Abstract
The purpose of this work was to obtain structural information about conformational changes in the membrane region of the sarcoplasmic reticulum (SERCA) and plasma membrane (PMCA) Ca2+ pumps. We have assessed changes in the overall exposure of these proteins to surrounding lipids by quantifying the extent of protein labeling by a photoactivatable phosphatidylcholine analog 1-palmitoyl-2-[9-[2′-[125I]iodo-4′-(trifluoromethyldiazirinyl)-benzyloxycarbonyl]-nonaoyl]-sn-glycero-3-phosphocholine ([125I]TID-PC/16) under different conditions. We determined the following. 1) Incorporation of [125I]TID-PC/16 to SERCA decreases 25% when labeling is performed in the presence of Ca2+. This decrease in labeling matches qualitatively the decrease in transmembrane surface exposed to the solvent calculated from crystallographic data for SERCA structures. 2) Labeling of PMCA incubated with Ca2+ and calmodulin decreases by approximately the same amount. However, incubation with Ca2+ alone increases labeling by more than 50%. Addition of C28, a peptide that prevents activation of PMCA by calmodulin, yields similar results. C28 has also been shown to inhibit ATPase SERCA activity. Interestingly, incubation of SERCA with C28 also increases [125I]TID-PC/16 incorporation to the protein. These results suggest that in both proteins there are two different E1 conformations as follows: one that is auto-inhibited and is in contact with a higher amount of lipids (Ca2+ + C28 for SERCA and Ca2+ alone for PMCA), and one in which the enzyme is fully active (Ca2+ for SERCA and Ca2+-calmodulin for PMCA) and that exhibits a more compact transmembrane arrangement. These results are the first evidence that there is an autoinhibited conformation in these P-type ATPases, which involves both the cytoplasmic regions and the transmembrane segments.
Although membrane proteins constitute more than 20% of the total proteins, the structure of only few of them is known in detail. An important group of integral membrane proteins are ion-motive ATPases. These proteins belong to the family of P-type ATPases, which share in common the formation of an acid-stable phosphorylated intermediate as part of its reaction cycle. Crystallographic information is available for a few members of this family. There are several crystal structures of the Ca2+ pump of sarcoplasmic reticulum (SERCA)2 revealing different conformations (1–5), and recently, crystal structures of the H+-ATPase (6) and of the Na,K-ATPase were reported as well (7).
We are interested in obtaining structural information about the plasma membrane calcium pump (PMCA). This pump is an integral part of the Ca2+ signaling mechanism (8). It is highly regulated by calmodulin, which activates this protein by binding to an auto-inhibitory region and changing the conformation of the pump from an inhibited state to an activated one (8, 9). Crystallization of PMCA is particularly challenging because there is no natural source from which this protein can be obtained in large quantities. Moreover, the presence of several isoforms in the same tissue further complicates efforts to obtain a homogeneous sample suitable for crystallization.
Information about the structure and assembly of the transmembrane domain of an integral membrane protein can also be obtained from the analysis of the lipid-protein interactions. In this work, we have used a hydrophobic photolabeling method to study the noncovalent interactions between PMCA and the surrounding phospholipids under different experimental conditions that lead to known conformations. We employed the photoactivatable phosphatidylcholine analog 1-palmitoyl-2-[9-[2′-[125I]iodo-4′-(trifluoromethyldiazirinyl)-benzyloxycarbonyl]-nonaoyl]-sn-glycero-3-phosphocholine ([125I]TID-PC/16) that has been previously used to analyze lipid-protein interfaces (10–12). This reagent is located in the phospholipidic milieu, and upon photolysis it reacts indiscriminately with its molecular neighbors. It is thus possible to directly analyze the interaction between a membrane protein and lipids belonging to its immediate environment (13–15). By measuring the amount of labeling of SERCA in conditions that promote conformations for which there are well resolved crystal structures, we were able to validate this photolabeling approach as a convenient tool for analyzing conformational changes within transmembrane regions. Furthermore, using this technique on PMCA and comparing the results obtained for SERCA, we were able to draw structural conclusions about these proteins under activated and inhibited states.
EXPERIMENTAL PROCEDURES
Reagents—All chemicals used in this work were of analytical grade and purchased mostly from Sigma. Recently drawn human blood for the isolation of PMCA was obtained from the Hematology Section of the Hospital de Clínicas General San Martín and from Fundación Fundosol (Argentina). Blood donation in Argentina is voluntary, and therefore the donor provides informed consent for the donation of blood and for the subsequent legitimate use of the blood by the transfusion service.
