Abstract
Protein S-glutathionylation is a reversible redox-dependent post-translational modification. Many cellular functions and signal transduction pathways involve proteins whose cysteine-dependent activities are modulated by glutathionylation. Glutaredoxin (Grx1) plays a key role in such regulation because it is a specific and efficient catalyst of deglutathionylation. We recently reported an increase in Grx1 in retinae of diabetic rats and in rat retinal Müller glial cells (rMC-1) cultured in high glucose. This up-regulation of Grx1 was concomitant with NFκB activation and induction of intercellular adhesion molecule-1 (ICAM-1). This proinflammatory response was replicated by adenoviral-directed up-regulation of Grx1 in cells in normal glucose. The site of regulation of NFκB was localized to the cytoplasm, where IκB kinase (IKK) is a master regulator of NFκB activation. In the current study, inhibition of IKK activity abrogated the increase in ICAM-1 induced by high glucose or by adenoviral-directed up-regulation of Grx1. Conditioned medium from the Müller cells overexpressing Grx1 was added to fresh cultures of Müller or endothelial cells and elicited increases in the Grx1 and ICAM-1 proteins in these cells. These effects correlate with a novel finding that secretion of interleukin-6 was elevated in the cultures of Grx overexpressing cells. Also, pure interleukin-6 increased Grx1 and ICAM-1 in the rMC-1 cells. Thus, Grx1 appears to play an important role in both autocrine and paracrine proinflammatory responses. Furthermore, IKKβ isolated from Müller cells in normal glucose medium was found to be glutathionylated on Cys-179. Hence Grx-mediated activation of IKK via deglutathionylation may play a central role in diabetic complications in vivo where Grx1 is increased.
S-glutathionylation on active cysteine residues is a redox-dependent post-translational modification that regulates many proteins important in cellular signaling. The enzymatic process of reversing and thus regulating steady state concentrations of S-glutathionylated proteins has been well documented and is attributed to glutaredoxin (Grx)3 (1–3).
Redox sensitivity of cysteine residues on proteins in the upstream pathway leading to NFκB-mediated transcription suggests that S-glutathionylation is an important regulatory mechanism in this pathway (4, 5). We previously reported induction of Grx1 in retinal homogenates from streptozotocin-diabetic rats. Grx1 was also up-regulated in retinal glial Müller cells cultured in diabetic-like (i.e. high glucose) conditions as a cell culture model for diabetic retinopathy (6). Increased Grx1 expression in high glucose medium or Grx1 overexpression via adenoviral-mediated transfection in normal glucose medium corresponded with increased nuclear translocation of the redox-sensitive transcription factor NFκB (p50-p65) and expression of intercellular adhesion molecule-1 (ICAM-1), a transcriptional product of NFκB. Furthermore, our previous study indicated that the site of NFκB regulation by Grx1 resides upstream in the cytoplasm, prompting us to elucidate further the molecular mechanism of Grx regulation of NFκB activation. IκB kinase (IKK) is a central regulator of NFκB signaling in the cytoplasm, and the proinflammatory effects of NFκB activation usually have been attributed to the IKKβ subunit specifically (7, 8). Here we report that IKKβ is glutathionylated site-specifically (Cys-179) in rat retinal glial (Müller) cells (rMC-1), implicating it as a Grx-regulated control point in NFκB-mediated ICAM-1 expression. Accordingly, inhibition of IKK abolished the effects of elevated Grx on ICAM-1 expression. Furthermore, conditioned medium from rMC-1 Müller glial cells overexpressing Grx elicited induction of ICAM-1 and Grx1 in freshly cultured rMC-1 Müller glial and in TRiBRB endothelial cells, suggesting an autoregulatory feedback mechanism for Grx1. Moreover, it was found that up-regulation of Grx1 leads to increased secretion of the IL-6 cytokine, implicating IL-6 as a key contributor to the induction of Grx1 and ICAM-1 in other cells. Accordingly, pure IL-6 also stimulated induction of Grx1 and ICAM-1 in the rMC-1 cells. Overall, these findings implicate Grx1 and corresponding glutathionylation events in the proinflammatory progression of diabetic retinopathy.
EXPERIMENTAL PROCEDURES
Cell Culture—Cell culture supplies were obtained from Invitrogen except where indicated. rMC-1 were a kind gift from Dr. Vijay Sarthy (Northwestern University, School of Medicine, Chicago, IL). Cells were cultured for up to 5 days in high glucose (25 mm) or normal glucose (5 mm) in Dulbecco's modified Eagle's medium with 2% heat-inactivated FBS (Fisher, Cellgro MT) with daily medium replacement in a humidified 37 °C incubator with 5% CO2. Glucose concentrations in the medium were monitored via the glucose oxidase kit as instructed by the manufacturer (Pointe Scientific). A transformed rat endothelial cell line isolated from the blood retinal barrier of transgenic mice (TRiBRB) was a kind gift from Dr. Tetsuya Terasaki (9). These cells were cultured in 5 mm glucose Dulbecco's modified Eagle's medium with 2% heat-inactivated FBS and 15 μg/ml endothelial cell growth supplement (Sigma) on 0.1% gelatin-coated plates in a humidified incubator with 5% CO2 at 33 °C.
Adenoviral Expression of Grx1 in rMC-1 Cells—rMC-1 cells (500,000 cells/100-mm dish) were grown in normal glucose medium for 2 days and infected with adenoviral vector containing a construct for expressing GRx1 (Ad-Grx) or an empty vector control (Ad-Empty) at a multiplicity of infection of 10 (m.o.i. of 10), except where indicated otherwise. The adenoviral infections were carried out in 1 ml of serum-free Dulbecco's modified Eagle's medium for 1 h, as described previously (6). Cells were cultured for two subsequent days in normal glucose medium and collected in 1% Nonidet P-40 lysis buffer (50 mm Tris, pH 8, 1% Nonidet P-40 detergent, and 150 mm NaCl).
Inhibition of IKK with Bay 11-7085 in Grx Overexpressing rMC-1 Cells—Cells treated with Bay 11-7085 inhibitor (BIOMOL International) were preincubated with the inhibitor for 30–40 min in normal glucose medium and subsequently infected with Ad-Grx or Ad-Empty in the absence of the inhibitor. These cells were then cultured for an additional 24–48 h in normal glucose medium in the absence of inhibitor and collected in 1% Nonidet P-40 lysis buffer.
