Abstract
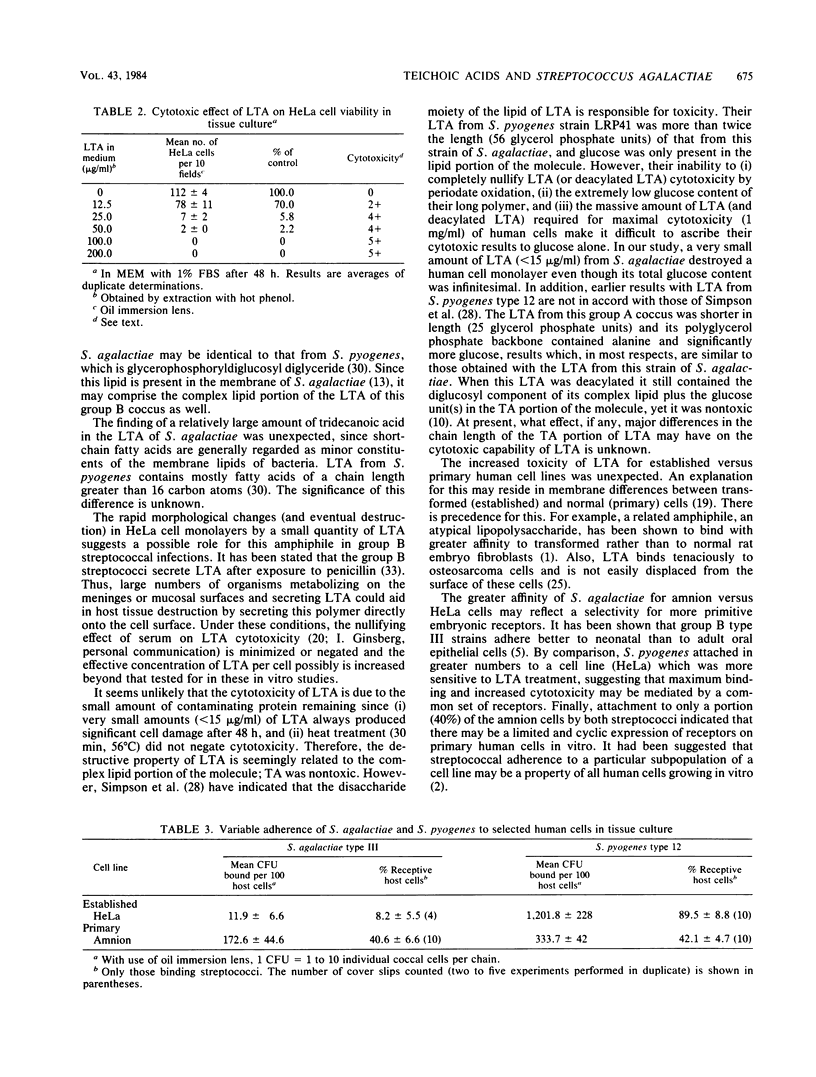
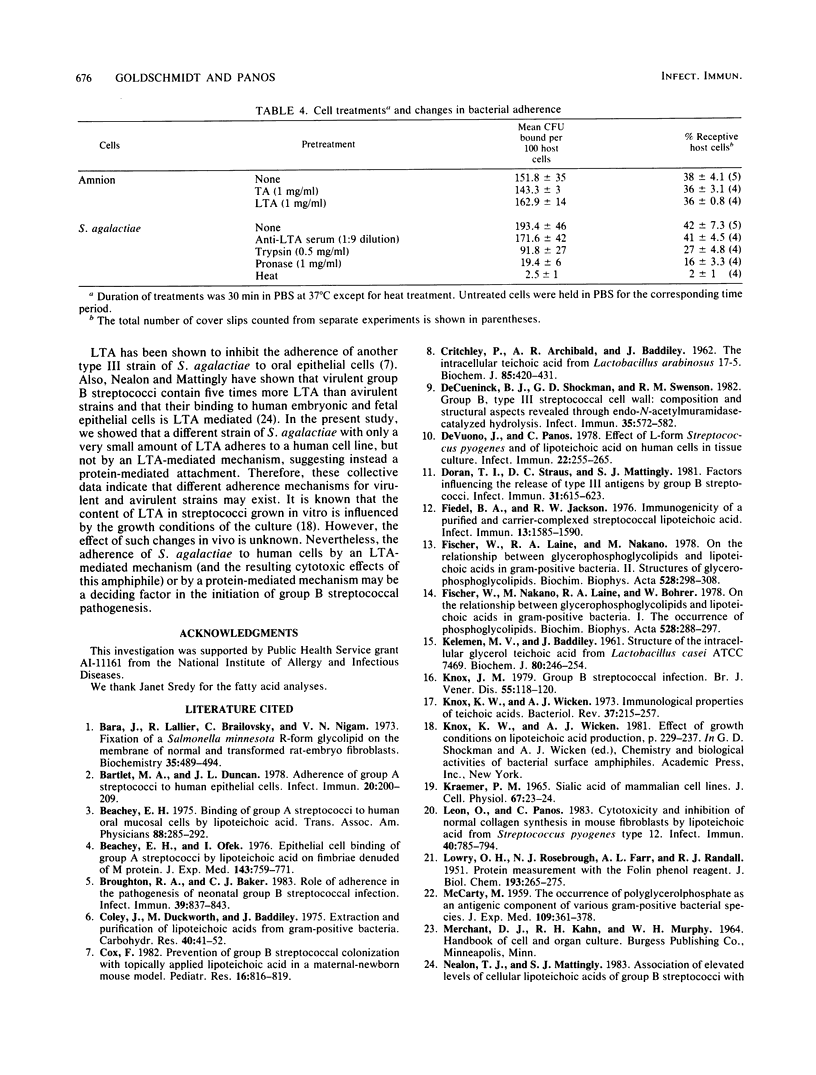
The ratio of teichoic acid to lipoteichoic acid (LTA) in a strain of Streptococcus agalactiae type III was found to be 8:1, with the total amount of LTA being 0.1% of the dry weight of the organism. Purified teichoic acid contained D-alanine and possibly a small amount of D-glucose and was approximately 22 glycerol phosphate units in length. The linkage between each of these units was 1-3. In addition, LTA contained a complex lipid, more glucose, and an unusually high content of a short-chain fatty acid, tridecanoic acid. This LTA was cytotoxic for a variety of human cell monolayers in tissue culture, including one derived from the human central nervous system. Established human cells were more sensitive than primary cell monolayers to this LTA, with as little as 12.5 micrograms of LTA per ml being cytotoxic for HeLa cells. Teichoic acid (250 micrograms/ml) was nontoxic under identical conditions. These cytotoxicity results suggest an LTA involvement in group B streptococcal pathogenesis. Also, the first model system for the study of group B streptococcal adherence to primary human embryonic amnion cells in tissue culture is detailed. This system was used to quantitate pronounced differences in tissue tropism between S. agalactiae and Streptococcus pyogenes and showed enhanced binding by this group A coccus over that of S. agalactiae for amnion cell monolayers. The adherence of both streptococcal species to only a portion (40%) of these amnion cells suggested that host cell receptor expression may vary for primary cells in vitro. Finally, this strain of S. agalactiae was shown to adhere to amnion cells by a non-LTA-mediated mechanism. The possibility of an LTA-mediated versus a protein-mediated adherence mechanism for host cells that is related to the virulence of S. agalactiae is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bara J., Lallier R., Brailovsky C., Nigam V. N. Fixation of a Salmonella minnesota R-form glycolipid on the membrane of normal and transformed rat-embryo fibroblasts. Eur J Biochem. 1973 Jun 15;35(3):489–494. doi: 10.1111/j.1432-1033.1973.tb02863.x. [DOI] [PubMed] [Google Scholar]
- Bartelt M. A., Duncan J. L. Adherence of group A streptococci to human epithelial cells. Infect Immun. 1978 Apr;20(1):200–208. doi: 10.1128/iai.20.1.200-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Binding of group A streptococci to human oral mucosal cells by lipoteichoic acid. Trans Assoc Am Physicians. 1975;88:285–292. [PubMed] [Google Scholar]
- Beachey E. H., Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med. 1976 Apr 1;143(4):759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton R. A., Baker C. J. Role of adherence in the pathogenesis of neonatal group B streptococcal infection. Infect Immun. 1983 Feb;39(2):837–843. doi: 10.1128/iai.39.2.837-843.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRITCHLEY P., ARCHIBALD A. R., BADDILEY J. The intracellular teichoic acid from Lactobacillus arabinosus 17-5. Biochem J. 1962 Dec;85:420–431. doi: 10.1042/bj0850420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley J., Duckworth M., Baddiley J. Extraction and purification of lipoteichoic acids from Gram-positive bacteria. Carbohydr Res. 1975 Mar;40(1):41–52. doi: 10.1016/s0008-6215(00)82667-1. [DOI] [PubMed] [Google Scholar]