Purification of PMCA from Human Erythrocytes—PMCA was isolated from calmodulin-depleted erythrocyte membranes by the calmodulin-affinity chromatography procedure (16). Briefly, membrane proteins were solubilized in a 0.5% C12E10-containing buffer. After centrifugation the supernatant was loaded into a Sepharose-calmodulin column in the presence of 1 mm Ca2+. The column was thoroughly washed with 0.05% C12E10-containing buffer. PMCA was eluted in 20% (w/v) glycerol, 0.005% C12E10, 120 mm KCl, 1 mm MgCl2, 10 mm MOPS-K, pH 7.4, 4 °C, 2 mm EGTA, 2 mm dithiothreitol and stored in the same buffer. It has been demonstrated previously that both the conformation and the activity of the protein are preserved in either solubilized or reconstituted purified preparation compared with that located in the erythrocyte (16). Protein concentration after purification was about 10 μg/ml. No phospholipids were added at any step along the purification procedure. By measuring inorganic phosphate (see under “Phospholipid Quantification”), we estimated that less than 1 mol of natural phospholipids per mol of PMCA is present in the purified enzyme. The purification procedure described preserves transport activity and maintains the kinetic properties and regulatory characteristics of the enzyme in its native milieu (16).
SERCA Preparation—SERCA was directly solubilized with C12E10 (0.5%) from sarcoplasmic reticulum membranes (prepared from rabbit skeletal muscle as described previously (17)). This sample was diluted 100 times in a medium containing 20% (w/v) glycerol, 120 mm KCl, 1 mm MgCl2, 10 mm MOPS-K, pH 7.4, 4 °C, 2 mm EGTA. Protein concentration after dilution was 10 μg/ml.
Measurement of Ca2+-ATPase Activity—Ca2+-ATPase activity was measured at 37 °C as the initial velocity of release of Pi from ATP as described previously (16). The incubation medium was 7 nm PMCA, 120 mm KCl, 30 mm MOPS, 3.75 mm MgCl2, 1 mm EGTA, 1.1 mm CaCl2, 140 μm soybean phospholipids, 800 μm C12E10, and 2 mm ATP (pH 7.4, and [Ca2+] = 140 μm). Release of Pi was estimated according to the procedure of Fiske and SubbaRow (18). Measurements were performed in a Jasco V-630 Bio spectrophotometer.
Preparation of [125I]TID-PC/16—TTD-PC/16 (tin precursor) was a kind gift of Dr. J. Brunner (ETHZentrum, Zürich, Switzerland). [125I]TID-PC/16 was prepared by radioiodination of its tin precursor according to Weber and Brunner (12). After the reaction was completed, the mixture was extracted with chloroform/methanol (2:1, v/v), and [125I]TID-PC/16 was purified by passage through a silica gel column (2.5 ml) using chloroform/methanol/water/acetic acid (65:25:4:1, v/v) as solvent. The elution was monitored by TLC/autoradiography, and the fractions containing the product were dried and stored at –20 °C.
Phospholipid Quantification—Phospholipid concentration was measured according to Chen et al. (19) with some modifications. Samples and standards containing 10–100 nmol of phosphorus were dried by heating at 100 °C. Mineralization was carried out by adding 0.1 ml of HNO3, 0.9 ml of HClO4 and incubating at 190 °C for 30 min. Inorganic phosphate was determined after Fiske and SubbaRow (18).
Labeling Procedure—A dried film of the photoactivatable reagent was suspended in 1,2-dimyristoyl-sn-glycero-3-phosphocholine/C12E10 mixed micelles containing 10 μg/ml of the membrane protein. The samples were incubated for 20 min at 37 °C before being irradiated for 15 min with light from a filtered UV source (λ ≈ 360 nm).
Radioactivity and Protein Determination—Electrophoresis was performed according to the Tris-Tricine SDS-PAGE method (20). Polypeptides were stained with Coomassie Blue R; the isolated bands were excised from the gel, and the incorporation of radioactivity was directly measured on a gamma counter. The amount of protein was quantified by eluting each stained band, as described previously (21), including serum albumin in each gel as a standard for protein quantification. Specific incorporation was calculated as the ratio between measured radioactivity and amount of protein determined for each band.
Proteolysis of PMCA—Proteolysis of PMCA was performed for 3 min in the presence of 25 mm Tris-HCl, pH 7.4, at 37 °C, 100 μm free Ca2+, and 0.22 μg/ml TLCK-treated chymotrypsin (stored as a stock in dimethylformamide). The reaction was stopped by a 10-fold excess of ovomucoid trypsin inhibitor solution at 4 °C.
Immunoblotting—The slabs obtained after SDS-PAGE were electroblotted onto a poly(vinylidene difluoride) microporous membrane (Immobilon) by means of a semi-dry electroblotting system as described by Laurière (22). Before immunostaining, the Immobilon sheets were treated with blocking solution and then incubated with appropriate dilutions of the primary antibodies (see below) in phosphate-buffered saline at room temperature for 1 h. After rinsing with phosphate-buffered saline, the streptavidin-phosphatase conjugate anti-mouse and anti-rabbit IgG (Sigma) were added for 1 h; this step was followed by rinsing. Color development was obtained using 3-amino-9-ethylcarbazole (Sigma).