Inhibition of IKK with Bay 11-7085 in rMC-1 Cells in High Glucose—Cells were cultured in high glucose (25 mm) medium for 2–3 days, treated with Bay 11-7085 for 5–10 min, and grown for an additional 2–3 days in high glucose medium for a total of 5 days. These cells were treated for less time with the inhibitor than those overexpressing Grx1 because the cells had to be less confluent to grow over the course of 5 days in culture. An incubation of 30–40 min with the inhibitor seemed to be cytotoxic on cells that were less confluent. All cells were grown for a total of 5 days in high glucose medium and collected in 1% Nonidet P-40 lysis buffer.
Nuclear Extraction—Samples were separated into nuclear and cytoplasmic fractions as described previously (6). rMC-1 cells from one 100-mm dish were collected in 1 ml of phosphate-buffered saline, centrifuged for 3 min at 800 × g, and lysed in 300 μl of low salt buffer (20 mm HEPES, pH 7.6, 20% glycerol (v/v), 10 mm NaCl, 1.5 mm MgCl2, 0.2 mm EDTA, and 0.1% (v/v) Triton X-100) for 20 min. Centrifugation at 800 × g for 3 min yielded a cytosolic supernatant. The nuclear pellet was washed twice in phosphate-buffered saline, incubated in 80 μl of high salt buffer (10 mm HEPES, pH 7.6, 10% glycerol (v/v), 0.5 m NaCl, 0.7 mm MgCl2, 0.1 mm EDTA, and 0.05% (v/v) Triton X-100) for 30 min 4 °C, and centrifuged at 16,000 × g for 15 min in 4 °C.
Immunoprecipitation of IKK—rMC-1 cells (100-mm dish, confluent) were collected and lysed in 500–600 μl of 1% Triton lysis buffer (1% Triton X-100, 250 mm Tris-HCl, pH 8, 150 mm NaCl, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin) for 10–15 min. Cells were lysed in 5 mm iodoacetamide, a thiol-blocking reagent, to prevent artifactual glutathione-protein oxidation or thiol exchange during processing. 5 μl of rabbit anti-IKKγ (0.2 μg/μl, Santa Cruz Biotechnology, Santa Cruz, CA) antibody and 40 μl of protein A/G bead 1:2 slurry (Santa Cruz Biotechnology) were added to cell lysates (∼1–2 mg) and incubated on a rotating platform at 4 °C overnight. For a nonspecific immunoprecipitation control, samples were incubated with 1 μg of rabbit IgG (Santa Cruz Biotechnology). Beads were centrifuged for 3 min at 3,000 × g, washed twice with phosphate-buffered saline, and boiled in 1× SDS sample buffer (0.5 m Tris-HCl, pH 6.8, 20% glycerol (v/v), 10% SDS (w/v), 1% bromphenol blue) for 15 min. Samples were processed by 12% SDS-PAGE and immunoblotted for IKKβ.
Detection of S-Glutathionylation of IKKβ by Mass Spectrometry—IKKβ was immunoprecipitated from 500–600 μl of lysate from a 100-mm dish of rMC-1 cells cultured in normal glucose medium as described above. The beads were eluted with 40 μl 5% (v/v) trifluoroacetic acid and 47.5% (v/v) acetonitrile. The eluates from four separate immunoprecipitations were combined and lyophilized. The sample was reconstituted in 50 μl of 100 mm ammonium bicarbonate and digested with ∼0.1 μg of trypsin/15 μl in 25 mm ammonium bicarbonate overnight at room temperature. Each sample was further digested with 5 μg of proteinase K to produce smaller molecular weight peptides. The sample was subsequently run on a ThermoElectron LTQ linear quadrupole ion trap spectrometer fitted with a GE Healthcare Ettan MDLC liquid chromatograph. The mobile phases were: (a) 0.1% formic acid (v/v) in HPLC grade water and (b) 0.1% formic acid (v/v) in HPLC grade acetonitrile. The gradient was run from 5 to 65% B over 50 min. All data files were searched against a rat protein subset taken from the NR data base using the ThermoElectron Bioworks 3.3 search program. In particular, the fragmentation patterns for peptides containing cysteine residues were additionally searched for specific masses (m/z) corresponding to addition of the glutathionyl moiety.
Western Blotting—Protein content of samples from whole cell lysates, nuclear extracts, or immunoprecipitations was determined via the Micro bicinchoninic acid method (BCA) (Pierce), according to the manufacturer's protocol. For Western blot analysis, lysates (100 μg) were mixed 4:1 with 4× SDS sample buffer (0.5 m Tris-HCl, pH 6.8, 20% glycerol (v/v), 10% SDS (w/v), 1% bromphenol blue, and unless indicated, 20 mm dithiothreitol), heated for 15 min at 95 °C, separated by 12% SDS-PAGE, and transferred to Immobilon-P membranes (Millipore Corp.). Membranes were immunoprobed with the appropriate antibodies: anti-Grx1 (1:1,000) (generated and purified via an adaptation of the McKinney and Parkinson caprylic acid method (10); anti-p50 (1:1,000) (ab7971 AbCam, Cambridge, MA); anti-p65 (1:3,000) (sc372, Santa Cruz Biotechnology); anti-ICAM-1 (1:500) (R&D Systems, Minneapolis, MN); anti-actin (1:30,000) (Sigma); and anti-YY1 (1:000) (Santa Cruz Biotechnology). Peroxidase conjugated secondary goat anti-rabbit or anti-mouse antibodies (1:10,000) (Jackson ImmunoResearch Laboratories, West Grove, PA) were used, and Western Lightning Plus chemiluminescence reagent (PerkinElmer Life Sciences) was used according to the manufacturer's protocol (PerkinElmer Life Sciences). Relative band intensities were quantified using a Bio-Rad calibrated imaging densitometer GS-710 with the Bio-Rad Quantity One software, version 4.1.1. Changes in band intensity are reported as ratios relative to loading controls (actin for cell lysates, and YY1 for nuclear fractions).
