Abstract
Secretory IgA (SIgA) is the most prevalent human antibody and is central to mucosal immunity. It exists as two subclasses, SIgA1 and SIgA2, where SIgA2 has a shorter hinge joining the Fab and Fc regions. Both forms of SIgA are predominantly dimeric and contain an additional protein called the secretory component (SC) that is attached during the secretory process and is believed to protect SIgA in harsh mucosal conditions. Here we locate the five SC domains relative to dimeric IgA2 within SIgA2 using constrained scattering modeling. The x-ray and sedimentation parameters showed that SIgA2 has an extended solution structure. The constrained modeling of SIgA2 was initiated using two IgA2 monomers that were positioned according to our best fit solution structure for dimeric IgA1. SC was best located along the convex edge of the Fc-Fc region. The best fit models showed that SIgA2 is significantly nonplanar in its structure, in distinction to our previous near planar SIgA1 structure. Both the shorter IgA2 hinges and the presence of SC appear to displace the four Fab regions out of the Fc plane in SIgA2. This may explain the noncovalent binding of SC in some SIgA2 molecules. This nonplanar structure is predicted to result in specific immune properties for SIgA2 and SIgA1. It may explain differences observed between the SIgA1 and SIgA2 subclasses in terms of their interactions with antigens, susceptibility to proteases, effects on receptors, and distribution in different tissues. The different structures account for the prevalence of both forms in mucosal secretions.
The human mucosal surfaces have an estimated surface area of 400 m2 and constitute the largest surface area in contact with the external environment when compared with the 1.8 m2 area of the external skin (1–3). Secretory immunoglobulin A (SIgA)2 acts as the first line of mucosal defense against pathogenic bacteria, their toxic products, and other antigens (4). SIgA binds and neutralizes these pathogens by a passive blocking mechanism but may also trigger specific cellular responses and complement-mediated effector functions. More IgA is produced daily than all the other antibody classes combined (1). In terms of its involvement with immunity, IgA is unique. It is the only human antibody that exists in multiple oligomeric states; different forms are predominant in different mucosal environments (1). Mucosal SIgA is mostly dimeric (with some trimeric and tetrameric forms). It is present as two subclasses, IgA1 and IgA2, the latter being found in at least two allotypic forms, IgA2m(1) and IgA2m(2). Slightly more SIgA1 is generally present than SIgA2, except in the colon where there is more SIgA2 (1–3). The major interest in IgA2 is that this is more similar to the IgA that is present in most other mammalian species including rodents, rabbits having up to 13 IgA2-like subclasses (5). IgA1 homologues have only been found in higher apes (1).
All of the IgA monomers contain two heavy and two light chains that possess 12 domains, two heavy chain C-terminal tailpieces, and N-linked glycosylation sites at Asn263 and Asn459 (Fig. 1) (6). The IgA2 allotypes contain two extra N-linked glycosylation sites at Asn166 and Asn337 in the CH1 and CH2 domains, respectively (Fig. 1). In the most common IgA2 allotype, IgA2m (1), the light chains are covalently linked by their C-terminal Cys214 residues rather than to the heavy chains (Fig. 1) (7). IgA1 and IgA2 differ the most in the hinge between the Fab and Fc regions (Fig. 1). The IgA1 hinge is 13 residues longer than the IgA2 hinge and is O-glycosylated (8, 9). The IgA1 hinge is uniquely sensitive to a specific group of microbial proteases that do not cleave IgA2. A dimer of two 12-domain IgA monomers is bound covalently through a glycosylated joining (J) chain at the base of the Fc regions (Fig. 1). This IgA dimer (dIgA) is transcytosed by the polymeric immunoglobulin receptor across the epithelium to the luminal membrane, at which the receptor is cleaved to release five extracellular domains that are now called secretory component (SC). The dimeric IgA-SC complex comprises SIgA (Fig. 1). SC is covalently bound to a CH2 domain in one IgA1 monomer in SIgA1, yet it has been observed that this covalent bond can be absent in SIgA2 (10, 11). When SIgA1 is formed, resistance of the α chain to proteolysis is significantly increased (12, 13). The protection against proteases is lower in SIgA2 (11).
FIGURE 1.
Domain structure of SIgA2. SIgA2 is shown as two IgA2m(1) monomers, a J chain, and a secretory component (domains D1–D5). Each IgA2 heavy chain contains the VH, CH1, CH2, and CH3 domains. Each light chain contains the VL and CL domains. The complementarity-determining regions (CDR), the 13-residue hinge and the 18-residue C-terminal tailpiece are highlighted. The IgA2m(2) allotype does not have a Cys214–Cys214 disulfide bridge. The possible interheavy chain disulfide bridges at Cys241, Cys242, Cys299, and Cys301 are denoted by an extended X. Cys471 in one tailpiece of each Fc region is disulfide-bridged with either Cys15 or Cys68 in the J chain, which is located on the convex edge of the Fc-Fc region. A Cys311–Cys502 bridge between the CH2 and D5 domains is shown. N-Linked oligosaccharide sites are denoted by filled symbols (•).
For over three decades after the original electron microscopy study showed the generally accepted end-to-end arrangement of two IgA monomers (14), the structural assembly of SIgA remained unclear. Electron microscopy could not locate SC within SIgA. Many cartoons suggested that the SC domains were wrapped around the Fc-Fc dimer to protect this (1, 3, 15, 16). A new method of constrained scattering modeling successively resulted in solution structures for monomeric IgA1 and IgA2m(1), dimeric IgA1, and SC (6, 17–19). Recently the first structural description of the assembly of SIgA1 has been reported (20). In this, the two IgA1 monomers form a near planar, end-to-end conformation with a slight bend in the Fc-Fc region, with SC located along the convex edge of the Fc-Fc region. Given its importance in mucosal immunity, the corresponding structure for human SIgA2 is also of great interest. The shorter hinge increases the rigidity of IgA2 compared with IgA1, with little independent Fab movement relative to the Fc region (17, 21). By again combining x-ray, neutron, and ultracentrifugation data, the first structural view of the assembly of SIgA2 shows that this is markedly different from that for SIgA1 in being nonplanar. These differences are discussed in reference to the function of SIgA in mucosal immunity, particularly its interactions with antigens and receptors and the efficient protection of mucosal surfaces.
MATERIALS AND METHODS
Purification and Composition of Human Colostrum SIgA2—Native SIgA2 was isolated from colostrum provided by consenting healthy volunteers. A thiophilic resin column was used to bind immunoglobulins, SIgA1 was removed from this pool by jacalin-agarose affinity chromatography, and a Sephyacryl S-300 size exclusion column separated dimeric SIgA2 as a well resolved peak from secretory IgM, higher polymers of SIgA2, and free SC (20, 22). After concentration, SIgA2 was checked by reducing and nonreducing SDS-PAGE and in Western blots using anti-SIgA2 α-chain, anti-SIgA2 κ and λ light chain, and anti-SC antibodies. Reducing SDS-PAGE showed only the three bands expected for the light chain and J-chain (25 kDa), the heavy chain (60 kDa), and SC (80 kDa) (11). For scattering and ultracentrifugation work, the buffer was as described previously (19). SIgA2 was assumed to comprise the IgA2m(1) and IgA2m(2) allotypes with both λ and κ class light chains. Its composition was approximated as IgA2m(1), plus J chain and SC (17–19). SIgA2 with four κ light chains has a molecular mass of 424 kDa, an unhydrated volume of 537 nm3, a hydrated volume of 711 nm3, an absorption coefficient (1%, 1 cm path length) of 12.2, and a v̄ of 0.721 ml/g. The κ light chains in SIgA2 are 7 kDa larger than the λ light chains.