Antibodies—The monoclonal antibodies used here were raised against different regions of human erythrocyte Ca2+ pump, i.e. 5F10 recognizes the central region (residues 719–738), JA9 the N terminus (residues 51–75), and JA3 the C terminus (residues 1156–1180). The numbering of residues corresponds to human PMCA4b. Specificity was demonstrated by their reaction with the purified enzyme in an enzyme-linked immunosorbent assay system and by their staining in a Western blot (23–25).
Analysis of SERCA Structure and Accessible Surface Area—The crystal structures used for comparison were as follows: PDB code 1iwo (3), PDB code 1su4 (2), and PDB code 2ear (1). The transmembrane regions were taken as explicitly defined in Uniprot for sarcoplasmic/endoplasmic reticulum calcium ATPase 2, accession number P20647. The ASA of the transmembrane helices was calculated with MolMol (26, 27) with a solvent radio of 1.4 Å. Superposition of crystal structures was performed with POSA (28).
Analysis of the Data—All measurements were performed in triplicate to quintuplicate unless is specified in the figures. SDS gels presented under “Results” were chosen as representative of at least three independent experiments.
RESULTS
The Probe
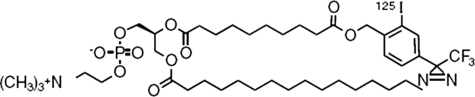
[125I]TID-PC/16 was previously used to identify and characterize regions within membrane proteins that interact with lipids (12, 13). This reagent is a PC analog endowed with a photoactivatable group at the end of one of the fatty acyl chains (Fig. 1). Its physicochemical behavior is indistinguishable from that of PC, i.e. it shows identical mobility on TLC plates using different solvent systems (data not shown). Moreover, its interaction with transmembrane regions has been shown to be identical to that of PC (14, 15). Additionally, regarding membrane protein-lipid interactions PC is considered the least selective lipid (29). In other words, transmembrane regions are expected to behave as an homogeneous surface displaying no specific binding sites for this phospholipid. This fact simplifies the interpretation of our results, allowing for a direct correlation between level of [125I]TID-PC/16 incorporation and amount of protein surface exposed to surrounding lipids.
FIGURE 1.
Photoactivatable phosphatidylcholine analog 1-palmitoyl-2-[9-[2′-[125I]iodo-4′-(trifluoromethyldiazirinyl)-benzyloxycarbonyl]-nonaoyl]-sn-glycero-3-phosphocholine, [125I]TID-PC/16.
Experiments with SERCA
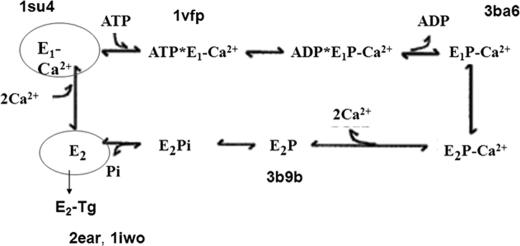
We have chosen SERCA as the P-type ATPase paradigm because a well defined three-dimensional structure is available for most of its conformations (1–5). Analysis of the results will be based on the SERCA reaction cycle proposed by de Meis and Vianna (30), as shown in Fig. 2. The cycle starts with binding of Ca2+ to E1. E1Ca is represented by the structure known as PDB code 1su4 (2). E1Ca binds ATP. E1Ca-ATP is represented by the PDB code 1vfp (4) structure. The release of ADP leads to E1PCa. The structure of E1PCa is known as PDB code 3ba6 (5). Conformational transition and release of two Ca2+ ions to the lumen yields E2P, represented by the PDB code 3b9b (5) structure. Finally, E2 conformation (stabilized by the inhibitor thapsigargin) is represented by PDB code structures 2ear (1) and 1iwo (3). The most striking rearrangement in protein structure occurs when the enzyme binds Ca2+, i.e. between 2ear (E2TG) and 1su4 (E1Ca). The different assembly of transmembrane segments in these conformations results in a decrease of the protein average exposure to surrounding lipids whenever Ca2+ is present. The extent of interaction between SERCA and membrane lipids under each condition was quantified by calculating the accessible surface area (ASA) of the transmembrane region. The results thus obtained are shown in Table 1.
FIGURE 2.
SERCA reaction cycle proposed by de Meis and Vianna (30) with the PDB names of the matching crystallized structures of the main reaction steps 2ear (1), 1iwo (3), 1su4 (2), 1vfp (4), 3ba6 (5), and 3b9b (5).
TABLE 1.
