Abstract
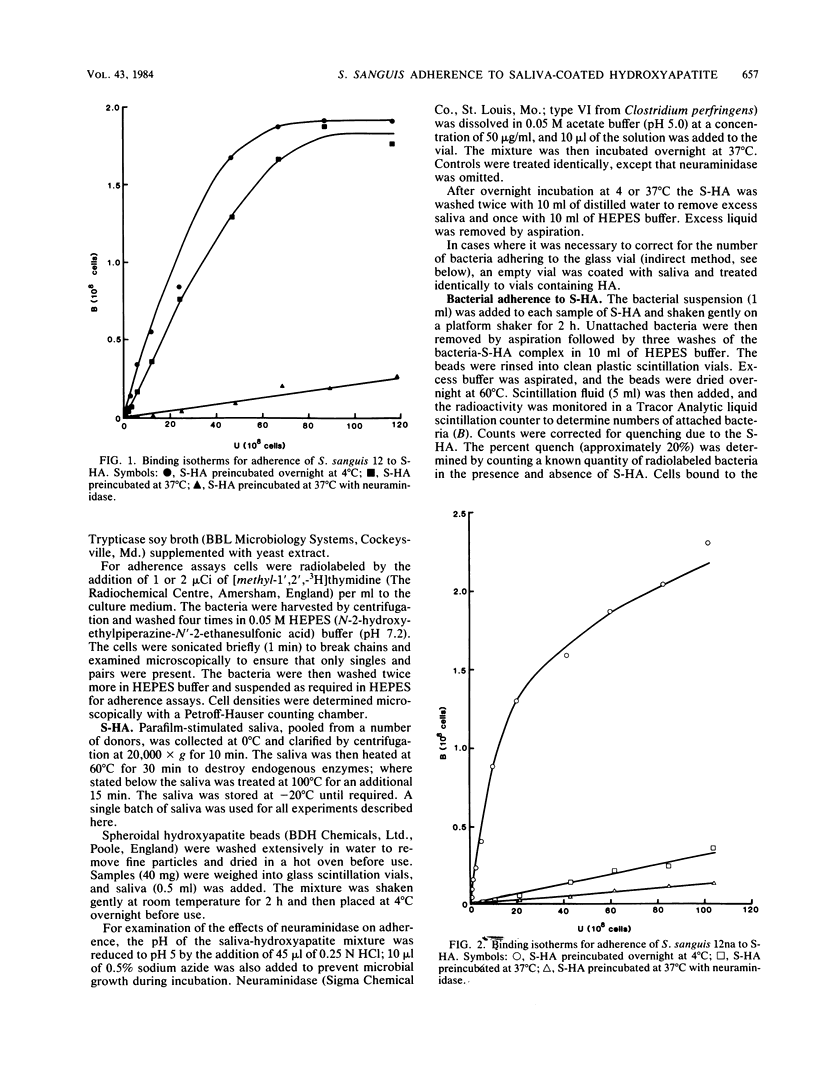
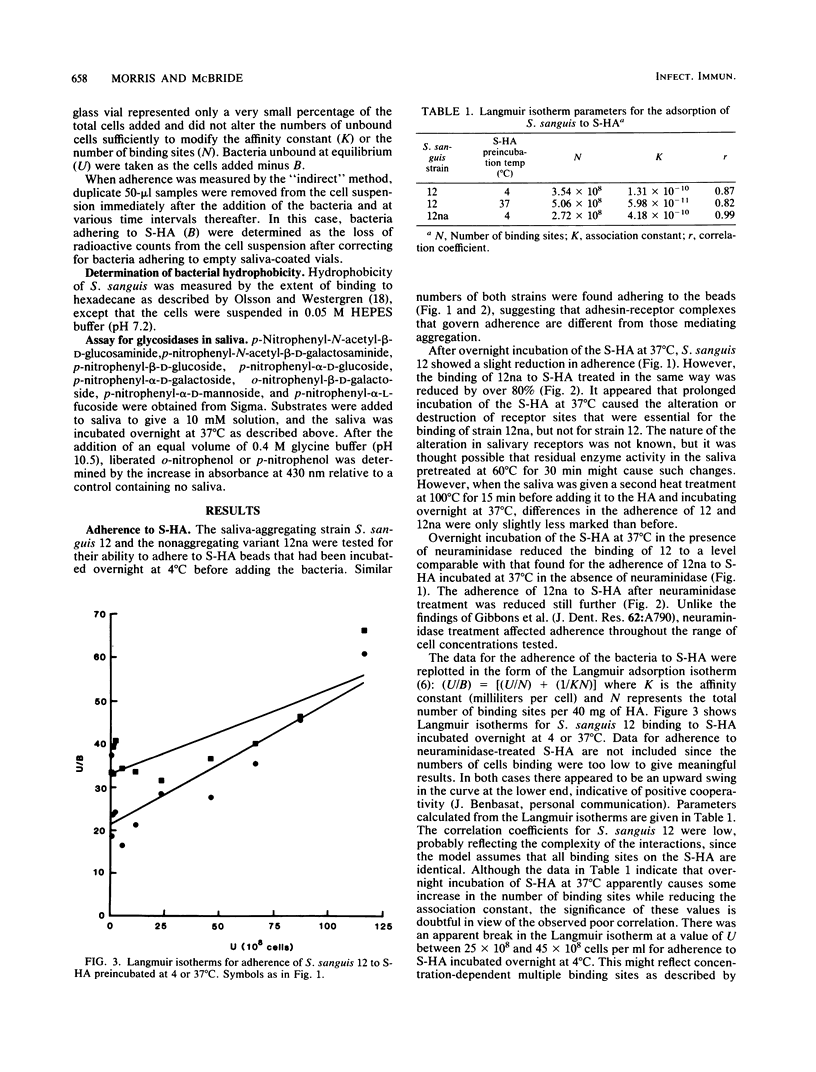
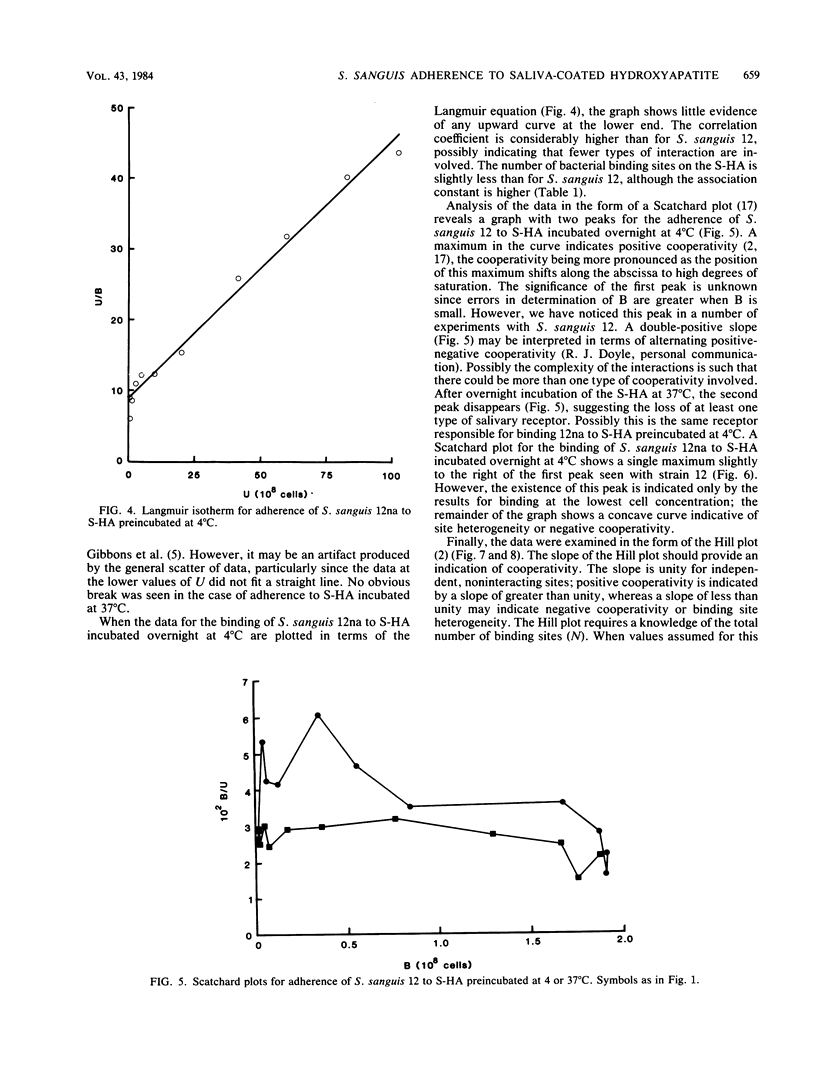
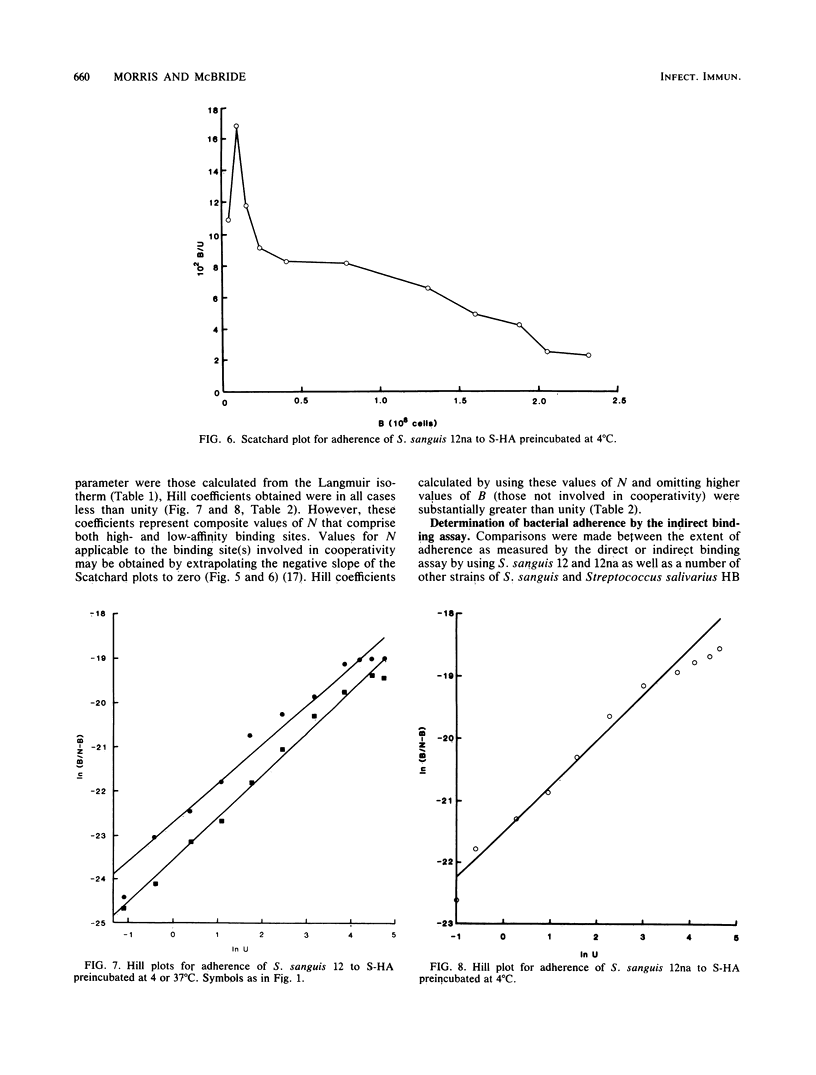
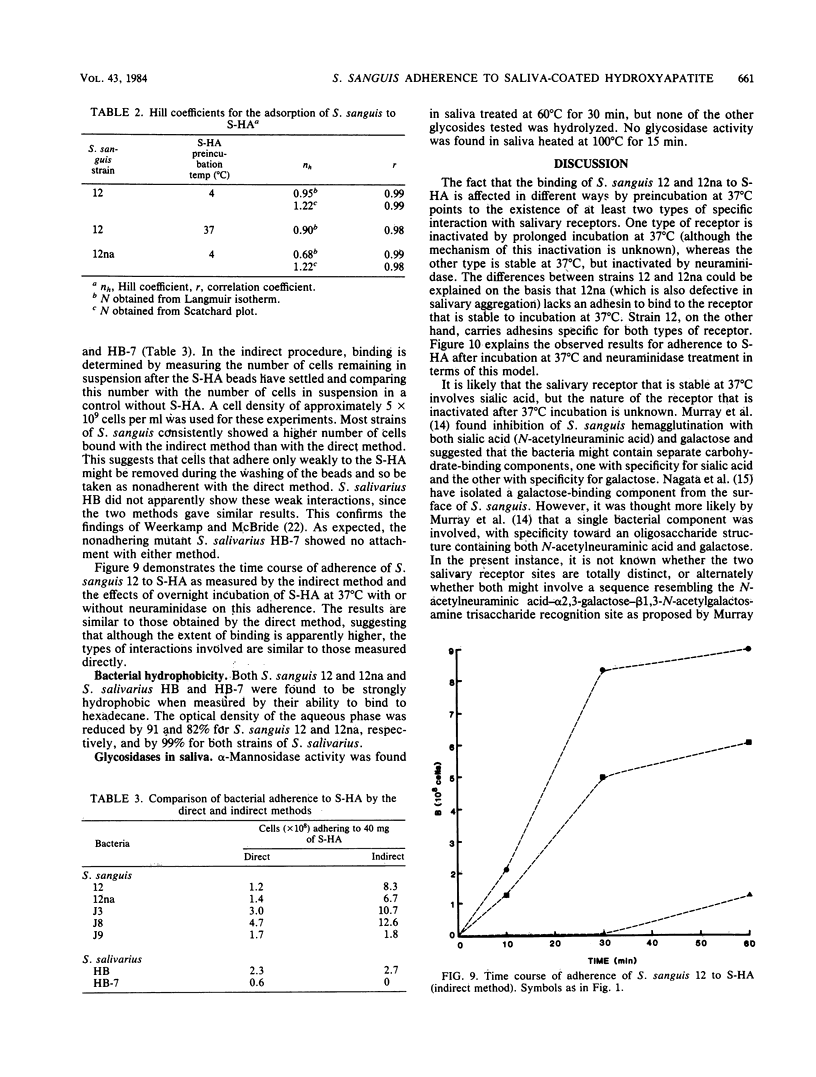
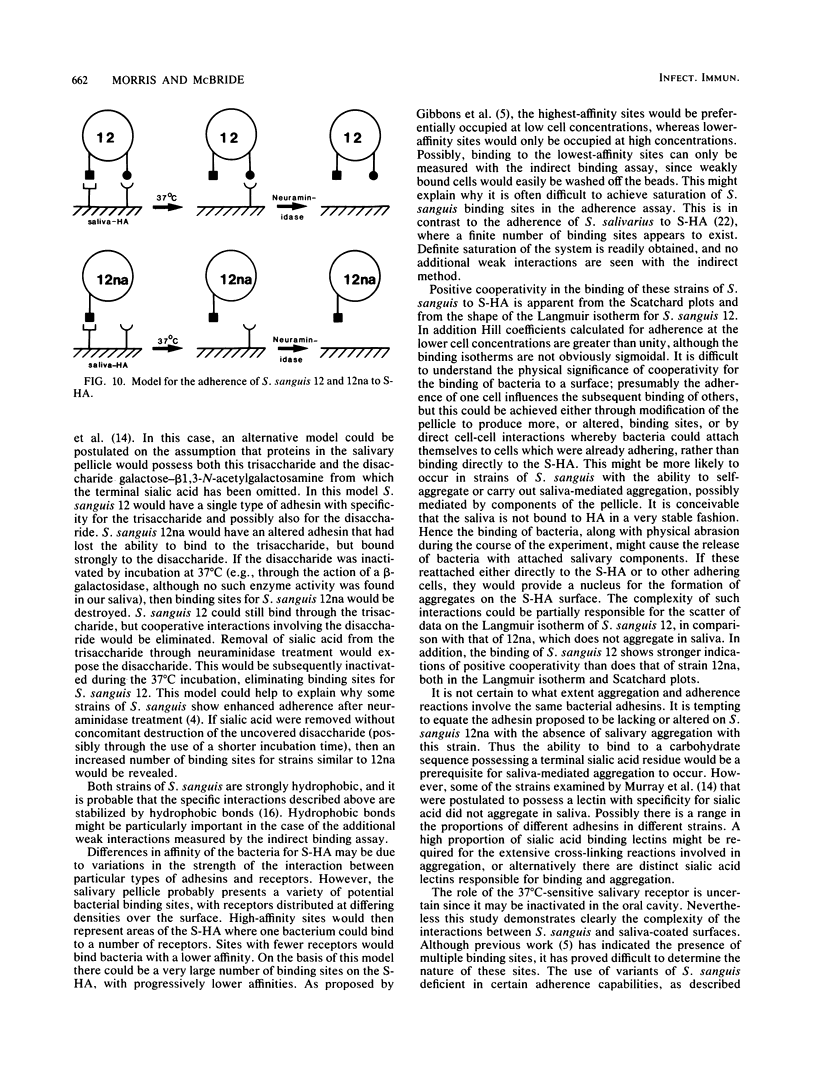
The characteristics of bacterial adherence to saliva-coated hydroxyapatite were examined for a salivary aggregating strain of Streptococcus sanguis, strain 12, and for its nonaggregating variant, strain 12na. Both strains were found to adhere in similar numbers to saliva-coated hydroxyapatite that had been preincubated at 4 degrees C overnight. Preincubation of saliva-coated hydroxyapatite overnight at 37 degrees C reduced subsequent adherence of S. sanguis 12 by approximately 10%, whereas adherence of S. sanguis 12na was reduced by over 80%. Preincubation at 37 degrees C in the presence of neuraminidase reduced adherence of S. sanguis 12 by over 90% and caused some additional reduction in adherence of S. sanguis 12na. The data were analyzed with Langmuir isotherms, Scatchard plots, and Hill plots. Some evidence