Scattering and Ultracentrifugation Data—X-ray scattering data were obtained in two different sessions on Beamline ID02 at the European Synchrotron Radiation Facility, Grenoble, France, using the synchrotron source in single-bunch mode to reduce the incident x-ray flux on the sample to minimize potential radiation effects (storage ring currents of 11–18 mA). The sample to detector distance was 3.0 m. SIgA2 samples at concentrations of 0.32–1.94 mg/ml were contained in water-cooled Perspex cells at 15 °C, with a path thickness of 1 mm and mica windows of thickness 25 μm. The samples were measured in sets of 10 consecutive time frames, each of 0.1, 1, or 2 s, to confirm the absence of radiation damage effects by comparing these. Neutron scattering data were obtained on Instrument LOQ at the ISIS pulsed neutron source (Didcot, UK). The proton beam current used to generate neutrons was ∼180 μA. Neutron data acquisition at 15 °C lasted 4–12 h at 1.94 mg/ml. To compute the distance distribution function P(r) that is a histogram of interatomic distances, the x-ray I(Q) curve contained 488 data points between Q values of 0.09 and 2.00 nm-1 and was fitted with Dmax set as 25 nm. The neutron I(Q) curve contained 71 data points between Q values of 0.13 and 2.3 nm-1 and was fitted with Dmax set as 24 nm to give the P(r) curve. Full details are described previously (6, 17).
Sedimentation equilibrium runs were measured at 20 °C on a Beckman XL-I instrument equipped with an AnTi50 rotor, recording both interference and 280-nm absorbance scans over 45 h. SIgA2 concentrations were 0.90, 0.60, and 0.32 mg/ml, using rotor speeds of 7,000, 9,000, 11,000, and 14,000 r.p.m. The molecular weights were determined from the curves at equilibrium by single species fits using Origin, version 4.1 (Microcal). Sedimentation velocity data were acquired at 0.93, 0.57, and 0.40 mg/ml at rotor speeds of 10,000, 15,000 20,000, 25,000, and 30,000 r.p.m The velocity data analyses utilized DCDT+ and SEDFIT, version 9.4 software. The continuous c(s) size distribution model of SEDFIT was used to identify the species present in the sample, and this model used a resolution of 150 and fixed the cell meniscus and bottom, the frictional ratio of 1.53, and the partial specific volume v̄, buffer density, and viscosity. The base line was allowed to float. Full details are described elsewhere (17, 19).
Constrained Modeling—The constrained modeling of SIgA2 to
fit the experimental scattering curve required molecular models to initiate
this procedure. This was derived from the recent dIgA1 structure (Protein Data
Bank code 2qtj) (19). Two
IgA2m(1) monomers (Protein Data Bank code 1r70)
(17) were superimposed on the
two Fc regions in dIgA1 to create a starting model for dimeric IgA2m(1). Next,
this dimer structure was combined with a library of 5,000 randomized
conformations in SC that was created from the unrestrained linker search
modeling described previously for SC
(18). Holding the dimeric IgA2
structure fixed, a Cycle 1 of modeling fits was performed using the same 10 SC
searches used for SIgA1 (20).
In each search, one SC domain was held fixed in position adjacent to the
dIgA2m(1) structure, and the resulting 5,000 SIgA2 models were evaluated for
their fits to the scattering curve. The subsequent Cycle 2 randomized the
Fab-Fc hinge in the best fit SIgA2 model obtained from Cycle 1. This hinge
between the CH1 and CH2 domains is
220CPVPPPPPCCHP244
(6,
17). For Cycle 2, six linker
libraries with 5000 hinge conformations in each were generated using molecular
dynamics for 12-residue hinge lengths set between 2.50 and 3.75 nm in six
0.25-nm steps. From the resulting 30,000 conformations, 3,000 were randomly
selected to create full SIgA2 coordinate models with four identical hinge
structures. In Cycles 1 and 2, each atomic model was converted into Debye
spheres to calculate the scattering curve
(17,
19). A cube side of 0.531 nm
and a four-atom cut-off reproduced the unhydrated SIgA2 volume, giving 3589
spheres for neutron fits, which visualizes a largely unhydrated structure. The
addition of the hydration volume corresponded to 4747 spheres and was used for
x-ray fits. To reduce computing times, the 5,000 SC models were first reduced
to 1500 using a generous 7% volume filter to remove significantly sterically
overlapped models. After calculation of the modeled x-ray scattering curve of
Fig. 6A, a flat
background correction of 0.2% of I(0) was subtracted from
the modeled curve as an empirical correction of different sample buffer
transmissions observed at large scattering angles No flat background
correction was required for the neutron fits. Sedimentation coefficients
 were calculated using
HYDROPRO, version 7c. Full details are given elsewhere
(6,
17,
18,
20).
were calculated using
HYDROPRO, version 7c. Full details are given elsewhere
(6,
17,
18,
20).
FIGURE 6.
X-ray and neutron scattering fits for the best fit and poor fit SIgA2 models from Cycle 1. The x-ray and neutron experimental I(Q) data (○) are compared with the best fit modeled SIgA2 curves (line). The P(r) curve is shown as an inset (experimental, dashed line). The models are shown as ribbon traces with the two IgA2 monomers shown in gray and SC in black. The fit parameters are reported in Table 1. A, the curve fits are shown for the best fit model from Search 1, where SC is positioned along the convex edge of the Fc-Fc dimer. B–D, the curve fits are shown for selected poor fit SIgA2 models. B, from Search 2 where SC is wrapped about the center of the Fc-Fc region at the J chain between the two IgA2 monomers. C, from Search 3, where SC is on the other (concave) edge of the Fc-Fc dimer. D, from Search 5, where SC is positioned on the central face of the Fc-Fc dimer.
RESULTS
X-ray and Neutron Scattering of SIgA2—Solution scattering is a diffraction technique that studies the overall structure of biological macromolecules in random orientations in solution (23). Comparison of the x-ray scattering I(Q) data as a function of scattering angle Q (Q = 4 π sin θ/λ;2θ = scattering angle; λ = wavelength) from a single time frame and the average of 10 consecutive frames showed no radiation damage effects. The averaged time frames showed improved signal-noise ratios; thus these were subsequently used for analyses. At the lowest Q values, Guinier analyses resulted in linear plots, from which the RG value (a monitor of macromolecular elongation) was obtained within satisfactory Q·RG limits (Fig. 2A). The mean x-ray RG value for SIgA2 was 8.13 ± 0.1 nm (five values) (Fig. 2A), which is slightly less than that of 8.29 ± 0.20 nm for SIgA1 (20). The SIgA2 anisotropy ratio RG/RO (where RO is the RG value of the sphere with the same volume as the glycoprotein) was 1.90, which is slightly less than that of 1.93 for SIgA1. The similarities between the two SIgA isoforms suggest that they both have a similar extended subunit arrangement (Fig. 1).