Treatment of rMC-1 Cells with Conditioned Medium from Grx1 Overexpressing rMC-1 Cells—400,000–600,000 rMC-1 cells/well in a 6-well dish were infected with Ad-Empty or Ad-Grx at m.o.i. of 10 or m.o.i. of 20 and cultured for 24 h in 1.5 ml of medium that contained 0.67% heat-inactivated FBS and 5 mm glucose. The conditioned medium was supplemented with glucose to re-establish a concentration of 5 mm and an additional 0.5 ml of medium that contained 2% heat-inactivated FBS and 5 mm glucose. The medium was then passed through a 0.22-μm syringe filter to remove cells and maintain sterility. The conditioned medium was then placed on newly cultured rMC-1 cells (200,000–400,000 cells/well in a 6-well dish) and cultured for 24 h. Cells were lysed in 1% Nonidet P-40 buffer and processed for Western blot analysis.
Treatment of TRiBRB Endothelial Cells with Conditioned Medium from Grx1 Overexpressing rMC-1 Cells—200,000–300,000 rMC-1 cells/well of a 6-well dish were infected with Ad-Empty or Ad-Grx at m.o.i. of 10 or m.o.i. of 20 and cultured for 24 h in 1.5 ml of medium that contained 0.67% heat-inactivated FBS and 5 mm glucose. The conditioned medium was supplemented with glucose to re-establish a concentration of 5 mm,20 μl of 15 mg/ml endothelial cell growth supplement, and 0.5 ml of medium that contained 2% heat-inactivated FBS and 5 mm glucose. The medium was then passed through a 0.22-μm syringe filter to remove cells and to maintain sterility. The conditioned medium was then placed on ∼200,000–300,000 TRiBRB endothelial cells/well of a 6-well dish and cultured for 24 h. Cells were lysed in 1% Nonidet P-40 buffer and processed for Western blot analysis.
Detection of Cytokines and Growth Factors in Conditioned Medium from Grx1 Overexpressing rMC-1 Cells or from High Glucose Cultured rMC-1 Cells—rMC-1 cells (750,000 cells/150-mm) were plated for a 3–4-day culture in normal or high glucose medium (30 ml/dish) containing 2% heat-inactivated FBS. Alternatively, 6 million rMC-1 cells/100-mm dish infected with Ad-Grx or Ad-Empty (both m.o.i. of 10) in normal glucose medium (8 ml/dish) were grown for 16–24 h in medium containing 0.67% heat-inactivated FBS and 5 mm glucose. To clear whole cells and cell debris, the medium from each type of experiment was passed through a 0.22-μm syringe filter. According to their initial volumes and cell density, medium from normal or high glucose was concentrated 60-fold, and medium from adenoviral infected cell cultures was concentrated 3-fold, with Amicon ultracentrifugal filter devices (molecular mass cutoff 10,000 Da). Concentrated medium was analyzed via a Fluorokine MultiAnalyte profiling cytokine multiplex kit, a rat IL-6 Quantikine immunoassay, and a rat VEGF immunoassay according to the manufacturer's instructions (R&D Systems).
Treatment of rMC-1 Cells with Pure IL-6—150,000–300,000 rMC-1 cells in wells of a 6-well dish were cultured for 16–24 h. Recombinant IL-6 (60 pg/ml) (R&D Systems) was added to 1–2 ml of normal glucose medium, passed through a 0.22-μm syringe filter, and applied to the cells. After 24 h of this treatment, the cells were lysed in 1% Nonidet P-40 buffer and processed for Western blot analysis with anti-Grx1 and anti-ICAM-1 antibodies.
Statistical Analysis—All values and graphs report means ± S.E. Statistical analysis of differences between control and experimental values were determined via the Student's t test. Differences displaying p values ≤0.05 are considered statistically significant.
RESULTS
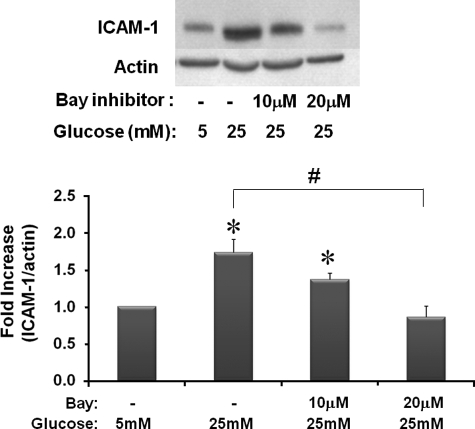
Inhibition of IKK Prevents ICAM-1 Increases in rMC-1 Cells Cultured in High Glucose Medium—The potential sites for glutathionylation in the cytosolic pathway of NFκB are extensive. We utilized inhibitors of IKK, a point of signaling convergence, to determine whether the protein(s) regulated by Grx1 are upstream, downstream, or directly on IKK. Specifically, to discover whether ICAM-1 induction was dependent on IKK or upstream mediators, we tested changes in ICAM-1 expression in cells cultured in normal or high glucose medium in the absence or presence of Bay 11-7085, an inhibitor of IKK activity. High glucose increased ICAM-1 expression nearly 2-fold, and this induction was blocked by increasing concentrations of Bay 11-7085 (Fig. 1), suggesting that IKK and/or upstream mediators are critical in the high glucose stimulation of NFκB-regulated transcription in rMC-1 cells.
FIGURE 1.
Effects of IKK inhibition on ICAM-1 expression in rMC-1 cells cultured in high glucose. rMC-1 cells were cultured in high glucose (25 mm) medium for 5 days and treated with Bay 11-7085 (Bay) for 5–10 min on days 2–3 of incubation. A representative Western blot for ICAM-1 and actin loading control and the quantification of blots is given in this figure (n = 12). High glucose cells expressed ICAM-1 nearly 2-fold (±0.6) over cells grown in normal glucose medium, *, p ≤ 0.002. 10 and 20 μm Bay inhibitor 11-7085 decreased ICAM-1 expression in high glucose-treated cells from that of high glucose-treated cells in the absence of inhibitor, #, p ≤ 0.03.
