Abstract
The novel α1D L-type Ca2+ channel is expressed in supraventricular tissue and has been implicated in the pacemaker activity of the heart and in atrial fibrillation. We recently demonstrated that PKA activation led to increased α1D Ca2+ channel activity in tsA201 cells by phosphorylation of the channel protein. Here we sought to identify the phosphorylated PKA consensus sites on the α1 subunit of the α1D Ca2+ channel by generating GST fusion proteins of the intracellular loops, N terminus, proximal and distal C termini of the α1 subunit of α1D Ca2+ channel. An in vitro PKA kinase assay was performed for the GST fusion proteins, and their phosphorylation was assessed by Western blotting using either anti-PKA substrate or anti-phosphoserine antibodies. Western blotting showed that the N terminus and C terminus were phosphorylated. Serines 1743 and 1816, two PKA consensus sites, were phosphorylated by PKA and identified by mass spectrometry. Site directed mutagenesis and patch clamp studies revealed that serines 1743 and 1816 were major functional PKA consensus sites. Altogether, biochemical and functional data revealed that serines 1743 and 1816 are major functional PKA consensus sites on the α1 subunit of α1D Ca2+ channel. These novel findings provide new insights into the autonomic regulation of the α1D Ca2+ channel in the heart.
L-type Ca2+ channels are essential for the generation of normal cardiac rhythm, for induction of rhythm propagation through the atrioventricular node and for the contraction of the atrial and ventricular muscles (1–5). L-type Ca2+ channel is a multisubunit complex including α1, β and α2/δ subunits (5–7). The α1 subunit contains the voltage sensor, the selectivity filter, the ion conduction pore, and the binding sites for all known Ca2+ channel blockers (6–9). While α1C Ca2+ channel is expressed in the atria and ventricles of the heart (10–13), expression of α1D Ca2+ channel is restricted to the sinoatrial (SA)2 and atrioventricular (AV) nodes, as well as in the atria, but not in the adult ventricles (2, 3, 10).
Only recently it has been realized that α1D along with α1C Ca2+ channels contribute to L-type Ca2+ current (ICa-L) and they both play important but unique roles in the physiology/pathophysiology of the heart (6–9). Compared with α1C, α1D L-type Ca2+ channel activates at a more negative voltage range and shows slower current inactivation during depolarization (14, 15). These properties may allow α1D Ca2+ channel to play critical roles in SA and AV nodes function. Indeed, α1D Ca2+ channel knock-out mice exhibit significant SA dysfunction and various degrees of AV block (12, 16–19).
The modulation of α1C Ca2+ channel by cAMP-dependent PKA phosphorylation has been extensively studied, and the C terminus of α1 was identified as the site of the modulation (20–22). Our group was the first to report that 8-bromo-cAMP (8-Br-cAMP), a membrane-permeable cAMP analog, increased α1D Ca2+ channel activity using patch clamp studies (2). However, very little is known about potential PKA phosphorylation consensus motifs on the α1D Ca2+ channel. We therefore hypothesized that the C terminus of the α1 subunit of the α1D Ca2+ channel mediates its modulation by cAMP-dependent PKA pathway.
EXPERIMENTAL PROCEDURES
Subcloning of Intracellular Loops, N Terminus, Proximal or Distal C Terminus of the α1 Subunit of the Rat α1D Ca2+ Channel into pGEX-6P-1 Vector—pCMV6b/rat α1D plasmid was kindly provided by Dr. Susumu Seino from Kobe University, Japan. Two sets of primers were used to amplify each of the intracellular loops, proximal, or distal C terminus of α1 subunit of rat α1D Ca2+ channel. Forward primer had a BamHI site and a reverse primer had a SalI site. Intracellular loop 1: forward: 5′-cgcggatccggtgtccttagtggagaattc-3′; reverse: 5′-acgcgtcgacgacagacttcacagctgc-3′. Intracellular loop 2: forward: 5′-cgcggatccctgaagctcttcttggccat-3′; reverse: 5′-acgcgtcgacgtggtggttgatgagtttgtg-3′. Intracellular loop 3: forward: 5′-cgcggatccatgaatatcttcgtgggcttcg-3′; reverse: 5′-acgcgtcgaccgaggagttcaccacgta-3′. Proximal C terminus: forward: 5′-cgcggatccgacaattttgactatctgac-3′ and reverse: 5′-acgcgtcgacaagctgctcgtctcctga-3′. Distal C terminus: forward: 5′-cgcggatccccaaccattttccgtgaag-3′, reverse: 5′-acgcgtcgacctacaaggtggtgatgcaaa-3′. For the subcloning of the N terminus of α1 subunit of rat α1D Ca2+ channel into pGEX 6P-1 vector, the forward primer had a BamHI site, and the reverse primer had an EcoRI site. Primers: forward: 5′-cgcggatcccagcatcaacggcagcagcaa-3′; reverse: 5′-ccggaattctttccaatccactatactaat-3′.