Accessible surface area of SERCA transmembrane region
Crystallographic data used for ASA calculations (26, 27) were taken from the following: 2ear (1), 1iwo (3), 1su4 (2), 1vfp (4), and 3ba6 and 3b9b (5). Tg means thapsigargin; AMPPCP means adenosine 5′-(β,γ -methylene)triphosphate.
| Crystal structure | Condition | ASA | Relative hydrophobicity | Conformation |
|---|---|---|---|---|
| Å2 | % | |||
| 2ear | Tg + EGTA | 8701 | 100 | E2 |
| 1iwo | Tg + EGTA | 8235 | 94.6 | E2 |
| 3b9b | BeF4- | 8164 | 93.8 | E2 |
| 1su4 | Ca2+ | 7114 | 81.7 | E1 |
| 1vfp | Ca2+ + AMPPCP | 7295 | 83.8 | E1 |
| 3ba6 | E1PCa | 7350 | 84.5 | E1 |
Effect of Ca2+
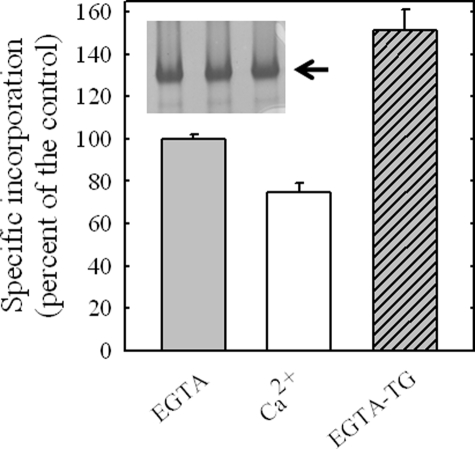
Fig. 3 shows the specific incorporation of [125I]TID-PC to SERCA in the presence of EGTA, Ca2+, and EGTA and thapsigargin. The extent of protein labeling measured in the presence of calcium was lower than that found in the presence of EGTA and considerably lower than that measured in the presence of EGTA and thapsigargin. This experimental result is in qualitative agreement with the predicted lipid-exposed area of the protein under each condition based on ASA calculations (Table 1). The effect of thapsigargin is probably the result of the stabilization of the enzyme in E2, i.e. under this condition SERCA is quantitatively fixed in a unique conformation. Indeed, it has only been possible to obtain SERCA crystals in the presence of EGTA provided that thapsigargin is also present.
FIGURE 3.
Relative specific incorporation of [125I]TID-PC/16 to SERCA in the presence of 1 mm EGTA and 300 nm thapsigargin (TG) and in the presence of 100 μm Ca2+. The incorporation of [125I]TID-PC/16 to SERCA in the presence of EGTA alone was taken as 100% (control). The inset shows a typical SDS-PAGE of SERCA. Values shown are the mean ± S.E. of six independent experiments.
Experiments with PMCA
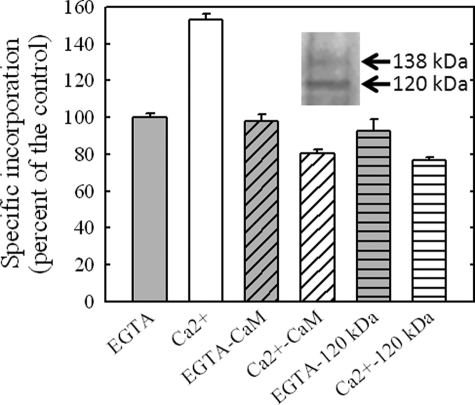
Effect of Ca2+ and Calmodulin—Because experiments with SERCA show that the incorporation of [125I]TID-PC/16 yields the results expected according to ASA calculations, we decided to quantify the amount of labeling of PMCA (for which no crystal structures are yet available) in different conditions. Fig. 4 shows that [125I]TID-PC/16 binds to PMCA in different amounts according to the conformational state of the enzyme. In the presence of Ca2+, the incorporation of [125I]TID-PC/16 is nearly 50% higher than the incorporation of the reagent in the absence of this ion. This result is opposite to that obtained for SERCA under identical conditions and indicates that in PMCA the amount of area directly exposed to membrane lipids increases upon Ca2+ binding.
FIGURE 4.
Effect of Ca2+, calmodulin, and partial proteolysis on PMCA-specific incorporation of [125I]TID-PC/16. PMCA was labeled in the presence of 1 mm EGTA and 100 μm Ca2+. Reagent incorporation was tested under these same conditions adding 1.6 μm CaM to the incubation medium or using a partially digested enzyme that lacks the CaM binding domain. Values are referred to that obtained for [125I]TID-PC/16 incorporation of PMCA in the presence of EGTA alone, which was taken as 100% (control). The inset shows the SDS-PAGE of the remaining undigested enzyme (138 kDa) and the resulting fragment (120 kDa) after partial proteolysis with TLCK-chymotrypsin. Values shown are the mean ± S.E. of at least three independent experiments.
