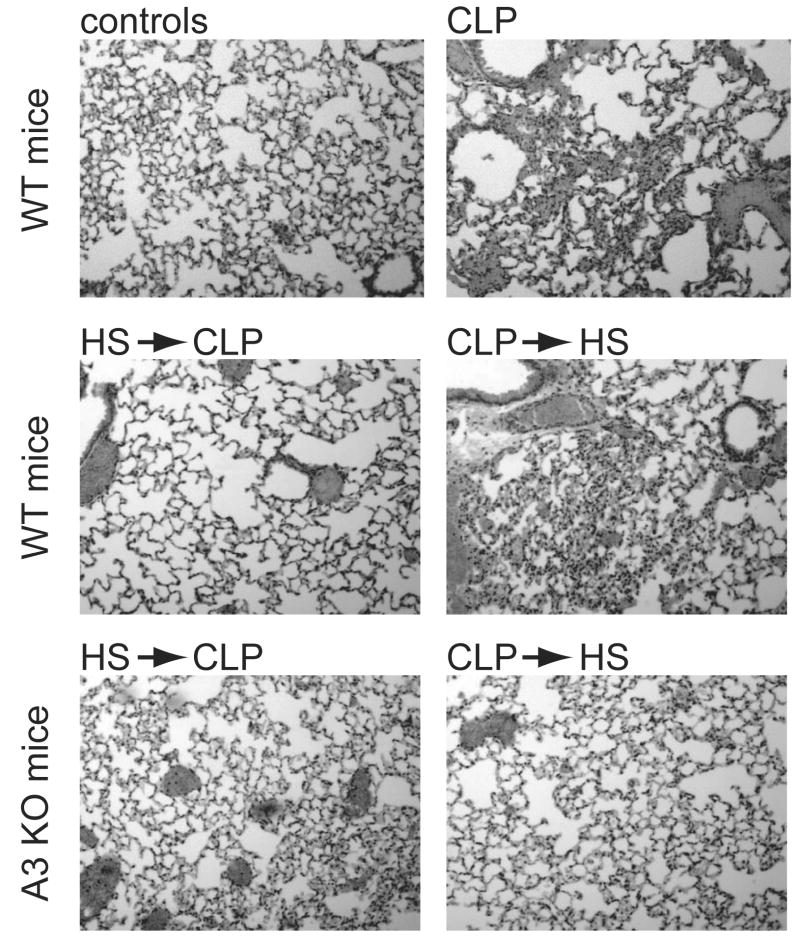
Representative lung tissue samples were obtained from an additional set of animals to evaluate lung tissue damage. Lung sections of WT mice subjected to CLP showed characteristic abnormalities indicative of acute lung injury, including focal atelectases, hyperemia with congestion, infiltration of PMN into widened alveolar septae, and alveolar spaces containing fluid, debris, and inflammatory cells (Fig. 6). These characteristics of acute lung damage were more severe in WT mice that received HS after CLP compared with mice that received HS before CLP. In comparison with WT mice, tissue damage was slighter in A3KO mice treated with HS before or after CLP.
Figure 6.
Lung histology samples of WT and A3KO mice treated with HS before or after CLP. Representative micrographs of histologic sections of lung tissue obtained from WT or A3KO mice 24 h after CLP. Representative tissue sections stained with H & E from WT control mice (WT control) and untreated WT mice (WT CLP), and WT or A3KO mice treated with HS either 5 min before or 60 min after CLP are shown (100-fold magnification).
DISCUSSION
Our study shows that early HS-treatment can reduce lung tissue damage, whereas delayed HS-treatment can significantly increase PMN accumulation in the lungs and aggravate acute lung tissue injury after sepsis. Moreover, mortality after delayed treatment with HS was also significantly higher compared with early HS-treatment. In addition, we found that A3 adenosine receptors play an important role in these undesirable effects of HS-treatment and that exposure of isolated human PMN to comparable levels of hypertonicity regulates the expression A3 receptors on the cell surface. We have previously reported that HS inhibits PMN functions when HS is given before or at the same time of fMLP-stimulation, whereas HS can enhance PMN degranulation when the cells are exposed to HS after stimulation with fMLP (7). Here, we confirm these findings in in vivo experiments using CLP as a trigger of PMN activation and acute lung tissue damage and mortality as outcome measures.