FIGURE 2.
Guinier analyses and distance distribution functions P(r). A–D, the Guinier plots are arbitrarily displaced on the intensity axis for reason of clarity. The Q·RG and Q·RXS ranges used to determine the RG, RXS-1, and RXS-2 values are represented by filled circles between the data points marked with arrows. The x-ray fits correspond to concentrations of 0.90 mg/ml (top), 0.60 mg/ml (middle), and 0.32 mg/ml (bottom). The neutron fit corresponds to a concentration of 1.0 mg/ml. A and B, the x-ray and neutron Q ranges used for the RG values were 0.10–0.17 and 0.10–0.18 nm-1, respectively. C and D, the x-ray Q ranges used for the RXS-1 and RXS-2 values were 0.20–0.28 and 0.72–1.04 nm-1, respectively. E and F, the two most frequently occurring distances in the P(r) curve are denoted by the maxima M1 and M2 at 7.3 and 10.0 nm, respectively (x-ray), and 7.2 and 9.6 nm, respectively (neutron). The maximum length L of SIgA2 is 25 nm (x-ray) and 24 nm (neutron) when P(r) reaches 0. The error bars are shown for the neutron P(r) curve.
The shorter axes of SIgA2 were monitored using the RXS-1 and RXS-2 parameters from x-ray cross-sectional Guinier analyses. The ln(I(Q)·Q) plots revealed two linear regions in Q ranges of 0.20–0.28 and 0.72–1.04 nm-1 that were similar to those for SIgA1 and dIgA1 (19, 20). The mean RXS-1 and RXS-2 value were 4.22 ± 0.09 nm (5 values) and 1.93 ± 0.03 nm (four values), respectively (Fig. 2, C and D). These values were slightly larger than the corresponding values of 3.90–3.94 and 1.27–1.43 nm for SIgA1 and dIgA1 (Table 1). The increase is attributed to an alteration in the relative arrangement of the Fab and Fc regions within the structure when compared with SIgA1 and dIgA1.
TABLE 1.
Comparison of x-ray and neutron parameters for human IgA and secretory component All of the proteins are from human sources except for SC, which is recombinant, and IgA2m(1), which is a recombinant human-mouse chimaera. The x-ray data were obtained on instrument ID02 at European Synchrotron Radiation Facility, except for monomeric IgA1, which was measured at Stations 2.1 and 8.2 at the SRS Daresbury. All of the neutron data were obtained on Instrument LOQ at ISIS. NA, not available.
|
Protein
|
Data
|
Guinier analyses
|
P(r) analyses
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| RG | RXS-1 | RXS-2 | RG/RO | RG | L | M1 | M2 | ||
| nm | nm | nm | nm | nm | nm | nm | |||
| Secretory IgA2 (present study) | X-ray | 8.13 ± 0.10 | 4.22 ± 0.09 | 1.93 ± 0.03 | 1.90 | 8.19 ± 0.37 | 25.0 | 7.3 | 10.0 |
| Neutrons | 7.57 | NA | NA | 1.77 | 7.95 | 24.0 | 7.2 | 9.6 | |
| Secretory IgA1 (20) | X-ray | 8.29 ± 0.20 | 3.90 ± 0.13 | 1.27 ± 0.03 | 1.93 | 8.30 ± 0.17 | 26.0 | 7.0 | 9.6 |
| Neutrons | 7.22 ± 0.33 | NA | NA | 1.85 | 8.04 | 24.0 | 6.8 | 10.0 | |
| Dimeric IgA1 (19) | X-ray | 8.65 ± 0.27 | 3.94 ± 0.18 | 1.43 ± 0.07 | 2.16 | 8.67 ± 0.17 | 26.0 | 4.9 | 9.9 |
| Neutrons | 7.60 ± 0.05 | NA | NA | 2.08 | 7.47 | 23.0 | 5.1 | 10.1 | |
| Monomeric IgA2m(1) (17) | X-ray | 5.18 ± 0.09 | 2.47 ± 0.09 | 1.47 ± 0.08 | 1.66 | 5.21 ± 0.15 | 17.0 | 4.5 ± 0.3 | 6.8 ± 0.7 |
| Neutrons | 5.03 ± 0.01 | 2.21 ± 0.10 | 1.04 ± 0.06 | NA | 5.12 ± 0.01 | 17.0 | 4.5 | NA | |
| Monomeric IgA1 (6) | X-ray | 6.20 ± 0.13 | 2.20 ± 0.26 | 1.56 ± 0.16 | 1.99 | 6.12 | 21.0 | 3.7 | 9.1 |
| Neutrons | 6.11 ± 0.18 | 2.17 ± 0.23 | 1.18 ± 0.12 | NA | NA | NA | NA | NA | |
| Secretory component (18) | X-ray | 3.53 ± 0.43 | 1.76 ± 0.08 | NA | 1.47 | 3.66 ± 0.19 | 13.0 | 3.8 | NA |
| Neutrons | 3.63 ± 0.28 | 1.30 ± 0.10 | NA | NA | 3.73 ± 0.17 | 12.0 | 3.2 | NA | |
Neutron scattering data acts as a control for radiation damage and structural inhomogeneity and/or hydration effects (21, 24). The SIgA2 neutron RG value in a 2H2O buffer was 7.57 nm (Fig. 2B), which is 0.56 nm lower than the x-ray value, and attributed to a largely invisible hydration shell observed by neutrons (24). The neutron RG/RO was 1.77, which is again smaller than the values of 1.85 and 2.08 for SIgA1 and dIgA1 (Table 1). No neutron RXS-1 and RXS-2 values were available for reason of an insufficient signal-noise ratio. The Guinier I(0)/c value leads to the protein molecular mass M (where M = I(0)/c × 9.105) (6). The I(0)/c value of 0.47 resulted in a mass of 425 kDa for SIgA2, in good agreement with the composition-derived value of 424 kDa for SIgA2, and confirms the monodispersity of the protein.
The distance distribution function P(r) provides structural information in real space. The mean RG values determined from the x-ray and neutron P(r) curves were 8.19 ± 0.37 nm (four values) and 7.95 nm, respectively (Fig. 2, E and F), in good agreement with the Guinier analyses. The x-ray and neutron P(r) curves reproducibly showed two broad maxima M1 located at 7.3 and 7.2 nm and M2 located at 10.0 and 9.6 nm, respectively. Both peaks were also seen for SIgA1 and dIgA1 (Table 1) and indicate similarity in their overall solution structures. The maximum length (L) is determined from where the P(r) curve reaches zero at larger r values and was 27 nm (x-ray) and 24 nm (neutron) (Fig. 2, E and F). Their similarity to those for SIgA1 and dIgA1 (Table 1) provides further evidence of an extended structure for SIgA2.