The effect of 1.6 μm calmodulin on the incorporation of [125I]TID-PC/16 to PMCA is also shown. Labeling in the presence of calmodulin was carried out either in the presence of EGTA or in the presence of 100 μm Ca2+. The amount of labeling of PMCA in the presence of EGTA and calmodulin is identical to that obtained in the presence of EGTA alone. Under this condition in which no binding of calmodulin to PMCA occurs, a possible sequestering effect of the reagent by calmodulin is tested. Binding of calmodulin to the enzyme dramatically reduces the extent of protein labeling to about 52% of the labeling in the presence of Ca2+ alone, indicating that in this condition PMCA exhibits less lipid exposure than in any other conformational state. This behavior is similar to that observed for SERCA after addition of Ca2+ that leads E2 to E1. This suggests that regarding the transmembrane domain, the PMCA conformation in which the enzyme is no longer auto-inhibited, i.e. in the presence of calmodulin and Ca2+, is similar to that adopted by SERCA after addition of Ca2+ alone.
Ca2+ concentration used in these labeling experiments was similar to that routinely used for Ca2+-ATPase measurements. Experiments performed under the same conditions but lowering Ca2+ concentration to 1 μm yielded identical results to those shown in Fig. 4. Furthermore, Mg2+ failed to mimic the effects observed with Ca2+, i.e. protein labeling in the presence of 3.75 mm Mg2+ was indistinguishable from that determined in the presence of EGTA. This evidence strongly supports the specificity of the effects exerted by Ca2+ on the extent of protein labeling.
In addition, Fig. 4 shows the effect of removal of the PMCA C terminus by proteolysis with TLCK-chymotrypsin. It is well known that proteolysis of PMCA by chymotrypsin yields a pump that is fully active lacking the C-terminal region, and hence does not bind calmodulin. The inset of Fig. 4 shows the SDS-PAGE of the experiment. An immunoblot of the truncated PMCA (data not shown) reveals that JA3, a monoclonal antibody raised against residues 1156–1180, did not react with the truncated protein indicating the complete removal of the C terminus. In the presence of EGTA, the level of reagent incorporation to truncated PMCA is indistinguishable from that obtained under the same condition for the undigested enzyme. On the other hand, Ca2+ decreased the incorporation of [125I]TID-PC/16 in the truncated PMCA to 83%. This result indicates that cleavage of the calmodulin binding region of PMCA mimics not only its activation by calmodulin but also the effect of calmodulin on the incorporation of [125I]TID-PC/16 in its transmembrane domain.
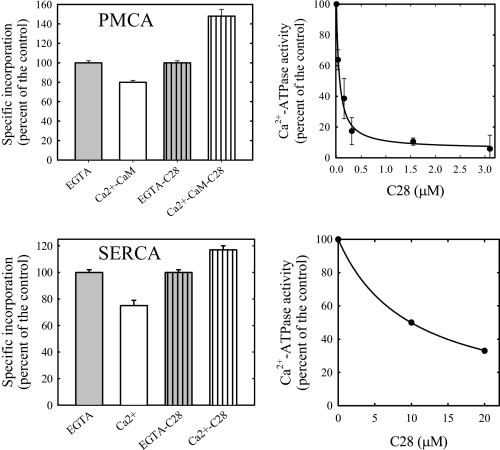
Effect of C28—To further explore the structural relationship between the level of exposure to phospholipids and activation by calmodulin, we assayed the effect of C28, a peptide made after the sequence of the calmodulin binding domain of PMCA. Fig. 5 shows that, as reported previously (31), C28 inhibits with high affinity the calmodulin activation of PMCA. This behavior is interpreted as the consequence of C28 binding to calmodulin, which preserves an auto-inhibited conformation of PMCA. In such conditions, C28 also reverts the effect of calmodulin on [125I]TID-PC/16 incorporation to PMCA. It has also been reported that at micromolar concentrations, C28 partially inhibits the Ca2+-ATPase activity of SERCA (31). As shown in Fig. 5, the presence of C28 results not only in the described inhibitory effect on SERCA activity but also in an almost 20% increment of [125I]TID-PC/16 incorporation. This result suggests that indeed C28 is able to promote an inhibited conformation in SERCA analogous to the one observed in PMCA in the absence of calmodulin.
FIGURE 5.
Effect of C28 on [125I]TID-PC/16 incorporation. PMCA and SERCA were labeled in the presence of 1 μm and 20 μm C28 respectively. These concentrations of C28 effectively inhibit CaM activation of PMCA and ATPase activity of SERCA as shown in the right panel. Values shown are the mean ± S.E. of at least three independent experiments.
Comparison between SERCA and PMCA
Effect of Other Ligands—Table 2 summarizes the relative incorporation of [125I]TID-PC/16 in the presence of different ligands, both in SERCA and in PMCA.
TABLE 2.