We have previously studied the mechanisms by which HS regulates PMN function. These studies revealed that HS-induced cell shrinkage results in the release of ATP from PMN and other cells. The released ATP controls PMN function either directly through P2 receptors or indirectly by the activation of a set of adenosine receptors expressed by PMN (8, 17). We have found that PMN express predominantly the A2a and A3 adenosine receptor subtypes and that PMN are able to rapidly convert extracellular ATP to adenosine using either ecto-ATPases or released enzymes that can hydrolyze ATP to adenosine (21). A2a receptors inhibit fMLP-stimulated PMN degranulation, whereas A3 receptors enhance this response (17). It is therefore likely that HS can control PMN functions by the opposing effects of these two adenosine receptor subtypes.
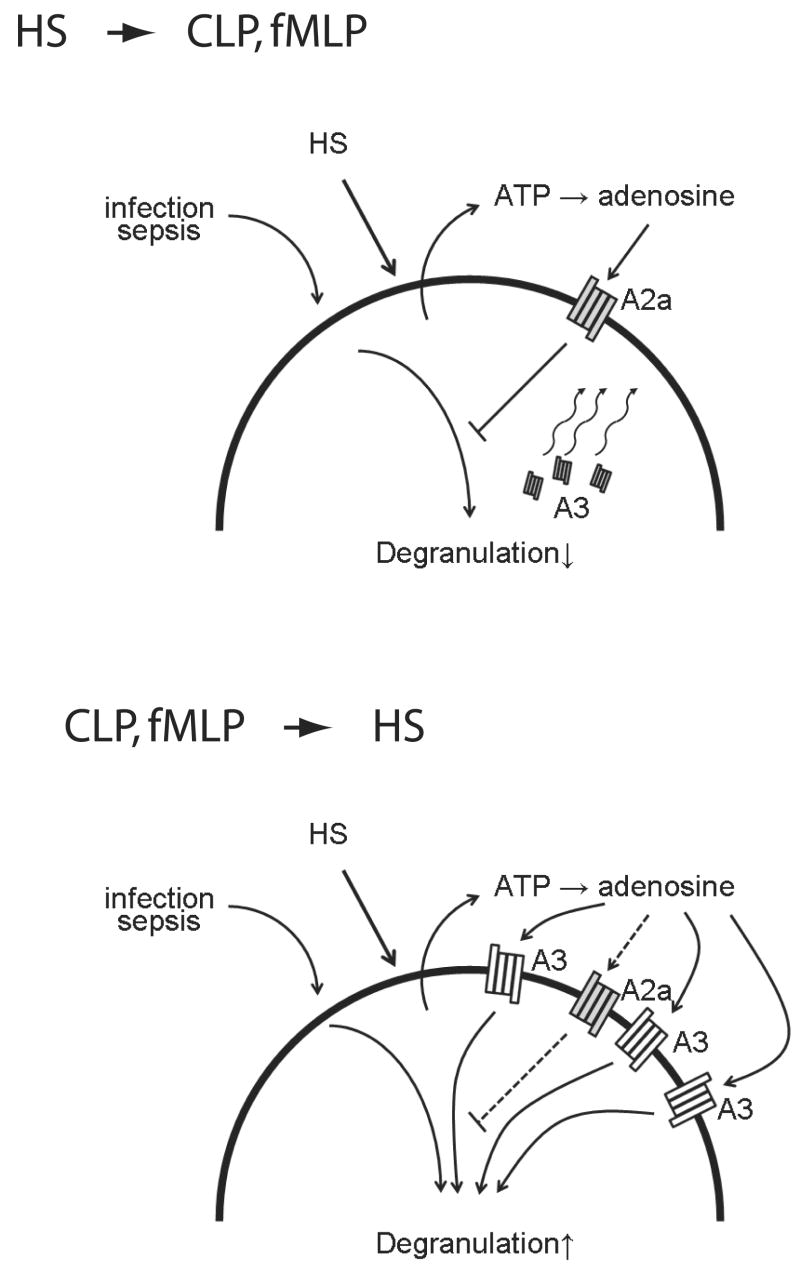
Our current data show that PMN stimulation causes rapid expression of A3 receptors on the cell surface of PMN. The mechanism through which A3 receptors are expressed is not known, however, it is likely that fusion of granule membranes containing A3 receptors with the cell surface is involved in this process. Our results showed that pretreatment of PMN with HS inhibited A3 receptor expression, whereas HS-treatment of previously stimulated PMN increased A3 receptor expression. These findings suggest that HS-treatment may shift the ratio of the co-stimulatory A3 receptors and the inhibitory A2a receptors in a manner that could account for the observation that pretreatment of cells with HS augments PMN responses (Fig. 7).
Figure 7.