Analytical Ultracentrifugation of SIgA2—Analytical ultracentrifugation studies macromolecular structures in solution by following their sedimentation behavior on subjecting these to a high centrifugal force (25). The molecular weight of SIgA2 was determined by sedimentation equilibrium at three concentrations and four rotor speeds (“Materials and Methods”). Individual fits at each concentration and speed gave 320–490 kDa (Fig. 3). Extrapolation to zero concentration gave a mean molecular mass of 415 ± 60 kDa, which is within error of the sequence-derived value of 425 kDa and confirms this.
FIGURE 3.
Sedimentation equilibrium analyses. SIgA2 was analyzed using interference (A) and absorbance optics (B) at concentrations of 0.90 mg/ml (top), 0.60 mg/ml (center), and 0.32 mg/ml (bottom) at a rotor speed of 8,000 r.p.m. The circles represent the experimental data and the continuous black lines represent their fits. The corresponding curve fit residuals are shown above the exponential fits. C, the rotor speed dependence of the fitted molecular mass gave a mean value of 415 ± 4 kDa denoted by 7,000 (•), 9,000 (▪), 11,000 (▵), and 14,000 r.p.m. (▿).
Sedimentation velocity provides an independent measure of structural
elongation and the absence of polydispersity. Good agreement between the
experimental and fitted boundaries in SEDFIT analyses revealed only a single
SIgA2 species in the size distribution analyses c(s)
(Fig. 4). The sedimentation
coefficients 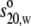 were 12.2
and 12.1 S. The conversion to c(M) mass distributions gave
422 kDa, in agreement with the determinations above. The
g(s*) time derivative analyses of between 6 and
14 scans gave 12.1 S (Fig. 4, E
and F). The
were 12.2
and 12.1 S. The conversion to c(M) mass distributions gave
422 kDa, in agreement with the determinations above. The
g(s*) time derivative analyses of between 6 and
14 scans gave 12.1 S (Fig. 4, E
and F). The
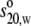 value resulted in a
frictional ratio f/fo, (where
fo is the frictional coefficient of a sphere with the same
hydrated volume) of 1.52. This f/fo is slightly
lower than that of 1.70 for SIgA1 and 1.63 for dIgA1, showing that SIgA2 is
more compact than these proteins in accordance with the Guinier
RG values.
value resulted in a
frictional ratio f/fo, (where
fo is the frictional coefficient of a sphere with the same
hydrated volume) of 1.52. This f/fo is slightly
lower than that of 1.70 for SIgA1 and 1.63 for dIgA1, showing that SIgA2 is
more compact than these proteins in accordance with the Guinier
RG values.
FIGURE 4.
Sedimentation velocity analyses. The black circles represent the experimental scans, and the continuous white or black lines represent the fits. A and C, in the SEDFIT c(s) analyses, only every fifth of the 120 scans is shown for reason of clarity. Interference scans at a concentration of 0.93 mg/ml and a rotor speed of 20,000 r.p.m. are shown in A, whereas the corresponding absorbance scans at 0.40 mg/ml at 280 nm are shown in C. The 120 scans were recorded at 5-min intervals. B and D, the c(s) plots from the fits of A and C are shown, from which the sedimentation coefficient was determined to be 12.2 S (interference) and 12.1 (absorbance). E and F, in the DCDT+ g(s*) analyses, s20,w was determined to be 12.1 S (arrow) using both absorbance and interference optics. Both fits are based on data at 0.93 mg/ml at rotor speeds of 10,000 r.p.m. (interference) and 15,000 r.p.m. (absorbance). The fits were determined using 4–14 scans recorded midway through the experiment shown in A and C. The goodness-of-fit residuals are shown above each analysis.
Constrained Scattering Modeling of SIgA2—The constrained modeling of the scattering and sedimentation data resulted in the SIgA2 solution structure (24). Even though the data corresponded to both the SIgA2m(1) and SIgA2m(2) allotypes, no allowance for this was necessary in the fits. Because no dimeric IgA2 structure was available, the modeling started from the best fit dIgA1 structure (19). The superimposition of the Fc α-carbon atoms in the IgA2m(1) structure (17) onto the Fc region of dIgA1 gave a low root mean square deviation value of 0.58 nm and provided the starting model for dimeric IgA2 (dIgA2).
The first of three search cycles localized the position of SC relative to dIgA2. This procedure followed the same strategy for the SIgA1 structure determination, in which the SC D3 or D5 domain was held fixed in position in all 10 searches (20). The same 10 different locations of 1500 randomized domain arrangements for SC relative to the fixed dimeric structure were evaluated (20). Thus Searches 1 and 2 explored SC positions on the convex edge of the Fc dimer, Searches 3 and 4 explored those on the concave edge of the Fc dimer, and Searches 5 and 6 explored those on top of the Fc-Fc dimer plane (Fig. 5A). Searches 7–10 explored SC locations where D5 is held proximate to each of the four CH2 domains (Fig. 5A). As for SIgA1, Search 1 gave the most favorable x-ray fit outcome, with extended SC structures aligned along the convex edge of the Fc-Fc plane. The distributions of RG values in Fig. 5B as a function of the goodness-of-fit R factors showed that the best fit models (arrow) had RG values close to the experimental value of 8.13 nm and were close to the minimum R factors. These best models also gave RXS-1 and RXS-2 values close to the global minimum R factors in Fig. 5C. Search 2 resulted in a best fit SIgA2 structure very similar to that from Search 1 (Fig. 5B). Searches 3–10 gave worsened fits. Searches 3 and 4 gave too low RG values of 7.5–8.1 nm (Fig. 5D), and many SC models sterically clashed with the two Fab regions. Searches 5 and 6 gave even lower RG values of 7.5–7.9 nm (Fig. 5E). Searches 7–10 gave a reasonable range of SIgA2 structures with RG values close to the minimum R factor (Fig. 5F). However, 29% of the 6,000 models showed significant steric overlap with the Fab regions, and the best of the remaining structures showed a fully extended SC with its D1–D4 domains extended along the edge of the Fc-Fc plane.
FIGURE 5.
Constrained modeling analyses of SIgA2 (Cycle 1). A, Searches 1–10 are denoted by shaded circles labeled D3 or D5 to correspond to the SC domain fixed in each search. The searches are shown against a reduced outline of Fig. 1. B and C, the x-ray RG, RXS-1, and RXS-2 values calculated from Search 1 are compared with the R factors from the curve fits (Table 1). Search 2 is shown in gray in B. The dashed lines correspond to the experimental RG and RXS values. The 10 best fit models are shown in gray, and the best fit model is shown in black (arrow). Three poor fit models are shown as a black circle, square, and triangle (Table 1). D–F, the RG values from Searches 3–10 are compared with their R-factors.