- Cox F. Prevention of group B streptococcal colonization with topically applied lipoteichoic acid in a maternal-newborn mouse model. Pediatr Res. 1982 Oct;16(10):816–819. doi: 10.1203/00006450-198210000-00003. [DOI] [PubMed] [Google Scholar]
- De Cueninck B. J., Shockman G. D., Swenson R. M. Group B, type III streptococcal cell wall: composition and structural aspects revealed through endo-N-acetylmuramidase-catalyzed hydrolysis. Infect Immun. 1982 Feb;35(2):572–581. doi: 10.1128/iai.35.2.572-581.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVuono J., Panos C. Effect of L-form Streptococcus pyogenes and of lipoteichoic acid on human cells in tissue culture. Infect Immun. 1978 Oct;22(1):255–265. doi: 10.1128/iai.22.1.255-265.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran T. I., Straus D. C., Mattingly S. J. Factors influencing release of type III antigens by group B streptococci. Infect Immun. 1981 Feb;31(2):615–623. doi: 10.1128/iai.31.2.615-623.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedel B. A., Jackson R. W. Immunogenicity of a purified and carrier-complexed streptococcal lipoteichoic acid. Infect Immun. 1976 Jun;13(6):1585–1590. doi: 10.1128/iai.13.6.1585-1590.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Laine R. A., Nakano M. On the relationship between glycerophosphoglycolipids and lipoteichoic acids in Gram-positive bacteria. II. Structures of glycerophosphoglycolipids. Biochim Biophys Acta. 1978 Mar 30;528(3):298–308. doi: 10.1016/0005-2760(78)90019-x. [DOI] [PubMed] [Google Scholar]
- Fischer W., Nakano M., Laine R. A., Bohrer W. On the relationship between glycerophosphoglycolipids and lipoteichoic acids in Gram-positive bacteria. I. The occurrence of phosphoglycolipids. Biochim Biophys Acta. 1978 Mar 30;528(3):288–297. doi: 10.1016/0005-2760(78)90018-8. [DOI] [PubMed] [Google Scholar]
- KELEMEN M. V., BADDILEY J. Structure of the intracellular glycerol teichoic acid from Lactobacillus casei A.T.C.C. 7469. Biochem J. 1961 Aug;80:246–254. doi: 10.1042/bj0800246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox J. M. Group B streptococcal infection: a review and update. Br J Vener Dis. 1979 Apr;55(2):118–120. doi: 10.1136/sti.55.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer P. M. Sialic acid of mammalian cell lines. J Cell Physiol. 1966 Feb;67(1):23–34. doi: 10.1002/jcp.1040670104. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leon O., Panos C. Cytotoxicity and inhibition of normal collagen synthesis in mouse fibroblasts by lipoteichoic acid from Streptococcus pyogenes type 12. Infect Immun. 1983 May;40(2):785–794. doi: 10.1128/iai.40.2.785-794.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCARTY M. The occurrence of polyglycerophosphate as an antigenic component of various gram-positive bacterial species. J Exp Med. 1959 Apr 1;109(4):361–378. doi: 10.1084/jem.109.