All amplified fragments were then subcloned into a glutathione S-transferase (GST) bacterial expression vector pGEX-6p-1. The DH5α bacterial strain of Escherichia coli was transformed with the recombinant pGEX-6P-1 vectors, and sequencing of clones with recombinant vectors were confirmed by Genemed (South San Francisco, CA).
Expression of GST Fusion Proteins of Intracellular Loops, N Terminus, Proximal and Distal C Terminus of α1 Subunit of Rat α1D Ca2+ Channel—GST fusion proteins were expressed under the induction of the lactose analog isopropyl β-d-thiogalactoside as described before (Handbook of GST Gene Fusion System, 18-1157-58, Amersham Biosciences). Purification of the fusion proteins was performed as previously described (23). All samples were run on 12% Bis-acrylamide gel under reducing conditions and the gel was stained with Coomassie Blue. To check the success of the isopropyl-1-thio-β-d-galactopyranoside induction, DH5α transformed with pGEX-6P-1 served as a positive control. Untransformed DH5α E. coli and uninduced cultures of the transformed DH5α E. coli served as a negative control.
Western Blot with Anti-GST Antibody—Equal amounts of GST fusion proteins were loaded on 10% SDS-polyacrylamide gel. The samples were transferred to a nitrocellulose membrane, and the membrane was blocked with 5% nonfat dry milk and 3% bovine serum albumin in 0.1% Tween-20 TBS (TBST). The membrane was washed with 0.1% TBST, and was then probed with mouse monoclonal anti-GST at a dilution of 1:1000. The membrane was washed with 0.1% TBST before being probed with rabbit anti-mouse horseradish peroxidase conjugate antibody at 1:6000. The membrane was washed again with 0.1% TBST and was developed with the ECL Plus Western blotting detection system (Amersham Biosciences) and Kodak developer.
In Vitro Kinase Assay for PKA—The kinase assay was performed on immobilized GST fusion proteins as described by the manufacturer (PKA assay kit, 17-134, Millipore). Briefly, immobilized GST fusion proteins were washed five times with 1× Tris-buffered saline (TBS). Then 25 μl of kinase assay mixture was added to each tube. The mixture consisted of 5 μl of 5× reaction buffer, 2 ng of recombinant PKA catalytic subunit, 7.5 μl of double distilled water, and diluted 1:1 magnesium/ATP (PKA assay kit, 17–134, Millipore). The reaction was allowed to occur for 30 min at 30 °C in a water bath with shaking. The reaction was stopped by adding 2 × SDS loading buffer, and boiling for 5 min. Negative controls consisted of GST fusion proteins subjected to an in vitro kinase assay without the catalytic subunits of PKA.
Western Blot with Anti-PKA Substrate and Anti-phosphoserine Antibody—Equal amounts of phosphorylated GST fusion proteins or negative controls of GST fusion proteins, as outlined in the in vitro kinase assay, were loaded on 10% SDS-polyacrylamide gel. The samples were transferred to a nitrocellulose membrane, and the membrane was blocked with 5% non-fat dry milk and 3% bovine serum albumin in 0.1% TBST. The membrane was probed with mouse monoclonal anti-PKA substrate antibody at 1:200 for negative controls and at 1:500 for the remaining blots (PhosphoDetect™, 700 μg/ml, Calbiochem) or with mouse monoclonal anti-phosphoserine antibody at 1:500 for negative controls and at 1:1000 for the remaining blots (mouse ascites fluid, Sigma). The membrane was washed with 0.1% TBST before being probed with rabbit anti-mouse horseradish peroxidase conjugate antibody (1:2000 or 1:4000, Sigma). Membrane was washed again with 0.1% TBST and was developed with the ECL Plus Western blotting detection system (Amersham Biosciences) and a Kodak developer.
Mass Spectrometry—Protein bands of GST fusion proteins for intracellular loops, N terminus, proximal and distal C terminus of the α1 subunit of α1D Ca2+ channel were excised from Coomassie Blue-stained 12% polyacrylamide gels and subjected to in-gel trypsin or chymotrypsin digestion for liquid chromatography-mass spectrometry analysis protocol at the Proteomics & Mass Spectrometry Facility of the University of Massachusetts Medical School (UMMS). Negative controls, which consisted of GST fusion proteins subjected to an in vitro kinase assay without the catalytic subunits of PKA were also sent for mass spectrometric analysis.