The filtering of the Search 1 models showed that 505 SIgA2 structures were satisfactory. The best fit 10 structures from Search 1 were selected from their lowest R factors and were visually checked for the absence of steric overlap between SC and dIgA2 (shaded in Fig. 5B). The best fit SIgA2 structure has a good R factor of 6.6% (arrow in Fig. 5B) and showed a good visual x-ray curve fit in a Q range extending to 1.4 nm-1 provided that a small flat background correction was applied (Fig. 6A). The corresponding neutron curve fit also gave a good R factor of 7.8% (Fig. 6A). The P(r) curves were well reproduced. Selected viable models from Searches 2, 3, and 5 were used to illustrate poor fits (Fig. 6, B–D) in which SC was wrapped around the Fc regions, aligned on the Fc concave edge, or placed on one planar Fc face. Small deviations in the curve fits in the Q range of 0.3–0.9 nm-1 were seen, causing the R factors to increase to 7.3, 8.9, and 11.5% (Table 2).
TABLE 2.
X-ray and neutron modeling fits for the SIgA2 solution structure The filters used for the 10 searches in Cycle 1 were 4509 hydrated spheres (N), RG values between 8.02 and 8.21 nm, RXS-1 values between 4.09 and 4.43 nm, and RXS-2 values between 1.80 and 2.04 nm. NA, not available.
| Filter for best fit models | Models | Hydrated spheres | X-ray RG | X-ray RXS-1 | X-ray RXS-2 | Neutron RG | X-ray R factor | Neutron R factor | s°20,w |
|---|---|---|---|---|---|---|---|---|---|
| N | nm | nm | nm | nm | % | % | s | ||
| Cycle 1: Search 1 | |||||||||
| None | 1500 | 4395–4737 | 7.68–8.42 | 3.70–4.72 | 1.46–2.04 | 6.64–7.25 | 5.8–10.6 | 6.5–12.0 | NA |
| N, RG, RXS-1, R factor | 10 | 4641–4705 | 8.03–8.14 | 4.25–4.37 | 1.81–1.92 | 6.93–7.05 | 6.6–7.1 | 7.4–8.2 | 11.3–11.9 |
| Best fit (Fig. 6A) | 1 | 4688 | 8.08 | 4.25 | 1.91 | 6.93 | 6.6 | 7.4 | 11.9 |
| Poor fit (Fig. 6B) | 1 | 4646 | 7.72 | 3.89 | 1.79 | 6.72 | 7.3 | 10.2 | 11.6 |
| Poor fit (Fig. 6C) | 1 | 4654 | 7.76 | 3.74 | 1.92 | 6.68 | 8.9 | 11.3 | 12.2 |
| Poor fit (Fig. 6D) | 1 | 4593 | 7.69 | 3.53 | 1.97 | 6.56 | 11.5 | 13.3 | 12.2 |
| Cycle 2: Fab reorientation | |||||||||
| None | 3000 | 2911–4641 | 4.65–8.76 | 0.05–4.42 | 0.55–2.59 | 4.30–7.63 | 6.7–33.8 | 8.0–33.5 | NA |
| N, RG, RXS-1, R factor (Fig. 7) | 31 | 4552–4640 | 7.95–8.30 | 4.00–4.37 | 1.48–1.84 | 7.09–7.34 | 7.3–9.1 | 8.1–10.1 | NA |
| Cycle 3: SC optimization | |||||||||
| None | 1500 | 4363–4689 | 7.80–8.55 | 3.83–4.89 | 1.46–1.95 | 6.99–7.59 | 6.1–11.3 | 5.8–10.6 | NA |
| N, RG, R factor (Fig. 8A) | 10 | 4647–4655 | 8.00–8.08 | 4.17–4.29 | 1.50–1.76 | 7.19–7.23 | 6.1–6.5 | 7.5–8.2 | 11.6–12.0 |
| Best fit (Fig. 8B) | 1 | 4655 | 8.04 | 4.22 | 1.50 | 7.20 | 6.1 | 7.5 | 11.94 |
| Experimental values | NA | 4757 | 8.13 ± 0.10 | 4.22 ± 0.09 | 1.93 ± 0.03 | 7.57 | NA | NA | 12.1 |
Cycle 2 investigated whether the positional randomization of the four Fab regions in SIgA2 would improve the curve fits. For this, SC and the Fc-Fc region in the best fit Search 1 model was held fixed, whereas the Fab regions were varied at the Fab-Fc hinges (17). A total of 3000 structures were generated, in which no Cys214–Cys214 disulfide bridge was present (Fig. 1). The minima seen in Fig. 7 showed that these tested a sufficient range of Fab orientations. Filtering of the 3,000 models identified 31 best fit models (Table 2 and Fig. 7). All 31 showed a similar Fab arrangement to that determined from Search 1 in the first modeling cycle. The Cys214–Cys214 Cα-Cα separation ranged from 2.5 to 5.6 nm, compared with a preferred value of 0.56 nm for disulfide bridge formation. This increased separation may reflect the presence of both IgA2m(1) and IgA2m(2), given that the Cys214–Cys214 bridge is found only in IgA2m(1). Although no improvement in the R factors of 7.3–9.1% was seen, all of the SIgA2 models displayed a T-shaped structure similar to that determined for the IgA2m(1) monomer (17). The most striking outcome of this modeling cycle is that the four Fab regions were optimally arranged almost perpendicular to the Fc plane, unlike that for SIgA1 (Fig. 8C). This accounted for the different RXS-1 and RXS-2 values of SIgA2 and SIgA1/dIgA1 (Table 1).
FIGURE 7.
Survey of best fit models for SIgA2 from Cycle 2. Comparison of the x-ray R factors with the RG and RXS values for 3000 models based on the randomization of the Fab regions. The dashed lines indicate the experimental x-ray RG, RXS-1, and RXS-2 values.
FIGURE 8.
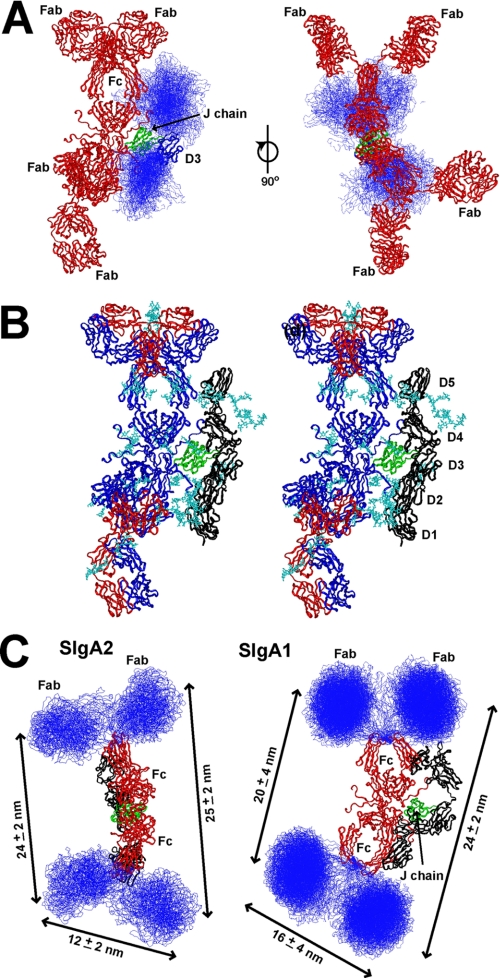
Molecular assembly of SIgA2. A, the 30 best fit SIgA2 models from Cycle 3 are shown in two orthogonal views in which the Fc regions in red are viewed face-on (left) and on their edge (right). The α-carbon views of SC are shown as a blue ribbon (D3) or trace (D1, D2, D4, and D5). J chain is in green. B, The best fit SIgA2 model is shown as a stereo pair in blue (heavy chains), red (light chains), and green (J chain). SC is shown in black. N-Linked carbohydrate chains are shown in cyan. C, the 31 best fit nonplanar models for SIgA2 from Cycle 2 are compared with the 50 best fit near planar models from the analogous fit cycle for SIgA1 (20). The Fab regions are in blue, the two Fc regions are in red, J chain is in green, and SC is in black. The mean ± S.D. of the distances between the complementarity-determining regions in each Fab are marked with arrows.