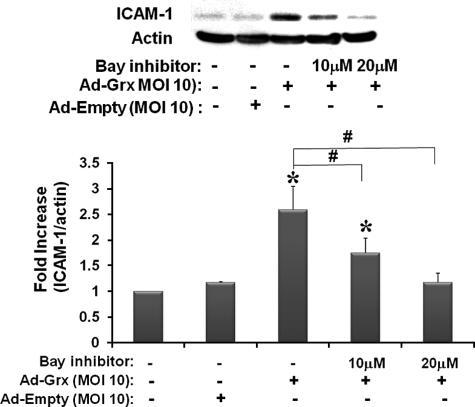
Inhibition of IKK Prevents Increases in ICAM-1 in rMC-1 Cells Overexpressing Grx1 in Normal Glucose Medium—Adenoviral Grx1 (Ad-Grx1)-mediated overexpression of Grx1 in rMC-1 cells in normal glucose medium increased ICAM-1 expression about 2.5-fold. This extent of induction at this particular amount of infection (m.o.i. of 10) agrees well with our previous observations (7). Because m.o.i. of 10 of Ad-Grx1 is the viral concentration that mimics the effect of high glucose with respect to induction of both Grx1 and ICAM-1 (7), it was used routinely in the present studies. We then tested whether the effects of IKK inhibition on ICAM-1 in the Grx1 overexpressing cells in normal glucose medium would mimic the effects seen in high glucose-treated cells. A concentration-dependent inhibition with Bay 11-7085 was observed, indicating that inhibition of IKK activity prevented the ICAM-1 increase (Fig. 2). These data indicate that the pathway of induction of ICAM-1 is the same in Grx1 overexpressing cells and in the diabetic model of high glucose medium.
FIGURE 2.
Effects of IKK inhibition on ICAM-1 expression in Grx1 overexpressing rMC-1 cells. rMC-1 cells were cultured in normal glucose (5 mm) medium and pretreated with Bay 11-7085 (Bay) for 30–40 min. Then the cells were infected immediately with m.o.i. of 10 of either Ad-Grx1 or Ad-Empty, cultured for 16–24 h in normal glucose medium, and collected in Nonidet P-40 lysis buffer for immunoblotting of ICAM-1 (1:1000) and actin (1:30,000) (n = 7). Ad-Grx1 increased ICAM-1 expression by 2.6-fold (±0.5), *, p < 0.01. ICAM-1 expression of Ad-Grx1-infected cells treated with 10 μm Bay 11-7085 was statistically increased, but cells treated with 20μm Bay 11-7085 expressed ICAM-1 in similar amounts as control cells (no adenovirus and Ad-Empty m.o.i. of 10). In addition, ICAM-1 of cells infected with Ad-Grx1 and treated with 10 and 20 μm inhibitor was significantly decreased from Ad-Grx1-infected cells with no inhibitor, #, p < 0.01.
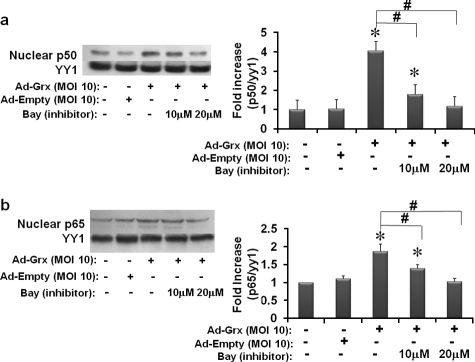
Inhibition of IKK Prevents Increases in Nuclear NFκB in Cells Overexpressing Grx1 in Normal Glucose Medium—To determine whether inhibition of IKK corresponds to an inhibition of NFκB, we tested effects of Bay 11-7085 on the nuclear translocation of the NFκB subunits, p50 and p65, in Grx1 overexpressing cells in normal glucose medium. Overexpression of Grx1 induced nuclear translocation of both NFκB subunits by 2–4-fold. These increases in NFκB in the nucleus were prevented by Bay 11-7085 in a concentration-dependent manner (Fig. 3, a and b). The empty vector control had no effect on ICAM-1 expression (Fig. 3, a and b). These results are consistent with NFκB being the predominant transcriptional regulator of ICAM-1 in this cell system and with the site of regulation by Grx1 taking place on and/or upstream of IKK.
FIGURE 3.
Effects of IKK inhibition on nuclear translocation of NFκB p50 and p65 in Grx1 overexpressing rMC-1 cells. rMC-1 cells were cultured in normal glucose (5 mm) medium, pretreated with Bay 11-7085 (Bay) for 30 min prior to Grx1 overexpression, immediately infected with m.o.i. of 10 of either Ad-Grx1 or Ad-Empty, cultured for two more days in normal glucose medium, and collected. Cells were separated into nuclear fractions, run on a 12% SDS-PAGE, and immunoprobed for p50 (1:1,000), p65 (1:3,000), and the nuclear loading control YY1 (1:1,000). Ad-Grx1 increased p50 in the nucleus by 4-fold (±0.7) (n = 9) (panel a), *, p ≤ 0.002. Cells overexpressing Grx1 treated with 10 and 20 μm Bay 11-7085 were significantly decreased from Grx1-overexpressing cells with no inhibitor (n = 9) (panel a), #, p < 0.02. Ad-Grx1 increased p65 in the nucleus by nearly 2-fold (±0.7) (n = 11) (panel b), *, p ≤ 0.002. Cells overexpressing Grx1 treated with 10 and 20 μm Bay 11-7085 were significantly decreased from Grx1-overexpressing cells with no inhibitor (n = 11) (panel b), #, p < 0.02.
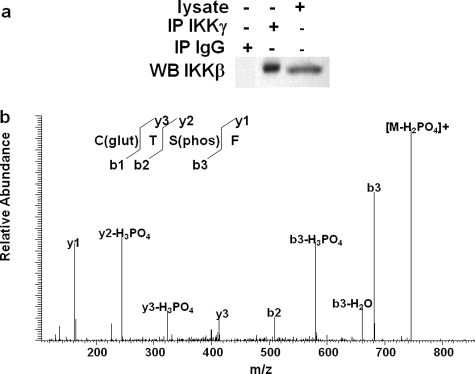
IKKβ Is S-Glutathionylated at Cys-179 in rMC-1 Cells in Normal Glucose Medium—To determine whether IKKβ is glutathionylated in rMC-1 cells and to identify the specific residue(s) that undergoes this modification, cellular IKKβ was co-immunoprecipitated with IKKγ and analyzed via mass spectrometry. Western blot analysis of IKKβ isolated from rMC-1 cells cultured in normal glucose conditions confirmed successful pull-down of IKKβ (Fig. 4a). The immunoprecipitated sample was digested sequentially with trypsin and proteinase K and analyzed by mass spectrometry. Peptide sequences from the digested sample matched to those of IKKβ. Tandem MS analysis identified a particular modified peptide. Fig. 4b shows the tandem MS spectrum with the fragment ions of the peptide sequence labeled according to Biemann nomenclature. The presence of cysteinyl-glutathione was documented by the loss of its mass (409 m/z) from the intact glutathionylated peptide. The remaining peptide fragment (Tphospho-SF) corresponds to the y3 ion. Of the 20 cysteine residues within IKKβ, only Cys-179 is located within the activation loop, and it was the single site of S-glutathionylation in IKKβ immunoprecipitated from lysate of rMC-1 cells grown in normal glucose medium. This result suggests that Cys-179 of endogenous IKK is modified by glutathionylation in cells under physiological conditions (i.e. cells that are unstimulated and not overexpressing proteins). Thus, Grx-mediated deglutathionylation of IKK is likely an important regulatory mechanism of NFκB-driven up-regulation of ICAM-1 in Müller cells under diabetic-like conditions.