Proposed model of the mechanisms through which HS regulates PMN functions. The results of our present and previous studies suggest that HS regulates PMN function via the release of ATP, production of extracellular adenosine, and the activation of suppressive A2a adenosine receptors and stimulatory A3 adenosine receptors, which blunt or aggravate PMN functions, respectively. Although treatment with HS before stimulation of PMN activates predominantly A2a receptors, late treatment with HS leads increasingly to the activation of A3 receptors, which are expressed on the cell surface in response to cell stimulation. Thus, the balance between A2a and A3 receptors seems to control whether HS-treatment inhibits or augments PMN function and tissue damage in trauma patients treated with HS resuscitation.
In support of the concept that A3 receptors play an important role in the up-regulation of PMN responses by HS, we found that delayed HS-treatment did not enhance PMN accumulation in the lungs of A3KO mice. These findings indicate that A3 receptors are required for the aggravating effects of HS, but not for its inhibitory effects when administered before PMN activation.
We found that PMN accumulation in the lungs increased in a time-dependent fashion when HS was added after CLP. These findings correspond with the results of our in vitro experiments using human PMN (Fig. 2), indicating that the addition of HS after initial stimulation with fMLP enhances degranulation. Moreover, previous work by our group has revealed similar findings in a mouse model of hemorrhagic shock (16) and in ex vivo experiments performed with blood samples obtained from trauma patients (18). All these findings can be explained by the results of the current study that show that the timing of HS-treatment modulates A3 receptor expression on the cell surface of PMN.
Inflammation leading to the influx of PMN into the lungs and subsequent development of acute lung injury and multiple organ failure are key contributors to the morbidity and mortality of intensive care patients (2, 3). This sequence of events is seems to be paralleled in our current study. Our findings suggest a role for A3 receptors in the sequestration of PMN into the lungs of mice that were treated with HS after the onset of sepsis. However, when interpreting our present findings, other possible systemic effects of HS should also be taken into consideration. Besides its immunomodulatory effects on PMN, HS also influences a number of hemodynamic (25), it affects adhesion molecule expression (26), HS has well-described effects on T cell functions (7, 27), red blood cells, platelets, and coagulation factors (26, 28–30), and HS influences vasopressin (31), and other endocrine systems (32). Along with our findings, the sum of all these other effects of HS is likely to determine the clinical efficacy of HS resuscitation in septic conditions. Indeed, previous studies have reported that HS resuscitation ameliorated organ damage and improved survival (32–34). These reports seem to be in conflicts with the findings of our current study, which indicates that delayed HS-treatment aggravates lung damage when HS is administered after the onset of sepsis. Further studies are needed to clarify the reasons for this discrepancy. However, many factors are likely responsible, including the type, momentum, and severity of sepsis, the nature of the underlying microbial flora, the focal point of sepsis and the rate of spreading, as well as the dosage and timing of HS-treatment relative to the onset and the characteristics of sepsis described above. Dose and timing of HS-treatment are other intangible quantities that depend, for example, on the infusion rate. Differences between in vitro and in vivo HS-treatments must also be taken into consideration in our current study. Although the level of hypertonicity in vitro was chosen to resemble plasma hypertonicity levels after HS infusion in vivo, both treatments still differ significantly, as the latter results in transient effects due to rapid fluid shifts after HS infusion. Thus, further studies should focus on establishing ideal treatment modalities such as the most appropriate dosages of HS to improve beneficial effects, while reducing the risks of potential negative side such as those identified in our study. The possibility of species-related differences in the adenosine receptor responses of mouse and human PMN must be considered and future studies should be designed to take such differences into account.
We believe that our current findings are of immediate clinical importance, as hypertonic resuscitation fluids are increasingly used to treat trauma patients in military and civilian settings. The use of these fluids, however, may carry the risk of excessively activating PMN, leading to aggravated tissue damage and posttraumatic complications in a subset of trauma patients with activated PMN. In the clinical reality, especially in patients prone to sepsis, it is likely that HS could be detrimental. Unfortunately, at the present time it is not easily discernable which patients are likely to benefit from HS-treatment and which patients or subgroups of patients may be at risk of increased side effects brought on by the ability of HS to aggravate tissue damage caused by previously activated PMN. Based on our findings, feedback through A3 receptors that are expressed on the cell membrane of PMN seems to play an important role in the enhancing effects of HS. Thus, pharmaceutical approaches to block A3 receptors may be a suitable approach to improve the clinical efficacy of HS resuscitation by preventing possible negative side effects in the subpopulation of patients in whom PMN may be activated before HS resuscitation can be administered.