Cycle 3 optimized the position of SC in the best fit SIgA2 model from Cycle 2 (Fig. 8A). This procedure followed Search 1 of Cycle 1. The 10 best fit SIgA2 models had x-ray R factor values of 6.1–6.5%, therefore improving the outcome of both Cycles 1 and 2. The α-carbon coordinates from these 10 models were deposited in the Protein Data Bank with the accession code 3cm9 (supplemental Fig. S1).
The experimental 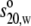 value of 12.1 S was compared with those calculated from the best fit SIgA2
models (Table 2). Those from
Cycle 1 Search 1 gave between 11.3 and 11.9 S. The poor fit Cycle 1 models
gave slightly larger
value of 12.1 S was compared with those calculated from the best fit SIgA2
models (Table 2). Those from
Cycle 1 Search 1 gave between 11.3 and 11.9 S. The poor fit Cycle 1 models
gave slightly larger 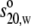 values of 11.6–12.2 S (Table
2). Those for the Cycle 3 models gave slightly improved agreement
between 11.6 S and 12.0 S (Table
2). These agreements support the x-ray modeling fits.
values of 11.6–12.2 S (Table
2). Those for the Cycle 3 models gave slightly improved agreement
between 11.6 S and 12.0 S (Table
2). These agreements support the x-ray modeling fits.
DISCUSSION
SIgA is central for mucosal immunity. Our solution structure for SIgA2 alongside those for dIgA1 and SIgA1 completes a new understanding of the three major forms of polymeric human IgA. The structural work was performed with a homogenous SIgA2 preparation, confirmed here by analytical ultracentrifugation (Fig. 4, B and D). This is functional; it binds to FcαR1 (CD89) with the same affinity as SIgA1, triggering a respiratory burst in neutrophils in the same way as SIgA1 and dIgA1 (22, 26, 27).
One of the two most striking features of the SIgA2 structure is the similar location of SC along the outer convex edge of the Fc-Fc region to that seen in our SIgA1 structure. The stereo view of Fig. 8B shows that carbohydrate does not prevent SC binding. The experimental scattering data demonstrate an extended solution structure. The small differences in SC position from SIgA1 (Table 1) are attributable to the shorter hinge in SIgA2. It is emphasized that the specific molecular interactions between the SC domains and the Fc-Fc region cannot be identified at the resolution of this method. This best fit location for SC was identified through the comparison of the modeled and experimental RG values (Fig. 5). Comparison with Searches 1–10 for SIgA1 showed that an even clearer location for SC had been obtained for SIgA2. Many earlier hypothetical models for SIgA placed SC on one planar face of the Fc-Fc region (1, 3, 15, 16), and these models were ruled out by Searches 5 and 6 (Figs. 5E and 6D).
The other striking outcome of this work is the nonplanarity of the SIgA2 solution structure compared with the near planar SIgA1 structure (Fig. 8C). Despite the similar location of SC in both isoforms, experimentally this outcome was indicated by differences in their RXS values (Table 1). The observed RXS values are influenced by the spatial arrangement of the Fab and Fc regions. Previously, for monomeric IgA2m(1), no tendency for the two Fab regions to lie in the Fc plane was identified, unlike that for the IgA1 monomer (17). The optimization of the Fab orientations in Cycle 2 clarified the existence of a nonplanar SIgA2 structure (Fig. 7). Inspection of the SIgA2 models suggested that nonplanarity resulted from the location of SC along the convex edge of the Fc-Fc region. This results in crowding between the D1 and D5 domains of SC and the adjacent Fab regions; hence the four Fab regions become displaced out of the Fc-Fc plane. This does not occur in SIgA1 because of its longer hinge.
The most significant biological consequence of the nonplanar SIgA2 structure with the different arrangement of the Fab arms when compared with SIgA1 is the prediction that the interaction with antigens will differ between SIgA2 and SIgA1. Although human serum IgA1 has been studied in the most detail because of its relative ease of purification, it should be stressed that IgA1 (including SIgA1) is only found in humans and in higher apes. Thus SIgA2 is more like the SIgA of rodents including those used in models of human disease. Laboratory rodents also lack the most fully characterized FcαRI receptor for IgA. In humans, SIgA2 is present in concentrations equal to or greater than SIgA1 in many secretions. However, the relative ratio varies considerably at different mucosal sites, suggesting that the two subclasses may have selective advantages in controlling infectious organisms affecting those different sites. IgA2 concentrations are relatively higher than IgA1 when the total IgA level is high (28). SIgA2 production is relatively enhanced mainly in the distal gut (29). Around 62% of IgA in colon is SIgA2 (30), and SIgA2 is also prominent in mammary and salivary glands and in the female urogenital tract. The ratio of IgA1/IgA2 at different sites may also change during development (31). There are significant differences between colostrum and transitional milk and between colostrum and mature milk (32). In the most extensive study, the mean ratio of total IgA1 to IgA2 in colostrum was around 53:47, although significant individual variations were observed (33). SIgA2 was detected in SIgA1-deficient individuals (33).
Differences in the ratio of SIgA1 and SIgA2 in secretions depend upon the nature of the specific antigen inducing their production. IgA1 and IgA2 antibody activities determined against a panel of antigens showed that although the IgA antibody activity directed against polysaccharides was almost equally distributed between the two subclasses, antibody specific for protein antigens was predominantly IgA1, whereas anti-lipopolysaccharide activity was mostly IgA2 (33). The IgA2-dominant mucosal response to polysaccharide and lipopolysaccharide antigens and to lipid A has been confirmed in numerous studies (34–38). The lower levels of specific SIgA2 antibodies in some secretions are often a reflection of lower levels of total SIgA2. However, in many cases, for example the colostral response to human immunodeficiency virus gp160, higher specific activity is observed for IgA2. The subclass-restricted response is not limited to infectious organisms. For example in celiac disease, the relative percentage of serum IgA2 endomysial antibodies is close to that of total IgA2 in serum (6.2%), which is significantly less than the percentage of IgA2 anti-gliadin (39). Although IgA1 is also the predominant anti-tissue transglutaminase subclass in patients with celiac disease, a positive association has been observed between a higher ratio of IgA2 and severe (Marsh 2-3) mucosal abnormalities (40). Remarkably, in a recent study it has been shown that grass pollen immunotherapy induces an allergen-specific IgA2 antibody response (41).