Relative incorporation of [125I]TID-PC/16
Results are the mean ± S.E. of 3–7 independent experiments. ND means not determined. E1A is an activated state of E1; E1I is an inhibited state of E1. TG means thapsigargin.
|
Condition
|
SERCA
|
PMCA
|
||
|---|---|---|---|---|
| Conformation | Specific incorporation | Conformation | Specific incorporation | |
| EGTA | E2 | 100 ± 3 | E2 | 100 ± 3 |
| EGTA + TG | E2 | 151 ± 10 | E2 | 100 ± 3 |
| EGTA + CaM | E2 | ND | E2 | 99 ± 3 |
| EGTA + C28 | E2 | 98 ± 3 | E2 | 99 ± 3 |
| EGTA + vanadate | E2 | 97 ± 5 | E2 | 100 ± 7 |
| Ca2+ | E1 | 75 ± 4 | E1I | 155 ± 2 |
| Ca2+ + CaM | E1 | 70 ± 7 | E1A | 80 ± 2 |
| Ca2+ + CaM + C28 | E1I | ND | E1I | 144 ± 3 |
| Ca2+ + C28 | E1I | 117 ± 3 | E1I | 150 ± 9 |
| Ca2+ + TG | E1; E2 | 91 ± 3 | E1I | 154 ± 8 |
| Ca2+ + LaIII | E1 | ND | E1I | 156 ± 4 |
| Ca2+ + ATP + LaIII | E1P | 89 ± 4 | E1P | 155 ± 8 |
| Ca2+ + vanadate | E1; E2 | 92 ± 3 | E1; E2 | 125 ± 9 |
From the observation of Table 2 we can draw the following conclusions. 1) Ca2+ has opposite effects on SERCA and PMCA when compared with pumps that had been labeled in the presence of EGTA or EGTA and thapsigargin. In SERCA Ca2+ decreases the amount of labeling, whereas in the case of PMCA it increases it. 2) Addition of calmodulin and Ca2+ (CaM + Ca2+) to PMCA decreases the amount of labeling in manner similar to how Ca2+ does to SERCA. 3) Addition of C28 to SERCA produces a larger level of incorporation of [125I]TID-PC/16, in a way similar to the effect of Ca2+ on PMCA in the absence of calmodulin. 4) Addition of La3+ and Ca2+ increases the labeling as Ca2+ alone does. 5) Finally, addition of La3+ + Ca2+ and ATP, a condition that blocks the interconversion between E1P and E2P (32), yields the same amount of labeling as Ca2+ alone. This suggests that E1P, the conformation stabilized by La3+ and ATP, has similar exposition to phospholipids than E1CaI. La3+ alone was not tested in SERCA.
Finally, vanadate has no effect on the incorporation if [125I]TID-PC/16 in the absence of Ca2+, and in the presence of Ca2+ has opposite effects in SERCA and PMCA. In SERCA, it increases the level of incorporation when compared with Ca2+ alone, and in the case of PMCA it decreases the incorporation level, again, when compared with Ca2+ alone. These results are consistent with the idea that vanadate partially antagonizes the effects of Ca2+ (33, 34).
DISCUSSION
We have developed a methodological approach based on the use of a photoactivatable probe to study the structure of the transmembrane region of PMCA in different conformations. By quantifying the amount of labeling by [125I]TID-PC/16, we were able to estimate the differential interaction of PMCA conformers with surrounding phospholipids. The procedure is validated by making comparisons with the known crystal structures of SERCA, for which a well defined three-dimensional structure is available (1–5).
A major structural difference between SERCA and PMCA is the presence of a C-terminal auto-inhibitory region in the latter. Activation of PMCA by calmodulin has been explained by binding of this protein to the C-terminal end, thus removing the inhibitory interactions from the cytosolic core. It should be noted that this hypothesis for auto-inhibition does not predict changes in the transmembrane region.
However, the results in this paper show for the first time that the auto-inhibited conformation is a distinct one (at least in the E1Ca phase of the PMCA reaction cycle) and that the conformational changes induced by auto-inhibition do expose additional surfaces to phospholipids. Because the vast majority of phospholipids present in our experiments is phosphatidylcholine and the photoactivatable reagent used is a phosphatidylcholine analog, no higher affinity sites are expected to be sensed under our experimental conditions. Nevertheless, the possibility that upon Ca2+ binding sites with different affinity for phospholipids are either exposed or buried cannot be ruled out. Efforts are currently underway in our laboratory to explore this alternative. It is worth noting that although PMCA has specific sites for acidic phospholipids (35), no such sites have been identified for neutral phospholipids. The auto-inhibited conformation was also obtained by adding the calmodulin-binding peptide C28 to an E1Ca-CaM state of PMCA, showing the reversibility of the conformational transition (36).