FIGURE 4.
Glutathionylation of IKKβ isolated from rMC-1 cells cultured in normal glucose medium. IKKβ was immunoprecipitated (IP) in the presence of iodoacetamide from rMC-1 cells cultured in normal glucose medium and immunoblotted (WB) under non-reducing conditions (a). Immunoprecipitated IKKβ is S-glutathionylated at Cys-179 as shown by the peak on the spectrum at 409 m/z of the peptide sequence obtained by liquid chromatography-tandem MS (b). C(glut), S-glutathionyl cysteine; T, threonine; S(phos), phosphoserine; F, phenylalanine.
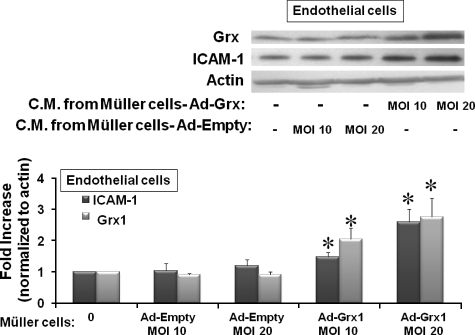
Conditioned Medium from Grx1 Overexpressing rMC-1 Cells Increases the Expression of ICAM-1 and Grx1 in TRiBRB Endothelial Cells—To test whether Grx activation of the NFκB pathway up-regulates expression of soluble mediators (e.g. cytokines) to an extent sufficient to induce an inflammatory response in other cells, we tested for effects of conditioned medium obtained from rMC-1 cells overexpressing Grx1 on TRiBRB endothelial cells. ICAM-1 was up-regulated in endothelial cells in a dose-dependent fashion in response to conditioned medium from rMC-1 cells overexpressing Grx1 at two different concentrations (Ad-Grx at m.o.i. of 10 and m.o.i. of 20) (Fig. 5). The empty control vector construct did not induce expression of ICAM-1 (Fig. 5). These data indicate that increased expression of Grx1 in Müller cells leads to an increase in release of a signaling medium capable of inducing a proinflammatory paracrine response in endothelial cells.
FIGURE 5.
ICAM-1 expression in rat TRiBRB endothelial cells treated with conditioned medium from Grx1 overexpressing rat rMC-1 cells. rMC-1 cells overexpressing Grx1 with Ad-Grx at m.o.i. of 10 or m.o.i. of 20 were cultured for 24 h, and the conditioned medium (C.M.) was collected. The C.M. was placed on TRiBRB endothelial cells for 24 h, and the cell lysate was processed for Western blot analysis. C.M. from rMC-1 cells overexpressing two concentrations of Grx1 (Ad-Grx1 m.o.i. of 10 and m.o.i. of 20) led to an increase of 2.1-fold (±0.4) and 2.8-fold (±0.6) in Grx1, respectively (n = 4), *, p ≤ 0.05. ICAM-1 was increased by 1.5-fold (±0.2) and 2.6-fold (±0.4) in response to conditioned medium from rMC-1 cells overexpressing Grx1 at m.o.i. of 10 or m.o.i. of 20, respectively (n = 7), *, p ≤ 0.03.
To test whether a corresponding increase in Grx1 occurred, Western blot analysis was run on TRiBRB endothelial cells that had been cultured in the conditioned medium from rMC-1 cells overexpressing Grx1. Grx1 was up-regulated in a similar dose-dependent manner in response to conditioned the medium from rMC-1 cells overexpressing Grx1 at m.o.i. of 10 or m.o.i. of 20 (Fig. 5). The empty vector control did not induce expression of Grx1 (Fig. 5). These data suggest that increased expression of Grx1 in rMC-1 cells induces NFκB-driven production of cytokines that activate Grx1 in endothelial cells, leading to increased NFκB activation and subsequent expression of ICAM-1.
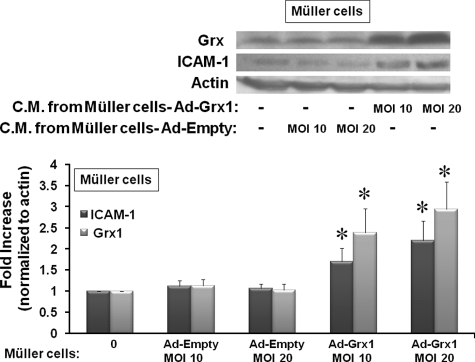
Conditioned Medium from Grx1 Overexpressing rMC-1 Cells Increases the Expression of ICAM-1 and Grx1 in Natural rMC-1 Cells—To test whether the signaling mediator(s) in the conditioned medium could act in a positive feedback mechanism in Müller cells analogous to the paracrine event observed in the TRiBRB endothelial cells, Western blot analysis of ICAM-1 and Grx1 was carried out on rMC-1 cells treated for 24 h with conditioned medium from rMC-1 cells overexpressing Grx1. ICAM-1 and Grx1 were both increased in rMC-1 cells in response to the conditioned medium from rMC-1 cells overexpressing Grx1 at two different concentrations (Ad-Grx1 at m.o.i. of 10 and m.o.i. of 20), consistent with autocrine regulation (Fig. 6). Again, conditioned medium from rMC-1 cells containing an empty adenoviral vector construct did not cause an up-regulation of ICAM-1 or Grx1 (Fig. 6).