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealon T. J., Mattingly S. J. Association of elevated levels of cellular lipoteichoic acids of group B streptococci with human neonatal disease. Infect Immun. 1983 Mar;39(3):1243–1251. doi: 10.1128/iai.39.3.1243-1251.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady R. L., Harrop P. J., Knox K. W., Wicken A. J. Studies on the binding of lipoteichoic acid to osseous tissue. J Periodontal Res. 1980 Mar;15(2):206–215. doi: 10.1111/j.1600-0765.1980.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Panos C., Leon O. Replacement of the octadecenoic acid growth-requirement for Acholeplasma laidlawii A by cis-9,10-methylenehexadecanoic acid, a cyclopropane fatty acid. J Gen Microbiol. 1974 Jan;80(1):93–100. doi: 10.1099/00221287-80-1-93. [DOI] [PubMed] [Google Scholar]
- Silvestri L. J., Craig R. A., Ingram L. O., Hoffmann E. M., Bleiweis A. S. Purification of lipoteichoic acids by using phosphatidyl choline vesicles. Infect Immun. 1978 Oct;22(1):107–118. doi: 10.1128/iai.22.1.107-118.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. A., Dale J. B., Beachey E. H. Cytotoxicity of the glycolipid region of streptococcal lipoteichoic acid for cultures of human heart cells. J Lab Clin Med. 1982 Jan;99(1):118–126. [PubMed] [Google Scholar]
- Slabyj B. M., Panos C. Membrane lipoteichoic acid of Streptococcus pyogenes and its stabilized L-form and the effect of two antibiotics upon its cellular content. J Bacteriol. 1976 Aug;127(2):855–862. doi: 10.1128/jb.127.2.855-862.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabyj B. M., Panos C. Teichoic acid of a stabilized L-form of Streptococcus pyogenes. J Bacteriol. 1973 Jun;114(3):934–942. doi: 10.1128/jb.114.3.934-942.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D. C., Mattingly S. J., Milligan T. W., Doran T. I., Nealon T. J. Protease production by clinical isolates of type III group B streptococci. J Clin Microbiol. 1980 Sep;12(3):421–423. doi: 10.1128/jcm.12.3.421-423.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synge R. L. A further study of hydrolysates of gramicidin. Biochem J. 1949;44(5):542–548. doi: 10.1042/bj0440542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. From penicillin-binding proteins to the lysis and death of bacteria: a 1979 view. Rev Infect Dis. 1979 May-Jun;1(3):434–467. doi: 10.1093/clinids/1.3.434. [DOI] [PubMed] [Google Scholar]
- Tomlinson K., Leon O., Panos C. Morphological changes and pathology of mouse glomeruli infected with a streptococcal L-form or exposed to lipoteichoic acid. Infect Immun. 1983 Dec;42(3):1144–1151. doi: 10.1128/iai.42.3.1144-1151.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Bacterial cell surface amphiphiles. Biochim Biophys Acta. 1980 May 27;604(1):1–26. doi: 10.1016/0005-2736(80)90583-0. [DOI] [PubMed] [Google Scholar]
- Wilkinson H. W. Analysis of group B streptococcal types associated with disease in human infants and adults. J Clin Microbiol. 1978 Feb;7(2):176–179. doi: 10.1128/jcm.7.2.176-179.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]