Calmodulin-binding peptides from related transporters have shown to inhibit SERCA as well (31). We show that addition of C28 in conditions in which this peptide inhibits SERCA also induces an increase in [125I]TID-PC/16 labeling in this pump as well. This fact suggests that C28 can induce an inhibitory conformation like the one present in PMCA in the absence of calmodulin. This evidence allows us to propose the existence of two different E1 conformations as follows: E1I is the auto-inhibited PMCA and E1A is the activated one (in the presence of calmodulin or after removing the C-terminal tail by controlled proteolysis). In this sense, the E1A is the conformation naturally present in SERCA. Addition of C28 to SERCA mimics the E1I conformation present in the auto-inhibited PMCA. It is noteworthy that a short hydrophilic peptide like C28 drives a profound change at the hydrophobic transmembrane region.
It is interesting to analyze the effects of two inhibitors of PMCA, La3+ and vanadate. La3+ is known to stabilize E1Ca, and in the presence of ATP it blocks the reaction cycle in E1P (32). The fact that La3+ and ATP produce the same level of incorporation than Ca2+ alone suggests that the transmembrane arrangement of E1P is similar to the one of E1Ca (E1I). On the other hand, early experiments on the kinetics of vanadate inhibition showed that it is antagonized by Ca2+ (33, 34), a result consistent with the idea of Ca2+ and vanadate binding to alternate conformations. In addition, vanadate binds to the E2 conformation of SERCA, and thus the apparent vanadate affinity also depends upon the El to E2 equilibrium. The intermediate level of reagent incorporation measured in the presence of vanadate and Ca2+ is indeed indicative of the coexistence of E1 and E2 states.
In conclusion, by detecting differences in the access of the photoactivatable probe [125I]TID-PC/16 to both PMCA and SERCA, we were able to assess lipid exposure of the transmembrane domain of well characterized kinetic intermediates. Moreover, we were able to uncover the involvement of the transmembrane region in the auto-inhibition of PMCA. Remarkably, we found out a similar conformational state generated by C28 in SERCA.
Acknowledgments
We are greatly indebted to Dr. J. Brunner, Department of Biochemistry, Swiss Federal Institute of Technology Zürich (ETHZ), Zürich, Switzerland, for the kind gift of TTD-PC/16 (tin precursor) and to Dr. Delia Takara from the School of Dentistry, Buenos Aires University, for the generous gift of purified SERCA.
This work was supported, in whole or in part, by a National Institutes of Health grant. This work was also supported by Fogarty International Center Grant R03TW006837 and by Agencia Nacional de Promoción Científica y Tecnológica, Consejo Nacional de Investigaciones Científicas y Técnicas, and Universidad de Buenos Aires Ciencia y Técnica from Argentina. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: SERCA, sarcoplasmic reticulum calcium pump; PMCA, plasma membrane calcium pump; ASA, accessible surface area; [125I]TID-PC/16, 1-O-hexadecanoyl-2-O-[9-[[[2-[125I]iodo-4-(trifluoromethyl-3H-diazirin-3-yl)benzyl]oxy]carbonyl] nonanoyl]-sn-glycero-3-phosphocholine; MOPS, 3-(N-morpholino)propanesulfonic acid; C12E10, polyoxyethylene(10)dodecyl ether/3,6,9,12,15,18,24,27,30-decaoxadotetracontan-1-ol; PDB, Protein Data Bank; TLCK, 1-chloro-3-tosylamido-7-amino-2-heptanone; Tricine, N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
References
- 1.Takahashi, M., Kondou, Y., and Toyoshima, C. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 5800–5805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toyoshima, C., Nakasako, M., Nomura, H., and Ogawa, H. (2000) Nature 405 647–655 [DOI] [PubMed] [Google Scholar]
- 3.Toyoshima, C., and Nomura, H. (2002) Nature 418 605–611 [DOI] [PubMed] [Google Scholar]
- 4.Toyoshima, C., and Mizutani, T. (2004) Nature 430 529–535 [DOI] [PubMed] [Google Scholar]