FIGURE 6.
ICAM-1 expression in rMC-1 cells treated with conditioned medium from Grx1 overexpressing rMC-1 cells. rMC-1 Müller cells were transfected with Ad-Grx or Ad-Empty at m.o.i. of 10 or m.o.i. of 20, and the C.M. was collected from these cells after 24 h. C.M. was then placed on newly cultured rMC-1 Müller cells for 24 h, and the cell lysate was processed for Western blot analysis. C.M. from rMC-1 overexpressing two concentrations of Grx1 (Ad-Grx1 m.o.i. of 10 and m.o.i. of 20) led to an increase of 2.4-fold (±0.6) and 2.9-fold (±0.7) in Grx1, respectively (n = 6), *, p ≤ 0.03. ICAM-1 was increased by 1.7-fold (±0.3) and 2.2-fold (±0.5) in response to conditioned medium from rMC-1 cells overexpressing Grx1 at m.o.i. of 10 or m.o.i. of 20, respectively (n = 6), *, p ≤ 0.04.
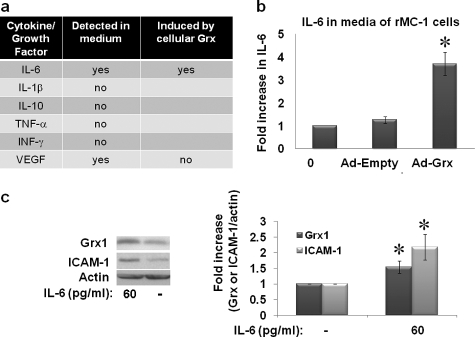
IL-6 Protein Is Increased in Conditioned Medium from rMC-1 Cells in Which Grx1 Is Elevated—We set out to identify the soluble mediator(s) in the conditioned medium of rMC-1 cells that leads to up-regulation of ICAM-1 and Grx1 in fresh cultures of Müller rMC-1 or endothelial cells. Target proteins to be screened were chosen according to their potential pro- or anti-inflammatory roles in diabetic retinopathy and/or retinal Müller cells. TNF-α, IL1-β, IFN-γ, and IL-10 were not detected in the conditioned medium of rMC-1 cells overexpressing Grx1 (Fig. 7a) or in the conditioned medium of the cells in high glucose medium. Although VEGF could be detected in the medium, up-regulation of Grx1 (Fig. 7a) or incubation in high glucose medium did not elicit an increase in VEGF secretion. In contrast, a 3.7-fold increase in IL-6 expression was observed in medium from rMC-1 Müller cells overexpressing Grx1 (Ad-Grx1, m.o.i. of 10) (Fig. 7b). Having identified IL-6 in the medium, we tested directly the effect of pure IL-6 on rMC-1 cells. At concentrations as low as 60 pg/ml, ∼1.5-fold induction of Grx1 and 2.1-fold induction of ICAM-1 were observed (Fig. 7c). In addition, in separate experiments, it was confirmed that IL-6 was also increased in the medium of rMC-1 cells cultured in high glucose (data not shown); however, the apparent -fold increase in IL-6 was substantially less than that of the Grx overexpressing cells due at least in part to the incomplete recovery of IL-6 during the more extensive processing of the medium that was required. Collectively, these data suggest that elevation of Grx1 leads to activation of NFκB via deglutathionylation of a protein(s) (e.g. IKK) in the cytosolic signaling pathway, leading to increased expression of IL-6 in Müller cells and its subsequent secretion.
FIGURE 7.
Cytokine regulation by Grx1 in rMC-1 cells. Cytokine expression in the culture medium of rMC-1 cells overexpressing Grx1 in normal glucose medium was measured via a multiplex cytokine Luminex enzyme-linked immunosorbent assay (ELISA), and VEGF protein was analyzed from the same samples of medium using a VEGF ELISA. Release of TNF-α, IFN-γ, IL-1β, and IL-10 into the medium was not detected (panel a). IL-6 and VEGF were both detected in the medium, but only IL-6 was induced by the overexpression of Grx1 in Müller cells (panel a). rMC-1 Müller cells overexpressing Grx1 with Ad-Grx at an m.o.i. of 10 were cultured for 24 h in normal glucose medium. Medium from the cells was then collected, concentrated 3-fold, and tested in an IL-6 ELISA. Medium from rMC-1 Müller cells overexpressing Grx1 showed a 3.7-fold (±0.5) increase in IL-6 expression over cells transfected with a control empty vector construct (Ad-Empty) or untransfected (0) (panel b)(n = 6) *, p = 0.003. Treatment with recombinant IL-6 induced expression of Grx by 1.5-fold (±0.2) and ICAM-1 by 2.1-fold (±0.4) *, p ≤ 0.03 (n = 8) (panel c).
DISCUSSION
Grx1 Regulation of NFκB Transcriptional Products (e.g. Cytokines)—Grx1 is an important cytosolic thiol redox enzyme that regulates reversible S-glutathionylation and corresponding activity of cellular proteins (1). Changes in Grx1 have been reported previously to regulate the secretion of proinflammatory chemokines, macrophage inflammatory protein-2, and keratinocyte-derived chemokine from tracheal epithelial cells (11). We have shown that Grx1 up-regulates ICAM-1 (Fig. 2) (6) and IL-6 in rMC-1 cells (Fig. 7b). Interestingly, the regulation by Grx1 appears not to be pleiotropic for NFκB transcriptional gene products. Detectable amounts of extracellular TNF-α, IL-10, IL1-β, or IFN-γ were not observed for rMC-1 cells in normal or high glucose medium or infected with Ad-Grx1 (Fig. 7a). Moreover, VEGF was released at substantial amounts by rMC-1 cells, but its secretion was not increased by overexpression of Grx1 (Fig. 7a) or by high glucose (data not shown). Therefore IL-6 and ICAM-1 appear to be selective targets for Grx-mediated NFκB activation. However, the possibility that Grx1 induces the expression and secretion of additional NFκB-regulated inflammatory mediators (e.g. IL-8) in Müller cells has not been ruled out.