Site-directed Mutagenesis—Site-directed mutagenesis was performed on pCMV6b/rat α1D using QuikChange TM site-directed mutagenesis kit from Stratagene (La Jolla, CA) according to the manufacturer's instructions. Serine residues were replaced by alanine in all substitution mutants. The following mutations were introduced by mutagenic primers: S1743A, S1816A, and S1964A. The presence of mutations was confirmed by sequencing at the Laval University sequencing facility (Quebec, Canada).
Transfection of the tsA201 Cell Line—Mammalian tsA201 cells were grown in high glucose Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, l-glutamine (2 mm), penicillin G (100 units/ml), and streptomycin (10 mg/ml, Invitrogen) from our laboratory. Cells were incubated in 5% CO2 humidified atmosphere. The tsA201 cells were transfected using the Ca2+ phosphate method with the following modification: to identify transfected cells, 7 μg of EBO/CD8 plasmid was cotransfected with 7 μg of each of the subunits of α1D Ca2+ channel: α1, β, and α2/δ cDNAs. For patch clamp experiments, 2–3 days post-transfection cells were incubated for 2 min in a medium containing anti-CD8a-coated beads (Dynabeads, M-450 CD8a). The unattached beads were removed by washing with extracellular solution (in mm): 135 choline chloride, 1 MgCl2, 2 CaCl2, and 10 HEPES; adjusted to pH 7.4 with tetraethylammonium hydroxide (TEA-OH). Cells expressing CD8a and therefore binding beads were distinguished from non-transfected cells by light microscopy.
Electrophysiology—Ca2+ currents were recorded in whole cell configuration of the patch clamp technique (pClamp 9, Axon Instrument) (2, 24). The internal solution contained (in mm): 135 CsCl, 4 MgCl2, 4 ATP, 10 HEPES, 10 EGTA, and 1 EDTA; adjusted to pH 7.2 with TEA-OH. Data were digitized at 5 kHz with an analog-to-digital converter. The recordings were filtered with a low-pass corner frequency of 2 kHz. For the time course, α1D Ca2+ current was continuously recorded at a test potential of 10 mV from a holding potential of 100 mV.
Statistics—Data were expressed as means ± S.E. Percent of inhibition was calculated as difference of the current amplitude by the intervention over the control value. When indicated, paired or unpaired Student's t test was performed. Differences were deemed significant at a p value of <0.05.
RESULTS
Generation of GST Fusion Proteins—Scansite showed that the α1 subunit of the α1D Ca2+ channel has a number of PKA consensus sites, mainly on the C terminus (Fig. 1A). To identify which of the potential PKA consensus sites is phosphorylated by PKA, constructs of GST fusion protein were designed in such a way that the GST tag was on the N terminus of different fragments of the α1 subunit of the α1D Ca2+ channel protein (Fig. 1B). GST fusion proteins spanning the intracellular loops, N terminus and C terminus of the α1 subunit of α1D Ca2+ channel were then generated (Fig. 2A). The expected size of GST fusion proteins of N terminus, proximal and distal C terminus is 43, 69, and 68 kDa, respectively. The expected size of GST fusion proteins of intracellular loops 1, 2, and 3, is 49, 44, and 36 kDa, respectively. Coomassie Blue staining showed that GST fusion proteins of N terminus, proximal and distal C terminus were migrating at higher than 40 kDa on polyacrylamide gel (Fig. 2A). Coomassie Blue stained gels also showed that GST fusion proteins of intracellular loops 1, 2, and 3 were migrating at 49, close to 49 and 36 kDa, respectively (Fig. 2A). Western blots showed the expression of GST fusion proteins of N terminus, proximal and distal C terminus, and intracellular loops (Fig. 2, B–D, respectively).
FIGURE 1.
Generation of GST fusion proteins of intracellular loops, N terminus and C terminus of the α1 subunit of α1D Ca2+ channel. A, sketch of the α1 subunit of α1D Ca2+ channel with the four domains, N terminus, proximal and distal C terminus. B, PKA consensus sites as determined by scansite on intracellular loops, proximal and distal C terminus of the α1 subunit of α1D Ca2+ channel. IC Loop, intracellular loop.
FIGURE 2.