- 5.Olesen, C., Picard, M., Winther, A. M. L., Gyrup, C., Morth, J. P., Oxvig, C., Moller, J. V., and Nissen, P. (2007) Nature 450 1036–1042 [DOI] [PubMed] [Google Scholar]
- 6.Pedersen, B. P., Buch-Pedersen, M. J., Morth, J. P., Palmgren, M. G., and Nissen, P. (2007) Nature 450 1111–1114 [DOI] [PubMed] [Google Scholar]
- 7.Morth, J. P., Pedersen, B. P., Toustrup-Jensen, M. S., Sørensen, T. L., Petersen, J., Andersen, J. P., Vilsen, B., and Nissen, P. (2007) Nature 450 1043–1049 [DOI] [PubMed] [Google Scholar]
- 8.Sarkadi, B., Enyedi, A., Foldes-Papp, Z., and Gardos, G. (1986). J. Biol. Chem. 261 9552–9557 [PubMed] [Google Scholar]
- 9.Corradi, G. R., and Adamo, H. P. (2007) J. Biol. Chem. 282 35440–35448 [DOI] [PubMed] [Google Scholar]
- 10.Brunner, J., and Semenza, G. (1981) Biochemistry 20 7174–7182 [DOI] [PubMed] [Google Scholar]
- 11.Brunner, J. (1993) Annu. Rev. Biochem. 62 483–514 [DOI] [PubMed] [Google Scholar]
- 12.Weber, T., and Brunner, J. (1995) J. Am. Chem. Soc. 117 3084–3095 [Google Scholar]
- 13.Durrer, P., Galli, C., Hoenke, S., Corti, C., Gluck, R., Vorherr, T., and Brunner, J. (1996) J. Biol. Chem. 271 13417–13421 [DOI] [PubMed] [Google Scholar]
- 14.Villamil Giraldo, A. M., Castello, P. R., González Flecha, F. L., Moeller, J. V., Delfino, J. M., and Rossi, J. P. F. C. (2006) FEBS 580 607–612 [DOI] [PubMed] [Google Scholar]
- 15.Villamil Giraldo, A. M, Castello, P. R., González Flecha, F. L., Delfino, J. M., and Rossi, J. P. F. C. (2006) Cell Biochem. Biophys. 44 431–437 [DOI] [PubMed] [Google Scholar]
- 16.Filomatori, C. V., and Rega, A. F. (2003) J. Biol. Chem. 278 22265–22271 [DOI] [PubMed] [Google Scholar]
- 17.Andersen, J. P., Lassen, K., and Moeller, J. V. (1985) J. Biol. Chem. 260 371–380 [PubMed] [Google Scholar]
- 18.Fiske, C. H., and SubbaRow, Y. (1925) J. Biol. Chem. 179 66–71 [Google Scholar]
- 19.Chen, P. S., Toribara, T. Y., and Warner, H. (1956) Anal. Chem. 28 1756–1758 [Google Scholar]
- 20.Schaegger, H., and von Jagow, G. (1987) Anal. Biochem. 166 368–379 [DOI] [PubMed] [Google Scholar]
- 21.Ball, E. H. (1986) Anal. Biochem. 155 26–27 [DOI] [PubMed] [Google Scholar]
- 22.Laurière, M. (1993) Anal. Biochem. 212 206–211 [DOI] [PubMed] [Google Scholar]
- 23.Filoteo, A. G., Elwess, N. L., Enyedi, A., Caride, A., Aung, H. H., and Penniston, J. T. (1997). J. Biol. Chem. 272 23741–23747 [DOI] [PubMed] [Google Scholar]
- 24.Caride, A. J., Filoteo, A. G., Enyedi, A., Verma, A. K., and Penniston, J. T. (1996) Biochem. J. 316 353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adamo, H. P., Caride, A. J., and Penniston, J. T. (1992) J. Biol. Chem. 267 14244–14249 [PubMed] [Google Scholar]
- 26.Lee, B., and Richards, F. M. (1971) J. Mol. Biol. 55 379–400 [DOI] [PubMed] [Google Scholar]
- 27.Koradi, R., Billeter, M., and Wüthrich, K. (1996) J. Mol. Graphics 14 51–55 [DOI] [PubMed] [Google Scholar]
- 28.Ye, Y., and Adam, G. (2005) Bioinformatics (Oxf.) 21 2362–2369 [DOI] [PubMed] [Google Scholar]
- 29.Marsh, D., and Horvath, L. I. (1998) Biochim. Biophys. Acta 1376 267–296 [DOI] [PubMed] [Google Scholar]
- 30.de Meis, L., and Vianna, A. L. (1979) Annu. Rev. Biochem. 48 275–292 [DOI] [PubMed] [Google Scholar]
- 31.Enyedi, A., and Penniston, J. T. (1993) J. Biol. Chem. 268 17120–17125 [PubMed] [Google Scholar]
- 32.Lutherbacher, S., and Schatzmann, H. J. (1983) Experientia (Basel) 39 311–312 [DOI] [PubMed] [Google Scholar]
- 33.Rossi, J. P., Garrahan, P. J., and Rega, A. F. (1981) Biochim. Biophys. Acta 648 145–150 [DOI] [PubMed] [Google Scholar]
- 34.Lytton, J., Westlin, M., Burk, S. E., Shull, G. E., and MacLennan, D. H. (1992) J. Biol. Chem. 267 14483–14489 [PubMed] [Google Scholar]
- 35.Filoteo, A. G., Enyedi, A., and Penniston, J. T. (1992) J. Biol. Chem. 267 11800–11805 [PubMed] [Google Scholar]
- 36.Penheiter, A. R., Filoteo, A. G., Penniston, J. T., and Caride, A. J. (2005) Biochemistry 44 2009–2020 [DOI] [PubMed] [Google Scholar]