Autocrine and Paracrine Mechanisms of Grx Regulation—Conditioned medium (IL-6) from glial (rMC-1) cells overexpressing Grx led to up-regulation of Grx1 in other rMC-1 cells (autocrine regulation) and in endothelial (TRiBRB) cells (paracrine regulation) (Figs. 5 and 6). These findings are distinguished from previous reports on cytokine regulation of Grx1 because Grx1 in this case is shown to be induced by an endogenous protein secreted from cells. The previous reports involved exogenous application of purified proteins to cultured cells. For example, pure IL-6 was reported to elicit a slight increase in Grx1 protein in a monocytic cell line (12). In another study, treatment with IFN-γ was shown to increase Grx1 protein in airway epithelial cells according to immunohistochemical staining (13). Transforming growth factor β1 led to a decrease in Grx activity and mRNA with a corresponding increase in total protein glutathionylation in mouse airway epithelium (13) and to a decrease in Grx1 protein in A549 cells (13, 14). In addition, in bovine aortic endothelial cells, TNF-α leads to an increase in activity of glutaredoxin and a corresponding decrease in glutathionylation of pro-caspase 3 without a change in Grx1 protein content (15). In contrast, no change in Grx1 activity in mouse airway epithelium and no change in Grx1 protein in A549 cells were found in response to TNF-α (13, 14). Other cytokines such as IL-4, IL-13, and TNF-α have been reported to change Grx-like activity and/or protein glutathionylation, but a change in Grx1 protein either was not tested or was not detected (13, 15, 16). Clearly, further studies are necessary to determine the scope and the mechanism(s) of cytokine regulation of Grx1.
Grx1 Regulation of the NFκB Pathway and S-Glutathionylation of IKKβ—A series of cytoplasmic proteins initiate nuclear NFκB activation through the canonical NFκB signaling pathway. Although these components represent a plethora of target sites for regulation by glutathionylation in the cytoplasm, signals from multiple upstream mediators converge in the cytoplasm on IKK, a well established master regulator of NFκB.
IKK is a 700–900-kDa heteromeric complex comprised of a core enzyme of two catalytic subunits (IKKα and IKKβ) and one regulatory subunit (IKKγ). The additional composition of the IKK complex is likely to be cell type-dependent because IKK can interact with at least 65 distinct proteins under various conditions (17). The redox-sensitive IKKβ is best characterized for mediating NFκB activation from proinflammatory stimuli (7, 8) and has the highest catalytic activity toward IκB (17). Glutathionylation of IKKβ has been correlated with NFκB activity and subsequent expression of keratinocyte-derived chemokine and macrophage inflammatory protein 2 in IKKβ overexpressing lung epithelial cells (11). Moreover, cells in which Grx1 is knocked down have decreased expression of these chemokines. These data suggest that Grx catalysis of IKKβ deglutathionylation leads to activation of NFκB and subsequent expression of chemokines.
Regulation of the NFκB pathway by Grx1 has been implicated in several contexts including diabetes (6, 11, 18, 19). Our studies indicate that IKK is a critical point of regulation of the NFκB pathway in rMC-1 cells exposed to high glucose (Figs. 1, 2, 3). Moreover, this is a novel documentation of the S-glutathionylation of endogenous IKKβ under physiological conditions, i.e. IKK isolated from untreated and non-overexpressing cells (Fig. 4). These data suggest that S-glutathionylation of IKKβ regulates the activity and downstream signaling of IKK and is involved in the inflammatory response of Müller cells in diabetic retinopathy.
Impact of IL-6 and Grx in Diabetic Retinopathy—Studies have suggested a neuroprotective role in retinal microglial cells for IL-6 in response to hydrostatic pressure and ischemia (20, 21) and specifically in rat Müller cells in response to pituitary adenylate cyclase-activating peptide (22). Despite the potential for beneficial effects of IL-6 in Müller cells in response to these stimuli, IL-6 has been associated with deleterious proinflammatory effects in diabetic retinopathy. IL-6 is increased in the vitreous humor of patients with diabetic retinopathy (23–25), in retinas from diabetic mice and rats (26, 27), and in serum from children with diabetic retinopathy (28). Our data implicate IL-6 for induction of ICAM-1 expression in Müller cells and endothelial cells (Figs. 5, 6, 7), and these findings are consistent with an overall proinflammatory effect. IL-6 is likely a key contributor in the initiation of the inflammatory response in the diabetic retina. We show here that Grx1 regulates expression of IL-6 and is thus a component of this response.
Grx1 has been reported previously to be increased in the hearts of diabetic rats (29), and we have documented that Grx1 is up-regulated in retinae of diabetic rats as well as retinal Müller cells in response to high glucose medium (6). Our findings in the current study are consistent with Grx regulation of NFκB activation and subsequent expression of ICAM-1 and IL-6 in retinal Müller cells via S-glutathionylated IKKβ. The NFκB pathway has been scrutinized for therapeutic potential in diabetes for many years (30, 31), but effective interventions have yet to be developed. In this context, Grx may emerge as a new therapeutic target for diabetic retinopathy.
Acknowledgments
We thank the Case Western Reserve University Inflammatory Core for help with the luminex detection of the cytokine multiplex. We are also very grateful to Michael Maguire, Ph.D. for critical review of the manuscript prior to submission.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 AG024413 and P01 AG 15885 (to J. J. M.) and by National Institutes of Health NEI Grant 5 T32 EY07157 (to M. D. S.). This work was also supported by a Merit Review grant from the Department of Veterans Affairs (to J. J. M.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: Grx, glutaredoxin; Ad-Empty, adenovirus vector-empty construct; Ad-Grx1, adenovirus vector containing glutaredoxin 1 cDNA construct; ICAM-1, intercellular adhesion molecule-1; IL-6, interleukin-6; IκB, inhibitor of NFκB; IKK, IκB kinase; NFκB, nuclear factor κB; m.o.i., multiplicity of infection; rMC-1, rat retinal Müller glial cells; FBS, fetal bovine serum; HPLC, high pressure liquid chromatography; TNF, tumor necrosis factor; IFN, interferon; VEGF, vascular endothelial growth factor; MS, mass spectrometry; C.M., conditioned medium.