Expression of GST fusion proteins. Representative Coomassie Blue staining showing GST fusion proteins. Two fragments that span the C terminus (proximal (69 kDa) and distal (68 kDa)), together with N terminus (43 kDa) and intracellular loops 1–3 (49, ∼49, and 36 kDa) of the α1 subunit of α1D Ca2+ channel were subcloned into the BamHI and SalI sites of pGEX vector. GST fusion proteins were separated by the GST affinity pull-down assay, run on 10% polyacrylamide gel under reducing conditions and stained with Coomassie Blue or probed with anti-GST antibodies. A, Coomassie Blue stain of GST fusion proteins of intracellular loops, N terminus, proximal and distal C terminus of α1 subunit of the α1D Ca2+ channel. B, Western blot of GST fusion proteins of N terminus, proximal, and distal C terminus probed with anti-GST antibody. C, Western blot of GST fusion protein of intracellular loops 1 and 2 probed with anti-GST antibody. D, Western blot of GST fusion proteins of intracellular loop 3 probed with anti-GST antibody. CT, C terminus; IC, intracellular. Note that on Coomassie gel (panel A), a 43-kDa marker was used. On the Western blot (panel B), a 45-kDa protein marker was used.
GST Fusion Proteins Were Phosphorylated—After successfully generating the GST fusion proteins of intracellular loops and the N terminus and C terminus of the α1 subunit of α1D Ca2+ channel, these fusion proteins were subjected to an in vitro PKA kinase assay followed by Western blot to determine which of the PKA consensus sites is phosphorylated by PKA. The phosphorylation status of the GST fusion proteins was tested by two antibodies. The first antibody was an anti-PKA substrate that is capable of detecting phosphorylated serines only in a PKA consensus site (arginine-arginine-x-serine, where x can be any amino acid). The second antibody was an anti-phosphoserine antibody that can detect phosphorylated serines regardless of the consensus site. Fig. 3 shows a Western blot using the two antibodies. The N terminus and proximal C terminus of the α1 subunit of α1D Ca2+ channel were phosphorylated (Fig. 3, A and B). The distal C terminus of the α1 subunit of α1D Ca2+ channel showed phosphorylation when probed with the anti-PKA substrate (Fig. 3A) and minimal when probed with anti-phosphoserine antibodies (Fig. 3B). These experiments were repeated four times, each giving identical results. In contrast, the intracellular loops of the α1 subunit of α1D Ca2+ channel were found to be minimally phosphorylated, both with the anti-PKA substrate and with the anti-phosphoserine antibodies (Fig. 3, C and D), while GST protein (negative control) was not found to be phosphorylated (Fig. 3, E and F).
FIGURE 3.
Western blot showing that the N terminus and proximal C terminus of the α1 subunit of α1D Ca2+ channel are phosphorylated by PKA. Immobilized GST fusion proteins of N terminus, proximal and distal C terminus of the α1 subunit of α1D Ca2+ channel were subjected to an in vitro PKA kinase assay. The fusion proteins were then run on 10% polyacrylamide gel and subjected to Western blotting using one of two antibodies: anti-PKA substrate or anti-phosphoserine. GST served as a negative control. A, Western blot of GST fusion proteins of N terminus, proximal and distal C terminus of the α1 subunit of α1D Ca2+ channel probed with anti-PKA substrate antibody. B, Western blot of GST fusion proteins of N terminus, proximal and distal C terminus of the α1 subunit of α1D Ca2+ channel probed with anti-phosphoserine antibodies. Distal C terminus of the α1 subunit of α1D Ca2+ channel was minimally phosphorylated. C, Western blot of GST fusion proteins of intracellular loops of the α1 subunit of α1D Ca2+ channel probed with anti-PKA substrate antibody. The antibodies detected minimal phosphorylation for intracellular loops 1 and 2 and almost none for intracellular loop 3. D, Western blot of GST fusion proteins of intracellular loops of the α1 subunit of α1D Ca2+ channel probed with anti-phosphoserine antibodies. The antibodies detected minimal phosphorylation of the three intracellular loops. E and F, Western blot of GST protein probed with anti-PKA substrate or anti-phosphoserine antibodies, respectively. CT, C terminus; IC, intracellular.
Mass Spectrometry Identified Phosphorylated Sites on GST Fusion Proteins—To identify the phosphorylated PKA consensus sites on the α1 subunit of α1D Ca2+ channel, mass spectrometry was performed on GST fusion proteins phosphorylated by PKA. The phosphorylated GST fusion proteins were run on a polyacrylamide gel, stained with Coomassie Blue, and the corresponding bands were excised. We performed an in-gel digestion with trypsin and chymotrypsin. Mass spectrometry identified two PKA consensus sites phosphorylated on the proximal C terminus of the α1 subunit of the α1D Ca2+ channel: serines 1743 and 1816 (Fig. 4, A–C). Mass spectrometry identified three more non-PKA consensus sites not identified by scansite and these were: serine 1703, serine 1788, and threonine 1795 (Fig. 4A). Mass spectrometric analysis did not detect any phosphorylated residues on GST (Fig. 4D) in agreement with our prior findings (Fig. 3, E and F).
FIGURE 4.