of cooperativity was seen. A peak in the Scatchard plot for S. sanguis 12 binding to saliva-coated hydroxyapatite preincubated at 4 degrees C disappeared after preincubation at 37 degrees C, suggesting the loss of a salivary receptor. Many more organisms were found to bind when adherence was measured by assays counting the number of organisms remaining in suspension after the beads had settled. These weakly binding organisms, which were removed by washing, demonstrated adherence characteristics similar to those of the firmly bound organisms. Both strains were strongly hydrophobic. It is proposed that the binding of S. sanguis 12 and 12na involves two types of receptor on the salivary pellicle. One type of receptor is stable at 37 degrees C, but sensitive to neuraminidase; the second type is inactivated by prolonged incubation at 37 degrees C. S. sanguis 12 may bind to both types of receptor, whereas S. sanguis 12na binds only to the second type. The neuraminidase-sensitive receptor might be involved in saliva-mediated aggregation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark W. B., Gibbons R. J. Influence of salivary components and extracellular polysaccharide synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surfaces. Infect Immun. 1977 Nov;18(2):514–523. doi: 10.1128/iai.18.2.514-523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlquist F. W. The meaning of Scatchard and Hill plots. Methods Enzymol. 1978;48:270–299. doi: 10.1016/s0076-6879(78)48015-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I. Enzymatic modification of bacterial receptors on saliva-treated hydroxyapatite surfaces. Infect Immun. 1982 Apr;36(1):52–58. doi: 10.1128/iai.36.1.52-58.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Etherden I. Concentration-dependent multiple binding sites on saliva-treated hydroxyapatite for Streptococcus sanguis. Infect Immun. 1983 Jan;39(1):280–289. doi: 10.1128/iai.39.1.280-289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Spinell D. M. Model delineating the effects of a salivary pellicle on the adsorption of Streptococcus miteor onto hydroxyapatite. Infect Immun. 