References
- 1.Chrestensen, C. A., Starke, D. W., and Mieyal, J. J. (2000) J. Biol. Chem. 275 26556–26565 [DOI] [PubMed] [Google Scholar]
- 2.Gravina, S. A., and Mieyal, J. J. (1993) Biochemistry 32 3368–3376 [DOI] [PubMed] [Google Scholar]
- 3.Gallogly, M. M., and Mieyal, J. J. (2007) Curr. Opin. Pharmacol. 7 381–391 [DOI] [PubMed] [Google Scholar]
- 4.Shelton, M. D., Chock, P. B., and Mieyal, J. J. (2005) Antioxid. Redox Signal. 7 348–366 [DOI] [PubMed] [Google Scholar]
- 5.Mieyal, J. J., Gallogly, M. M., Qanungo, S., Sabens, E. A., and Shelton, M. D. (2008) Antioxid. Redox Signal. 10 1941–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shelton, M. D., Kern, T. S., and Mieyal, J. J. (2007) J. Biol. Chem. 282 12467–12474 [DOI] [PubMed] [Google Scholar]
- 7.Rossi, A., Kapahi, P., Natoli, G., Takahashi, T., Chen, Y., Karin, M., and Santoro, M. G. (2000) Nature 403 103–108 [DOI] [PubMed] [Google Scholar]
- 8.Jeon, K. I., Byun, M. S., and Jue, D. M. (2003) Exp. Mol. Med. 35 61–66 [DOI] [PubMed] [Google Scholar]
- 9.Hosoya, K., Tomi, M., Ohtsuki, S., Takanaga, H., Ueda, M., Yanai, N., Obinata, M., and Terasaki, T. (2001) Exp. Eye Res. 72 163–172 [DOI] [PubMed] [Google Scholar]
- 10.Gravina, S. A. (1993) Characterization and Kinetic Mechanism of Thiol-transferase. Ph.D. thesis, Case Western Reserve University, Cleveland, OH
- 11.Reynaert, N. L., van der Vliet, A., Guala, A. S., McGovern, T., Hristova, M., Pantano, C., Heintz, N. H., Heim, J., Ho, Y. S., Matthews, D. E., Wouters, E. F., and Janssen-Heininger, Y. M. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 13086–13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takashima, Y., Hirota, K., Nakamura, H., Nakamura, T., Akiyama, K., Cheng, F. S., Maeda, M., and Yodoi, J. (1999) Immunol. Lett. 68 397–401 [DOI] [PubMed] [Google Scholar]
- 13.Reynaert, N. L., Wouters, E. F., and Janssen-Heininger, Y. M. (2007) Am. J. Respir. Cell Mol. Biol. 36 147–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peltoniemi, M., Kaarteenaho-Wiik, R., Saily, M., Sormunen, R., Paakko, P., Holmgren, A., Soini, Y., and Kinnula, V. L. (2004) Hum. Pathol. 35 1000–1007 [DOI] [PubMed] [Google Scholar]
- 15.Pan, S., and Berk, B. C. (2007) Circ. Res. 100 213–219 [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee, T. K., Mishra, A. K., Mukhopadhyay, S., and Hoidal, J. R. (2007) J. Immunol. 178 1835–1844 [DOI] [PubMed] [Google Scholar]
- 17.Scheidereit, C. (2006) Oncogene 25 6685–6705 [DOI] [PubMed] [Google Scholar]
- 18.Pineda-Molina, E., Klatt, P., Vazquez, J., Marina, A., Garcia, d. L., Perez-Sala, D., and Lamas, S. (2001) Biochemistry 40 14134–14142 [DOI] [PubMed] [Google Scholar]
- 19.Qanungo, S., Starke, D. W., Pai, H. V., Mieyal, J. J., and Nieminen, A. L. (2007) J. Biol. Chem. 282 18427–18436 [DOI] [PubMed] [Google Scholar]
- 20.Sappington, R. M., and Calkins, D. J. (2006) Investig. Ophthalmol. Vis. Sci. 47 3860–3869 [DOI] [PubMed] [Google Scholar]
- 21.Sanchez, R. N., Chan, C. K., Garg, S., Kwong, J. M., Wong, M. J., Sadun, A. A., and Lam, T. T. (2003) Investig. Ophthalmol. Vis. Sci. 44 4006–4011 [DOI] [PubMed] [Google Scholar]
- 22.Seki, T., Hinohara, Y., Taki, C., Nakatani, M., Ozawa, M., Nishimura, S., Takaki, A., Itho, H., Takenoya, F., and Shioda, S. (2006) Ann. N. Y. Acad. Sci. 1070 535–539 [DOI] [PubMed] [Google Scholar]
- 23.Abu el Asrar, A. M., Maimone, D., Morse, P. H., Gregory, S., and Reder, A. T. (1992) Am. J. Ophthalmol. 114 731–736 [DOI] [PubMed] [Google Scholar]
- 24.Murugeswari, P., Shukla, D., Rajendran, A., Kim, R., Namperumalsamy, P., and Muthukkaruppan, V. (2008) Retina 28 817–824 [DOI] [PubMed] [Google Scholar]
- 25.Kauffmann, D. J., van Meurs, J. C., Mertens, D. A., Peperkamp, E., Master, C., and Gerritsen, M. E. (1994) Investig. Ophthalmol. Vis. Sci. 35 900–906 [PubMed] [Google Scholar]
- 26.Gustavsson, C., Agardh, C. D., Hagert, P., and Agardh, E. (2008) Retina 28 645–652 [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan, S., Hatley, M. E., Reilly, K. B., Danziger, E. C., and Hedrick, C. C. (2004) Arterioscler. Thromb. Vasc. Biol. 24 851–857 [DOI] [PubMed] [Google Scholar]
- 28.Mysliwiec, M., Balcerska, A., Zorena, K., Mysliwska, J., Lipowski, P., and Raczynska, K. (2008) Diabetes Res. Clin. Pract. 79 141–146 [DOI] [PubMed] [Google Scholar]
- 29.Li, X., Xu, Z., Li, S., and Rozanski, G. J. (2005) Am. J. Physiol. 288 H1417–H1424 [DOI] [PubMed] [Google Scholar]
- 30.Cameron, N. E., and Cotter, M. A. (2008) Curr. Drug Targets 9 60–67 [DOI] [PubMed] [Google Scholar]
- 31.Shoelson, S. E., Lee, J., and Yuan, M. (2003) Int. J. Obes. Relat. Metab. Disord. 27 Suppl. 3, S49–S52 [DOI] [PubMed] [Google Scholar]