Phosphorylation of serine 1743 and serine 1816 identified by mass spectrometry on the proximal C terminus of the α1 subunit of α1D Ca2+ channel. Trypsinized proximal C terminus of the α1 subunit of α1D Ca2+ channel was subjected to mass spectrometry. A, amino acid sequence of proximal C terminus of α1 subunit of α1D Ca2+ channel with phosphorylated amino acid residues. Five sites were found to be phosphorylated by PKA (amino acids in red). Mass spectrometry covered 82% of the proximal C terminus of the α1 subunit of α1D Ca2+ channel. B and C, mass spectra for peptides containing serines 1816 and 1743, respectively. D, amino acid sequence of GST. No phosphorylated sites were detected on GST. Mass spectrometry covered 92% of GST.
The same approach applied for the proximal C terminus (mass spectrometry), we found that serine 1964 was phosphorylated. This residue is a PKA consensus site, phosphorylated in trypsinized digests of the distal C terminus of the α1 subunit of α1D Ca2+ channel. In addition, from our mass spectrometry studies, we identified a number of non-PKA consensus sites that were phosphorylated on the N terminus and distal C terminus of the α1 subunit of the α1D Ca2+ channel. These included serines 1944, 2152, 2165, and threonine 2147 phosphorylated on the distal C terminus (Fig. 5A). Like-wise, we found that serines 45, 46, 52, 121, and threonine 49 were all phosphorylated on the N terminus (Fig. 5B). These findings suggest that there are non-PKA consensus sites that can be phosphorylated by PKA on the N terminus and distal C terminus of the α1 subunit of the α1D Ca2+ channel.
FIGURE 5.
Mass spectrometry-identified phosphorylated sites on intracellular loop 1, N terminus, and distal C terminus on the α1 subunit of α1D Ca2+ channel. A, amino acid sequence of distal C terminus of the α1 subunit of α1D Ca2+ channel with phosphorylated amino acid residues. Four sites were found phosphorylated on distal C terminus of the α1 subunit of α1D Ca2+ channel by mass spectrometry (amino acids in gray and underlined). B, amino acid sequence of N terminus of the α1 subunit of α1D Ca2+ channel with phosphorylated amino acid residues. Four sites were found phosphorylated on the N terminus of the α1 subunit of α1D Ca2+ channel by mass spectrometry (amino acids in gray and underlined). Mass spectrometry covered 67% for both N terminus and distal C terminus ofα1 subunit ofα1D Ca2+ channel. C, amino acid sequence of intracellular loop 1 of the α1 subunit of α1D Ca2+ channel with phosphorylated amino acid residues. Four sites were found phosphorylated on intracellular loop 1 of the α1 subunit of α1D Ca2+ channel by mass spectrometry (amino acids in gray and underlined). Mass spectrometry covered 35% of intracellular loop 1 of the α1 subunit of α1D Ca2+ channel.
We also found non-PKA consensus sites on intracellular loop 1 of the α1 subunit of the α1D Ca2+ channel (Fig. 5C). On the other hand, we did not detect any phosphorylated residues on double digests of the other two loops. However, we identified phosphorylated non-PKA consensus sites on trypsinized digests of intracellular loop 2 of the α1 subunit of α1D Ca2+ channel. The phosphorylated residues on intracellular loop 1 were: serines 517, 519 and threonines 443, 504. For trypsinized digests of intracellular loop 2 the phosphorylated amino acid residues were: serines 857, 923, 929, and threonines 863.
Negative Controls of Intracellular Loop 1, N Terminus, Proximal and Distal C Terminus Were Not Phosphorylated—To test the antibodies and the mass spectrometric approaches, we performed an in vitro kinase assay without the catalytic subunit of PKA. No phosphorylated peptides were detected by Western blot (Fig. 6) nor by mass spectrometry.
FIGURE 6.
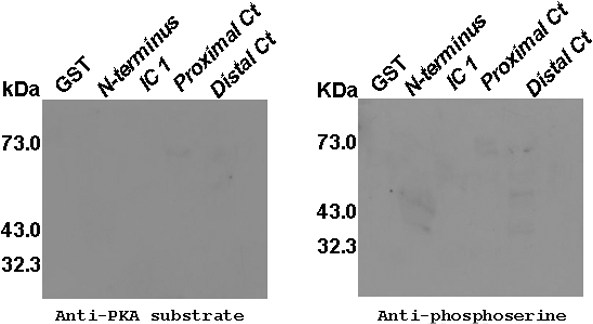
Negative controls of GST fusion proteins were not phosphorylated. Western blot of GST fusion proteins of intracellular loop 1, N terminus, proximal and distal C terminus of the α1 subunit of α1D Ca2+ channel after they have been subjected to an in vitro PKA kinase assay without PKA catalytic subunit. Western blots with either anti-PKA substrate or with anti-phosphoserine antibodies are shown with no bands. All of these fusion proteins were subjected to mass spectrometry, and no phosphorylated sites were detected.