1976 Oct;14(4):1109–1112. doi: 10.1128/iai.14.4.1109-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman J. D., Van Houte J., Gibbons R. J. Sorption of bacteria to human enamel powder. Arch Oral Biol. 1970 Sep;15(9):899–903. doi: 10.1016/0003-9969(70)90163-9. [DOI] [PubMed] [Google Scholar]
- Hogg S. D., Embery G. Blood-group-reactive glycoprotein from human saliva interacts with lipoteichoic acid on the surface of Streptococcus sanguis cells. Arch Oral Biol. 1982;27(3):261–268. doi: 10.1016/0003-9969(82)90060-7. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G. Isolation of a protein-containing cell surface component from Streptococcus sanguis which affects its adherence to saliva-coated hydroxyapatite. Infect Immun. 1981 Nov;34(2):428–434. doi: 10.1128/iai.34.2.428-434.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Gisslow M. T. Role of sialic acid in saliva-induced aggregation of Streptococcus sanguis. Infect Immun. 1977 Oct;18(1):35–40. doi: 10.1128/iai.18.1.35-40.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. A., Levine M. J., Tabak L. A., Reddy M. S. Specificity of salivary-bacterial interactions: II. Evidence for a lectin on Streptococcus sanguis with specificity for a NeuAc alpha 2, 3Ga1 beta 1, 3Ga1NAc sequence. Biochem Biophys Res Commun. 1982 May 31;106(2):390–396. doi: 10.1016/0006-291x(82)91122-6. [DOI] [PubMed] [Google Scholar]
- Nagata K., Nakao M., Shibata S., Shizukuishi S., Nakamura R., Tsunemitsu A. Purification and characterization of galactosephilic component present on the cell surfaces of Streptococcus sanguis ATCC 10557. J Periodontol. 1983 Mar;54(3):163–172. doi: 10.1902/jop.1983.54.3.163. [DOI] [PubMed] [Google Scholar]
- Nesbitt W. E., Doyle R. J., Taylor K. G. Hydrophobic interactions and the adherence of Streptococcus sanguis to hydroxylapatite. Infect Immun. 1982 Nov;38(2):637–644. doi: 10.1128/iai.38.2.637-644.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt W. E., Doyle R. J., Taylor K. G., Staat R. H., Arnold R. R. Positive coooperativity in the binding of Streptococcus sanguis to hydroxylapatite. Infect Immun. 1982 Jan;35(1):157–165. doi: 10.1128/iai.35.1.157-165.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B., Malamud D., Appelbaum B., Golub E. Characteristic differences between saliva-dependent aggregation and adhesion of streptococci. Infect Immun. 1982 Jan;35(1):86–90. doi: 10.1128/iai.35.1.86-90.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Levine M. J., Cavese J. M., Prakobphol A., Murray P. A., Tabak L. A., Reddy M. S. Adherence of Streptococcus sanguis to salivary mucin bound to glass. J Dent Res. 1982 Dec;61(12):1390–1393. doi: 10.1177/00220345820610120101. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Banghart S. B. Adherence as a determinant of the presence of Streptococcus salivarius and Streptococcus sanguis on the human tooth surface. Arch Oral Biol. 1970 Nov;15(11):1025–1034. doi: 10.1016/0003-9969(70)90115-9. [DOI] [PubMed] [Google Scholar]
- Weerkamp A. H., McBride B. C. Adherence of Streptococcus salivarius HB and HB-7 to oral surfaces and saliva-coated hydroxyapatite. Infect Immun. 1980 Oct;30(1):150–158. doi: 10.1128/iai.30.1.150-158.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