It is difficult to avoid the conclusion from the above data that the subclass specificity of SIgA responses is driven by antigen, as predicted from our SIgA2 and SIgA1 models. Although the mechanism is not clear, it is possible that the relative flexibility and rigidity of the two IgA subclasses does control antigen recognition. Spin labeling showed that monomeric serum IgA2 was considerably less flexible than IgA1 (42). The lack of flexibility of the IgA2 hinge was also demonstrated by electron microscopy of small immune complexes (21), which suggested that the IgA2 hinge was completely devoid of any features that would contribute to independent Fab-Fab or Fab-Fc movement. It appears that the rigid and nonplanar nature of SIgA2 facilitates its multivalent binding to antigens on relatively fixed bacterial surfaces, whereas the more flexible SIgA1 structure is able to interact more effectively with protein antigens positioned in a great range of different orientations.
The nonplanar SIgA2 structure clarifies its interactions with receptors. Little is known about the differences in effector functions of the two SIgA isotypes, although SIgA2 binding to the FcαRI receptor (CD89) consistently triggers a slightly slower (though similarly sized) neutrophil respiratory burst than SIgA1 (26, 43, 44). The location of SC along the outer convex edge of SIgA2 as in SIgA1 confirms that for both isotypes, the Fc region is more accessible than previous SIgA models have suggested. As a result, some but not all binding sites for FcαR1 are accessible, thus explaining the ability of aggregated SIgA to trigger FcαR1-mediated cellular functions. Like SIgA1, the SIgA2 structure shows that receptor and antigen binding will be independent events because the Fc and Fab regions are well separated. The FcαRI-Fc crystal structure shows that there is a binding site at each of the four CH2 and CH3 junctions (45). Two of these four FcαRI sites will be masked by SC in SIgA2, leaving two that will remain accessible. Of these two FcαRI sites, only one of these will be in the correct orientation to bind to cell surface receptors at any one time, as already discussed for dIgA1 and SIgA1 (19, 20). Molecular views of our best fit SIgA2 model (Fig. 8B) and the Fc-FcαRI complex (Protein Data Bank code 1ow0), where the two Fc regions are superimposed (not shown) confirm that this continues to be the case in SIgA2, even though the Fab regions have been reoriented.
SIgA2 binding to the polymeric immunoglobulin receptor appears to be facilitated by a nonplanar arrangement of the Fab regions relative to Fc. This nonplanarity makes the edge of the Fc-Fc region more accessible in SIgA2 (Fig. 8C). Thus the SIgA2 structure is consistent with the proposal for SIgA1 in which a “zipper effect” association of the unbound J-shaped SC domain structure occurs along the edge of the Fc regions (20, 46). The lack of the extended hinge in SIgA2 does, of course, mean that SIgA2 cannot perform the functions mediated by the O-linked sugars of the IgA1 hinge that serve as ligands for cellular receptors and bind pathogens such as S-fimbriated Escherichia coli (47, 48). SIgA2 also differs in that the presence of O-linked sugars in the hinge of IgA1, which are not found in that of IgA2, serve as ligands for cellular receptors and bind to pathogens such as S-fimbriated E. coli (47, 48).
Our SIgA2 structure also provides insight into the proteolysis of SIgA2, which is relevant to the harsh environment of mucosal surfaces. SC on its own is readily degraded (15, 18, 49). Proteases cleave monomeric IgA1 and dIgA1 more readily than SIgA1 (13, 50). SC delays cleavage in the hinge/Fc region of the α-chain (12). However, SC in SIgA2 is cleaved at a similar rate to free SC (11). Structurally, this may be explained in terms of the greater exposure of SC along the convex edge of the Fc-Fc region in SIgA2, because SC is no longer shielded by the Fab regions in the manner described for SIgA1 (20). The reported noncovalent link between the SC D5 and CH2 domains in SIgA2 (11) may result from hindrance by the Fab regions that restricts the interaction between these domains and may also contribute to this susceptibility. Although the heavier N-glycosylation of IgA2 at 24 sites (Fig. 1) may protect SIgA2 from proteolytic cleavage (Fig. 8B) (17), the evolution of the SIgA1 isotype has provided a more satisfactory solution to this issue. The extended hinge of SIgA1 does, however, make the molecule susceptible to a wide range of microbial proteases at this location (51).
The present study provides molecular explanations of some functional differences between the two human SIgA subclasses. It offers a framework on which to interpret future studies and a starting point for studies of nonprimate SIgA. In conclusion, the similar binding of SC along one edge (the convex edge) of the Fc regions in both subclasses explains the accessibility of the opposite edge (the concave edge) to ligands, receptors, and some proteases. The nonplanar SIgA2 and near planar SIgA1 solution structures explain different sensitivities to other proteases and suggest that differences in antigen specificity between the two subclasses might be a reflection not only of the length of the hinge and therefore the antigenic reach of the Fab regions (17), but also the flexibility and directionality of their antigen binding.
Supplementary Material
Acknowledgments
We thank J. Gor, Dr. A. Robertson, and Dr. P. W. Whitty for assistance with data collection and Dr. S. Finet, Dr. R. K. Heenan, and Dr. S. M. King for instrumental support.
The atomic coordinates and structure factors (code 3cm9) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
This work was supported by the Biological and Biotechnology Sciences Research Council and the Wellcome Trust. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: SIgA2, secretory immunoglobulin A2; dIgA, dimeric immunoglobulin A; SC, secretory component.