Acknowledgments
Supported, in part, by a Pfizer Fellowship (Y.I.), by National Institutes of Health Grants R01 GM 51477, GM-60475 (W.G.J.), and CDMRP Grant PR043034 (W.G.J.), and by a Novo Nordisk Fellowship (Y.C.).
Footnotes
The authors have not disclosed any potential conflicts of interest.
References
- 1.Botha AJ, Moore FA, Moore EE, et al. Early neutrophil sequestration after injury: A pathogenic mechanism for multiple organ failure. J Trauma. 1995;39:411–417. doi: 10.1097/00005373-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 3.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 4.Velasco IT, Pontieri V, Rocha-e-Silva M, et al. Hyperosmotic NaCl and severe hemorrhagic shock. Am J Physiol. 1980;239:H664–H673. doi: 10.1152/ajpheart.1980.239.5.H664. [DOI] [PubMed] [Google Scholar]
- 5.Velasco IT, Rocha e Silva M, Oliveira MA, et al. Hypertonic and hyperoncotic resuscitation from severe hemorrhagic shock in dogs: A comparative study. Crit Care Med. 1989;17:262–264. doi: 10.1097/00003246-198903000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Younes RN, Aun F, Tomida RM, et al. The role of lung innervation in the hemodynamic response to hypertonic sodium chloride solutions in hemorrhagic shock. Surgery. 1985;98:900–906. [PubMed] [Google Scholar]
- 7.Junger WG, Liu FC, Loomis WH, et al. Hypertonic saline enhances cellular immune function. Circ Shock. 1994;42:190–196. [PubMed] [Google Scholar]
- 8.Junger WG, Hoyt DB, Davis RE, et al. Hypertonicity regulates the function of human neutrophils by modulating chemoattractant receptor signaling and activating mitogen-activated protein kinase p38. J Clin Invest. 1998;101:2768–2779. doi: 10.1172/JCI1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angle N, Hoyt DB, Coimbra R, et al. Hypertonic saline resuscitation diminishes lung injury by suppressing neutrophil activation after hemorrhagic shock. Shock. 1998;9:164–170. doi: 10.1097/00024382-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Junger WG, Coimbra R, Liu FC, et al. Hypertonic saline resuscitation: A tool to modulate immune function in trauma patients? Shock. 1997;8:235–241. [PubMed] [Google Scholar]
- 11.Rizoli SB, Kapus A, Fan J, et al. Immunomodulatory effects of hypertonic resuscitation on the development of lung inflammation following hemorrhagic shock. J Immunol. 1998;161:6288–6296. [PubMed] [Google Scholar]
- 12.Rostein OD. Novel strategies for immunomodulation after trauma: Revisiting hypertonic saline as a resuscitation strategy for hemorrhagic shock. J Trauma. 2000;49:580–583. doi: 10.1097/00005373-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Ciesla DJ, Moore EE, Zallen G, et al. Hypertonic saline attenuation of polymorphonuclear neutrophil cytotoxicity: Timing is everything. J Trauma. 2000;48:388–395. doi: 10.1097/00005373-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Angle N, Hoyt DB, Cabello-Passini R, et al. Hypertonic resuscitation reduces neutrophil margination by suppressing neutrophil L selectin expression. J Trauma. 1998;45:7–13. doi: 10.1097/00005373-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Murao Y, Hoyt DB, Loomis W, et al. Does the timing of hypertonic saline resuscitation affect its potential to prevent lung damage? Shock. 2000;14:18–23. doi: 10.1097/00024382-200014010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Murao Y, Loomis W, Wolf P, et al. Effect of dose of hypertonic saline on its potential to prevent lung tissue damage in a mouse model of hemorrhagic shock. Shock. 2003;20:29–34. doi: 10.1097/01.shk.0000071060.78689.f1. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Hashiguchi N, Yip L, et al. Hypertonic saline enhances neutrophil elastase release through activation of P2 and A3 receptors. Am J Physiol Cell Physiol. 2006;290:C1051–C1059. doi: 10.1152/ajpcell.00216.2005. [DOI] [PubMed] [Google Scholar]
- 18.Hashiguchi N, Lum L, Romeril E, et al. Hypertonic saline resuscitation: Efficacy may require early treatment in severely injured patients. J Trauma. 2007;62:299–306. doi: 10.1097/01.ta.0000222956.88760.33. [DOI] [PubMed] [Google Scholar]