Site-directed Mutagenesis and Patch Clamp Studies Showed That Serine 1816 Is Functionally Important—Of the nine PKA sites identified by scansite, 3 were found to be phosphorylated by mass spectrometry. These were serines 1743, 1816, and 1964. To test for their functional relevance, deletion mutants, and site-directed mutagenesis combined with electrophysiology experiments were performed.
We first prepared two deletion mutants of the C terminus of the α1D Ca2+ channel and expressed them in tsA201 cells. We then measured the basal current of these cells with or without the presence of 50 μm 8-Br-cAMP, which activates PKA in tsA201 cells (2). To minimize the variation of the current measurements, all current comparisons were made under similar conditions, which included using the same set of tsA201 cells, same amount of plasmids and same post transfection recording time.
The first deletion mutant with a stop codon at amino acid residue 1517 of the C terminus of the α1D Ca2+ channel resulted in the abolition of α1D ICa-L (data not shown). The second deletion mutant had a stop codon at amino acid residue 1917 of the C terminus of α1D Ca2+ channel. Cells expressing this deletion mutant showed an increase of α1D ICaL in response to 50 μm 8-Br-cAMP similar to that observed in cells expressing the wild-type (WT) α1D channel. These findings further support the unique importance of the proximal region of the C terminus of α1D Ca2+ channel.
We finally examined the modulation of single site mutants in response to 8-Br-cAMP (50 μm) (3). While treatment of tsA201 cells expressing WT α1D Ca2+ channels with 8-Br-cAMP caused a significant increase in α1D ICa-L amplitude (43.64 ± 9.6%) (Fig. 7A), substitution of serine 1743 by alanine reduced α1D ICa-L increase by PKA activation to 22.0 ± 4.2% (Fig. 7B). However, substitution of serine 1816 by alanine almost completely abolished 8-Br-cAMP-induced current increase (10.70 ± 3.3%) (Fig. 7C). Substitution of serine 1964 by alanine did not show any alteration in 8-Br-cAMP-induced enhancement of α1D ICa-L (data not shown). Averaged data for WT α1D ICaL, α1D/S1743A and α1D/S1816A are shown in Fig. 7D.
FIGURE 7.
α1D/S1743A and α1D/S1816A mutants had a decreased response to 8-Br-cAMP in tsA201 cells. Time course for the effect of 8-Br-cAMP (50 μm) on ICa-L of wild-type α1D Ca2+ channel, α1D/S1743A and α1D/S1816A expressed in tsA201 cell, respectively. A, 8-Br-cAMP increased ICa-L of wild-type α1D Ca2+ channel. B, response to 8-Br-cAMP was decreased in mutant α1D/S1743A Ca2+ channel. C, response to 8-Br-cAMP was almost completely abolished in mutant α1D/S1816A Ca2+ channel. D, averaged data of the increase of α1D ICa-L by 8-Br-cAMP (50 μm) in tsA201 cells. Differences were deemed significant at a p value of < 0.05.
DISCUSSION
Here we provide a systematic biochemical and proteomic approach for identifying phosphorylated PKA consensus sites on the α1 subunit of the α1D Ca2+ channel. We have identified serines 1743 and 1816 as two major functional PKA consensus sites. We also showed that serine 1816 has a major functional role in the α1D Ca2+ channel response to PKA as compared with serine 1743.
We also showed that the distal C terminus is phosphorylated by PKA, but no functional site was identified. We found that serine 1964, a PKA consensus site identified by scansite and mass spectrometry, was not functionally relevant.
Several phosphorylated non-PKA consensus sites on the proximal and distal C terminus were identified. However, we were mainly interested in characterizing PKA consensus sites identified by scansite and phosphorylated by PKA. We did not identify any PKA consensus sites on intracellular loops of the α1 subunit of the α1D Ca2+ channel. Although scansite identified several PKA consensus sites on intracellular loops 1 and 2 of α1D Ca2+ channel, none of these sites was phosphorylated by PKA. The data obtained with the site-directed mutants with S1816A and S1743A of α1D Ca2+ channel argue against the functional relevance of other PKA consensus sites present on the α1D Ca2+ channel. In fact, when serine 1816 was mutated to alanine the response of the α1D Ca2+ channel to 8-Br-cAMP was almost abolished.