References
- 1.Mestecky, J., Moro, I., Kerr, M. A., and Woof, J. M. (2005) Mucosal Immunology, 3rd Ed., pp. 153-181, Academic Press, San Diego
- 2.Childers, N. K., Bruce, M. G., and McGhee, J. R. (1989) Annu. Rev. Microbiol. 43 503-536 [DOI] [PubMed] [Google Scholar]
- 3.Brandtzaeg, P. (2007) Vaccine 25 5467-5484 [DOI] [PubMed] [Google Scholar]
- 4.Nagler-Anderson, C. (2001) Nat. Rev. Immunol. 1 59-67 [DOI] [PubMed] [Google Scholar]
- 5.Sumiyama, K., Saitou, N., and Ueda, S. (2002) Mol. Biol. Evol. 19 1093-1099 [DOI] [PubMed] [Google Scholar]
- 6.Boehm, M. K., Woof, J. M., Kerr, M. A., and Perkins, S. J. (1999) J. Mol. Biol. 286 1421-1447 [DOI] [PubMed] [Google Scholar]
- 7.Grey, H. M., Abel, C. A., Yount, W. J., and Kinkel, H. G. (1968) J. Exp. Med. 128 1223-1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torano, A., and Putnam, F. W. (1978) Proc. Natl. Acad. Sci. U. S. A. 75 966-969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Putnam, F. W., Liu, Y.-S. V., and Low, T. L. K. (1979) J. Biol. Chem. 254 2865-2874 [PubMed] [Google Scholar]
- 10.Fallgreen-Gebauer, E., Gebauer, W., Bastian, A., Kratzin, H. D., Eiffert, H., Zimmermann, B., Karas, M., and Hilschmann, N. (1993) Biol. Chem. Hoppe-Seyler 374 1023-1028 [DOI] [PubMed] [Google Scholar]
- 11.Almogren, A., Senior, B. W., and Kerr, M. A. (2007) Immunology 120 273-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crottet, P., and Corthésy, B. (1998) J. Immunol. 161 5445-5453 [PubMed] [Google Scholar]
- 13.Almogren, A., Senior, B. W., Loomes, L. M., and Kerr, M. A. (2003) Infect. Immun. 71 3349-3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloth, B., and Svehag, S. E. (1971) J. Exp. Med. 133 1035-1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heremans, J. F. (1974) The Antigens, Vol. 2, pp. 365-522, Academic Press, New York [Google Scholar]
- 16.Mestecky, J., and McGhee, J. R. (1987) Adv. Immunol. 40 153-245 [DOI] [PubMed] [Google Scholar]
- 17.Furtado, P. B., Whitty, P. W., Robertson, A., Eaton, J. T., Almogren, A., Kerr, M. A., Woof, J. M., and Perkins, S. J. (2004) J. Mol. Biol. 338 921-941 [DOI] [PubMed] [Google Scholar]
- 18.Bonner, A., Perrier, C., Corthésy, B., and Perkins, S. J. (2007) J. Biol. Chem. 282 16969-16980 [DOI] [PubMed] [Google Scholar]
- 19.Bonner, A., Furtado, P. B., Almogren, A., Kerr, M. A., and Perkins, S. J. (2008) J. Immunol. 180 1008-1018 [DOI] [PubMed] [Google Scholar]
- 20.Bonner, A., Almogren, A., Furtado, P. B., Kerr, M. A., and Perkins, S. J. (2009) Mucosal Immunol., 2 74-84 [DOI] [PubMed] [Google Scholar]
- 21.Roux, K. H., Strelets, L., Brekke, O. H., Sandlie, I., and Michaelsen, T. E. (1998) J. Immunol. 161 4083-4090 [PubMed] [Google Scholar]
- 22.Almogren, A., and Kerr, M. A. (2008) Mol. Immunol. 45 87-94 [DOI] [PubMed] [Google Scholar]
- 23.Perkins, S. J., Okemefuna, A. I., Fernando, A. N., Bonner, A., Gilbert, H. E., and Furtado, P. B. (2008) Method. Cell Biol. 84 375-423 [DOI] [PubMed] [Google Scholar]
- 24.Perkins, S. J. (2001) Biophys. Chem. 93 129-139 [DOI] [PubMed] [Google Scholar]
- 25.Cole, J. L., Lary, J. W., Moody, T. P, and Laue, T. M. (2008) Mol. Cell Biol. 84 143-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazengera, R. L., and Kerr, M. A. (1990) Biochem. J. 272 159-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart, W. W., and Kerr, M. A. (1990) Immunology 71 328-334 [PMC free article] [PubMed] [Google Scholar]
- 28.Trégoat, V., Montagne, P., Béné, M. C., and Faure, G. (2001) J. Clin. Lab. Anal. 15 55-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kett, K., Brandtzaeg, P., Radl, J., and Haaijman, J. J. (1986) J. Immunol. 136 3631-3635 [PubMed] [Google Scholar]
- 30.Prigent-Delecourt, L., Coffin, B., Colombel, J. F., Dehennin, J. P., Vaerman, J. P., and Rambaud, J. C. (1995) Clin. Exp. Immunol. 99 221-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hacsek, G., and Savilahti, E. (1996) J. Pediatr. Gastroenterol. Nutr. 22 307-311 [DOI] [PubMed] [Google Scholar]
- 32.Fitzsimmons, S. P., Evans, M. K., Pearce, C. L., Sheridan, M. J., Wientzen, R., and Cole, M. F. (1994) J. Pediatr. 124 566-573 [DOI] [PubMed] [Google Scholar]
- 33.Ladjeva, I., Peterman, J. H., and Mestecky, J. (1989) Clin. Exp. Immunol. 78 85-90 [PMC free article] [PubMed] [Google Scholar]
- 34.Brown, T. A., and Mestecky, J. (1985) Infect. Immun. 49 459-462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lue, C., Tarkowski, A., and Mestecky, J. (1988) J. Immunol. 140 3793-3800 [PubMed] [Google Scholar]
- 36.Takeshita, S., Kawase, H., Shimizu, T., Yoshida, M., and Sekine, I. (2002) Pediatr. Int. 44 5-11 [DOI] [PubMed] [Google Scholar]
- 37.Obaro, S. K., Deubzer, H. E., Newman, V. O., Adegbola, R. A., Greenwood, B. M., and Henderson, D. C. (2004) Pediatr. Infect. Dis. J. 23 1023-1029 [DOI] [PubMed] [Google Scholar]
- 38.Simell, B., Kilpi, T., and Käyhty, H. (2006) Clin. Exp. Immunol. 143 543-549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osman, A. A., Richter, T., Stern, M., and Mothes, T. (1996) Clin. Chim. Acta 255 145-152 [DOI] [PubMed] [Google Scholar]
- 40.Schilling, J., Spiekerkoetter, U., Wohlrab, U., Wendel, U., and Seissler J. (2005) Scand. J. Immunol. 61 207-212 [DOI] [PubMed] [Google Scholar]
- 41.Pilette, C., Nouri-Aria, K. T., Jacobson, M. R., Wilcock, L. K., Detry, B., Walker, S. M., Francis, J. N., and Durham, S. R. (2007) J. Immunol. 178 4658-4666 [DOI] [PubMed] [Google Scholar]
- 42.Sykulev, Iu. K., Nezlin, R. S., German, G. P., Chernokhvostova, E. V., and Lavrent'ev, V. V. (1984) Biofizika 29 744-748 [PubMed] [Google Scholar]
- 43.Monteiro, R. C., and van de Winkel J. G. J. (2003) Annu. Rev. Immunol. 21 177-204 [DOI] [PubMed] [Google Scholar]
- 44.Woof, J. M., van Egmond, M., and Kerr, M. A. (2005) Mucosal Immunology, 3rd Ed., pp. 251-266, Academic Press, San Diego
- 45.Herr, A. B., Ballister, E. R., and Bjorkman, P. J. (2003) Nature 423 614-620 [DOI] [PubMed] [Google Scholar]
- 46.Crottet, P., and Corthésy, B. (1999) J. Biol. Chem. 274 31456-31462 [DOI] [PubMed] [Google Scholar]
- 47.Russell, M. W., and Kilian, M. (2005) Mucosal Immunology, 3rd Ed., pp. 267-290, Academic Press, San Diego
- 48.Arnold, J, N., Wormald, M. R., Sim, R. B., Rudd, P. M., and Dwek, R. A. (2007) Annu. Rev. Immunol. 25 21-50 [DOI] [PubMed] [Google Scholar]
- 49.Beale, D. (1985) Biochem. J. 229 759-763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindh, E. (1975) J. Immunol. 114 284-286 [PubMed] [Google Scholar]
- 51.Kilian, M., and Russell, M. W. (2005) Mucosal Immunology, 3rd Ed., pp. 291-304, Academic Press, San Diego
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.