- 19.Orlic T, Loomis WH, Namiki S, et al. Hypertonicity increases cAMP in PMN and blocks oxidative burst by PKA-dependent and -independent mechanisms. Am J Physiol Cell Physiol. 2002;282:C1261–C1269. doi: 10.1152/ajpcell.00479.2001. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Corriden R, Inoue Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Shukla A, Namiki S, et al. A putative osmoreceptor system that controls neutrophil function through the release of ATP, its conversion to adenosine, and activation of A2 adenosine and P2 receptors. J Leukocyto Biol. 2004;76:245–253. doi: 10.1189/jlb.0204066. [DOI] [PubMed] [Google Scholar]
- 22.Nick JA, Avdi NJ, Young SK, et al. Common and distinct intracellular signaling pathways in human neutrophils utilized by platelet activating factor and FMLP. J Clin Invest. 1997;99:975–986. doi: 10.1172/JCI119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato N, Kashima K, Shimazu H, et al. Hypertonic glucose inhibits the production of oxygen-derived free radicals by rat neutrophils. Life Sci. 1993;52:1481–1486. doi: 10.1016/0024-3205(93)90109-g. [DOI] [PubMed] [Google Scholar]
- 24.Baker CC, Chaudry IH, Gaines HO, et al. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 25.Chiara O, Pelosi P, Brazzi L, et al. Resuscitation from hemorrhagic shock: Experimental model comparing normal saline, dextran, and hypertonic saline solutions. Crit Care Med. 2003;31:1915–1922. doi: 10.1097/01.CCM.0000074725.62991.42. [DOI] [PubMed] [Google Scholar]
- 26.Vachharajani V, Vital S, Russell J, et al. Hypertonic saline and the cerebral microcirculation in obese septic mice. Microcirculation. 2007;14:223–231. doi: 10.1080/10739680601139153. [DOI] [PubMed] [Google Scholar]
- 27.Yip L, Cheung CW, Corriden R, et al. Hypertonic stress regulates T-cell function by the opposing actions of extracellular adenosine triphosphate and adenosine. Shock. 2007;27:242–250. doi: 10.1097/01.shk.0000245014.96419.3a. [DOI] [PubMed] [Google Scholar]
- 28.Fujiyoshi N, Deitch EA, Feketeova E, et al. Amiloride combined with small-volume resuscitation with hypertonic saline is superior in ameliorating trauma-hemorrhagic shock-induced lung injury in rats to the administration of either agent alone. Crit Care Med. 2005;33:2592–2598. doi: 10.1097/01.ccm.0000186770.59312.44. [DOI] [PubMed] [Google Scholar]
- 29.Homma H, Deitch EA, Feketeova E, et al. Small volume resuscitation with hypertonic saline is more effective in ameliorating trauma-hemorrhagic shock-induced lung injury, neutrophil activation and red blood cell dysfunction than pancreatitic protease inhibition. J Trauma. 2005;59:266–272. doi: 10.1097/01.ta.0000184582.55417.77. [DOI] [PubMed] [Google Scholar]
- 30.Wilder DM, Reid TJ, Bakaltcheva IB. Hypertonic resuscitation and blood coagulation: In vitro comparison of several hypertonic solutions for their action on platelets and plasma coagulation. Thromb Res. 2002;107:255–261. doi: 10.1016/s0049-3848(02)00335-3. [DOI] [PubMed] [Google Scholar]
- 31.Giusti-Paiva A, Martinez MR, Bispo-da-Silva LB, et al. Vasopressin mediates the pressor effect of hypertonic saline solution in endotoxic shock. Shock. 2007;27:416–421. doi: 10.1097/01.shk.0000239759.05583.fd. [DOI] [PubMed] [Google Scholar]
- 32.Poli-de-Figueiredo LF, Cruz RJ, Jr, Sannomiya P, et al. Mechanisms of action of hypertonic saline resuscitation in severe sepsis and septic shock. Endocr Metab Immune Disord Drug Targets. 2006;6:201–206. doi: 10.2174/187153006777442305. [DOI] [PubMed] [Google Scholar]
- 33.Oi Y, Aneman A, Svensson M, et al. Hypertonic saline-dextran improves intestinal perfusion and survival in porcine endotoxin shock. Crit Care Med. 2000;28:2843–2850. doi: 10.1097/00003246-200008000-00027. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira RP, Velasco I, Soriano FG, et al. Clinical review: Hypertonic saline resuscitation in sepsis. Crit Care. 2002;6:418–423. doi: 10.1186/cc1541. [DOI] [PMC free article] [PubMed] [Google Scholar]