The weak phosphorylation seen for intracellular loop 3 on Western blots can be accounted for by the presence of serines 1263 and 1264, two non-PKA consensus sites that were not detected by mass spectrometry. This occurred because treatment of the intracellular loop 3 peptide with trypsin or chymotrypsin generates small peptides that are of low mass to be detected. For example, tryptic digestion of the intracellular loop 3 peptide results in a small peptide, KFWYVVNSS, which has a molecular mass close to the detection threshold for mass spectrometry. Chymotrypsin generates peptides that are even smaller than the tryptic peptides.
Physiological Significance—The α1D Ca2+ channel has a more negative activation voltage (between -60 to -40 mV) than the α1C Ca2+ channel. This enables the channel to play an important role in phase 4 diastolic depolarization of the pacemaker (12, 15, 19, 25, 26). The restricted expression of the α1D Ca2+ channel to atria, SA, and AV nodes supports such a notion (2, 3, 10). Sinus bradycardia and second degree AV block seen in the α1D Ca2+ channel knock-out mice, shows that deletion of α1D Ca2+ channel seems to affect heart rate and conduction (12, 16, 17, 19). Furthermore, the α1D Ca2+ channel knock-out mice were prone to atrial fibrillation (12, 16, 17, 19).
The phosphorylation process constitutes one of the major regulatory pathways for cardiac L-type Ca2+ channels. Because the SA node is heavily innervated by the sympathetic nervous system (SNS) (29), stimulation of the SNS in response to exercise or stress insults results in a rapid and dramatic increase in heart rate. cAMP-dependent PKA is the point of convergence for the regulation of Ca2+ channels by many neurotransmitters and hormone receptors including the β1- and β2-adrenergic receptors and the serotoninergic 5-HT4 receptor, all of which are present in atrial cells (27, 28). PKA is a serine/threonine kinase that can be stimulated by extracellular signals that elevate intracellular cAMP concentrations which, in turn, can induce phosphorylation of the α1D L-type Ca2+ channel, leading to increased channel activity (30). Identifying consensus PKA phosphorylation sites α1D L-type Ca2+ channel is important to the understanding of cardiac rhythm regulation by the SNS.
A—In summary, the present work establishes that serines 1743 and 1816, two PKA consensus sites, are phosphorylated by PKA and are functionally relevant. The present data are the first to identify serine 1816 as a PKA consensus site that can be phosphorylated by PKA and is functionally relevant. Not all PKA consensus sites are functionally relevant, i.e. phosphorylation of these sites does not alter the activity of α1D Ca2+ channel in response to PKA activation and phosphorylation.
Acknowledgments
We thank Dr. Matthew Pincus for reading and editing this manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant HL 077494 from the NHLBI. This work was also supported by Veterans Administration Merit Grant Award (to M. B.) and the Veterans Administration MREP Grant (to Y. Q.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: SA, sinoatrial; AV, atrioventricular; PKA, cAMP-dependent protein kinase; GST, glutathione S-transferase; WT, wild type; SNS, sympathetic nervous system.
References
- 1.Brette, F., Leroy, J., Le Guennec, J. Y., and Salle, L. (2006) Prog. Biophys. Mol. Biol. 91 1-82 [DOI] [PubMed] [Google Scholar]
- 2.Qu, Y., Baroudi, G., Yue, Y., El-Sherif, N., and Boutjdir, M. (2005) Am. J. Physiol. Heart Circ. Physiol. 288 H2123-H2130 [DOI] [PubMed] [Google Scholar]
- 3.Qu, Y., Baroudi, G., Yue, Y., and Boutjdir, M. (2005) Circulation 111 3034-3041 [DOI] [PubMed] [Google Scholar]
- 4.Reuter, H. (1984) Annu. Rev. Physiol. 46 473-484 [DOI] [PubMed] [Google Scholar]
- 5.Hockerman, G. H., Peterson, B. Z., Johnson, B. D., and Catterall, W. A. (1997) Annu. Rev. Pharmacol. Toxicol. 37 361-396 [DOI] [PubMed] [Google Scholar]
- 6.Catterall, W. A. (2000) Annu. Rev. Cell Dev. Biol. 16 521-555 [DOI] [PubMed] [Google Scholar]
- 7.Zamponi, G. (2005) Voltage-Gated Calcium Channels, 1st Ed., pp. 61-94, Springer
- 8.Striessnig, J. (1999) Cell Physiol Biochem. 9 242-269 [DOI] [PubMed] [Google Scholar]
- 9.Dolphin, A. C. (2006) Br. J. Pharmacol. 147 Suppl. 1, S56-S62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takimoto, K., Li, D., Nerbonne, J. M., and Levitan, E. S. (1997) J. Mol. Cell Cardiol. 29 3035-3042 [DOI] [PubMed] [Google Scholar]
- 11.Marionneau, C., Couette, B., Liu, J., Li, H., Mangoni, M. E., Nargeot, J., Lei, M., Escande, D., and Demolombe, S. (2005) J. Physiol. 562 223-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang, Z., He, Y., Tuteja, D., Xu, D., Timofeyev, V., Zhang, Q., Glatter, K. A., Xu, Y., Shin, H. S., Low, R., and Chiamvimonvat, N. (2005) Circulation 112 1936-1944 [DOI] [PubMed] [Google Scholar]
- 13.Tellez, J. O., Dobrzynski, H., Greener, I. D., Graham, G. M., Laing, E., Honjo, H., Hubbard, S. J., Boyett, M. R., and Billeter, R. (2006) Circ. Res. 99 1384-1393 [DOI] [PubMed] [Google Scholar]
- 14.Bell, D. C., Butcher, A. J., Berrow, N. S., Page, K. M., Brust, P. F., Nesterova, A., Stauderman, K. A., Seabrook, G. R., Nurnberg, B., and Dolphin, A. C. (2001) J. Neurophysiol. 85 816-827 [DOI] [PubMed] [Google Scholar]
- 15.Xu, W., and Lipscombe, D. (2001) J. Neurosci. 21 5944-5951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platzer, J., Engel, J., Schrott-Fischer, A., Stephan, K., Bova, S., Chen, H., Zheng, H., and Striessnig, J. (2000) Cell 102 89-97 [DOI] [PubMed] [Google Scholar]
- 17.Mangoni, M. E., Traboulsie, A., Leoni, A. L., Couette, B., Marger, L., Le Quang, K., Kupfer, E., Cohen-Solal, A., Vilar, J., Shin, H. S., Escande, D., Charpentier, F., Nargeot, J., and Lory, P. (2006) Circ. Res. 98 1422-1430 [DOI] [PubMed] [Google Scholar]
- 18.Moosmang, S., Lenhardt, P., Haider, N., Hofmann, F., and Wegener, J. W. (2005) Pharmacol. Ther. 106 347-355 [DOI] [PubMed] [Google Scholar]
- 19.Zhang, Z., Xu, Y., Song, H., Rodriguez, J., Tuteja, D., Namkung, Y., Shin, H. S., and Chiamvimonvat, N. (2002) Circ. Res. 90 981-987 [DOI] [PubMed] [Google Scholar]
- 20.De Jongh, K. S., Murphy, B. J., Colvin, A. A., Hell, J. W., Takahashi, M., and Catterall, W. A. (1996) Biochemistry 35 10392-10402 [DOI] [PubMed] [Google Scholar]
- 21.Yang, L., Liu, G., Zakharov, S. I., Morrow, J. P., Rybin, V. O., Steinberg, S. F., and Marx, S. O. (2005) J. Biol. Chem. 280 207-214 [DOI] [PubMed] [Google Scholar]
- 22.Ganesan, A. N., Maack, C., Johns, D. C., Sidor, A., and O'Rourke, B. (2006) Circ. Res. 98 e11-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frangioni, J. V., and Neel, B. G. (1993) Anal. Biochem. 210 179-187 [DOI] [PubMed] [Google Scholar]
- 24.Baroudi, G., Qu, Y., Ramadan, O., Chahine, M., and Boutjdir, M. (2006) Am. J. Physiol. Heart Circ. Physiol. 291 H1614-H1622 [DOI] [PubMed] [Google Scholar]
- 25.Mangoni, M. E., Couette, B., Bourinet, E., Platzer, J., Reimer, D., Striessnig, J., and Nargeot, J. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 5543-5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthes, J., Yildirim, L., Wietzorrek, G., Reimer, D., Striessnig, J., and Herzig, S. (2004) Naunyn Schmiedebergs Arch. Pharmacol. 369 554-562 [DOI] [PubMed] [Google Scholar]
- 27.Ouadid, H., Seguin, J., Dumuis, A., Bockaert, J., and Nargeot, J. (1992) Mol. Pharmacol. 41 346-351 [PubMed] [Google Scholar]
- 28.Nargeot, J., Lory, P., and Richard, S. (1997) Eur. Heart J. 18 Suppl. A, A15-A26 [DOI] [PubMed] [Google Scholar]
- 29.Randall, W. C., Rinkema, L. E., and Jones, S. B. (1984) J. Auton. Nerv. Syst. 11 145-159 [DOI] [PubMed] [Google Scholar]
- 30.Stiles, G. L., and Lefkowitz, R. J. (1984) Annu. Rev. Med. 35 149-164 [DOI] [PubMed] [Google Scholar]








