Abstract
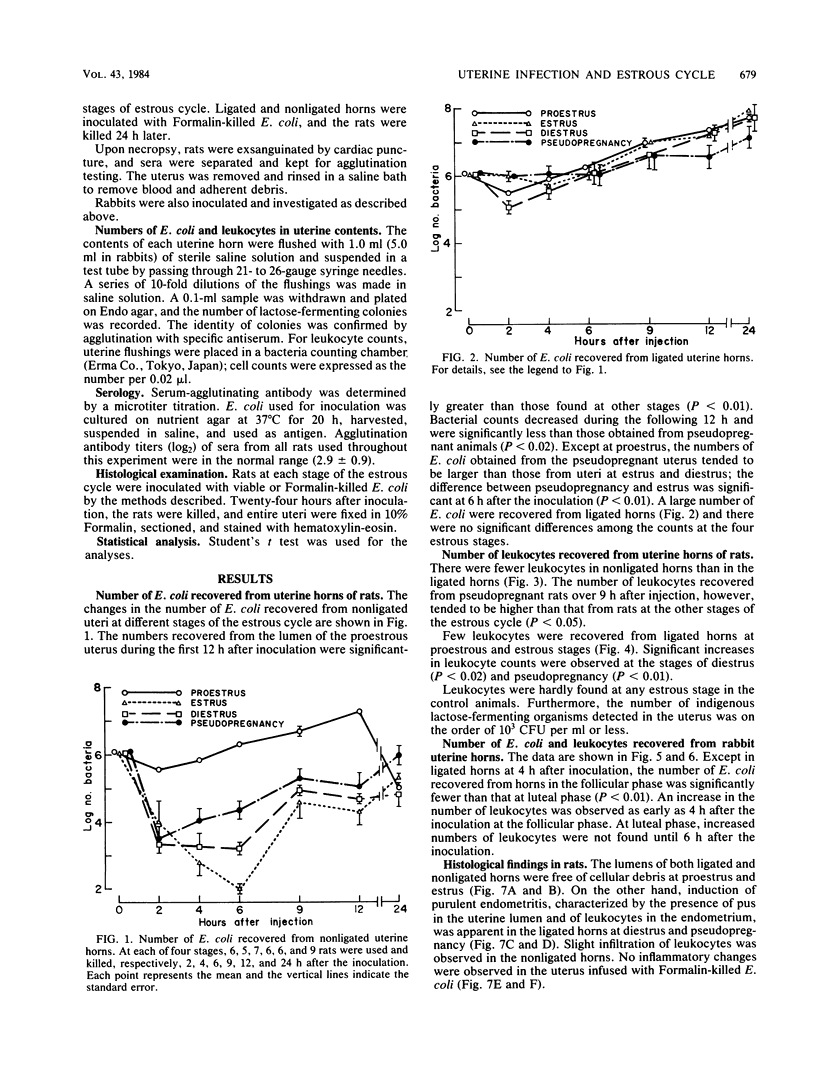
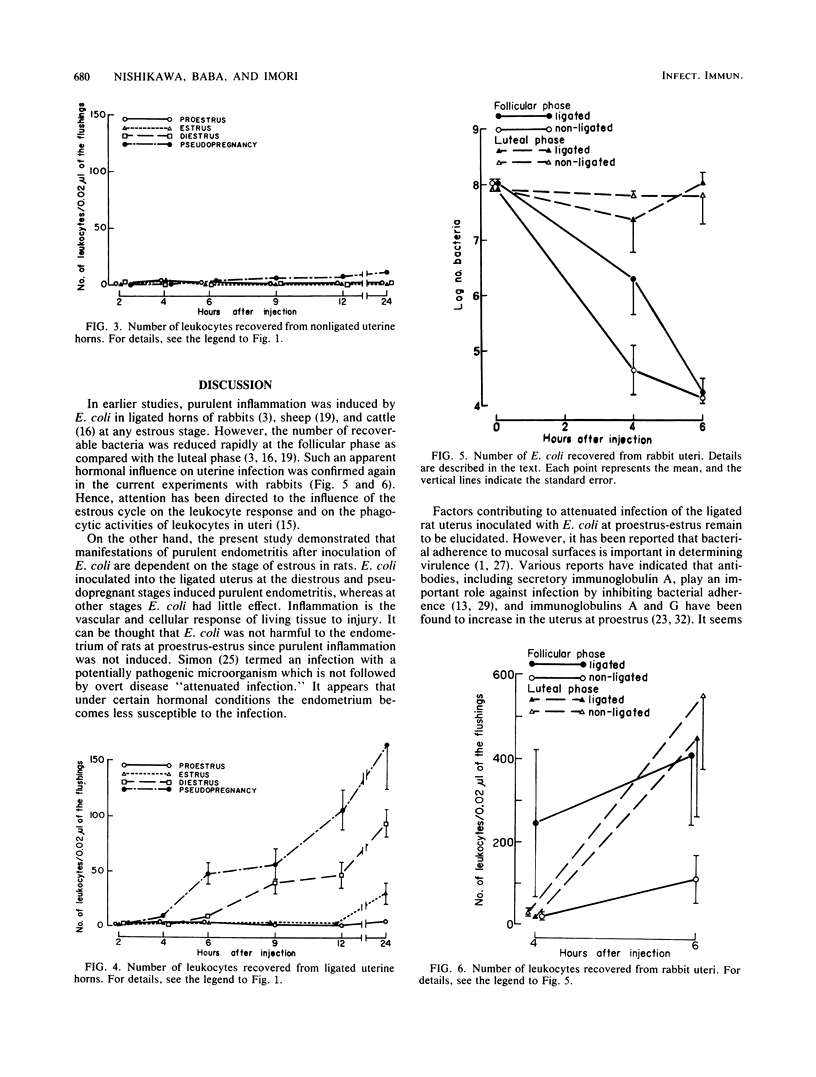
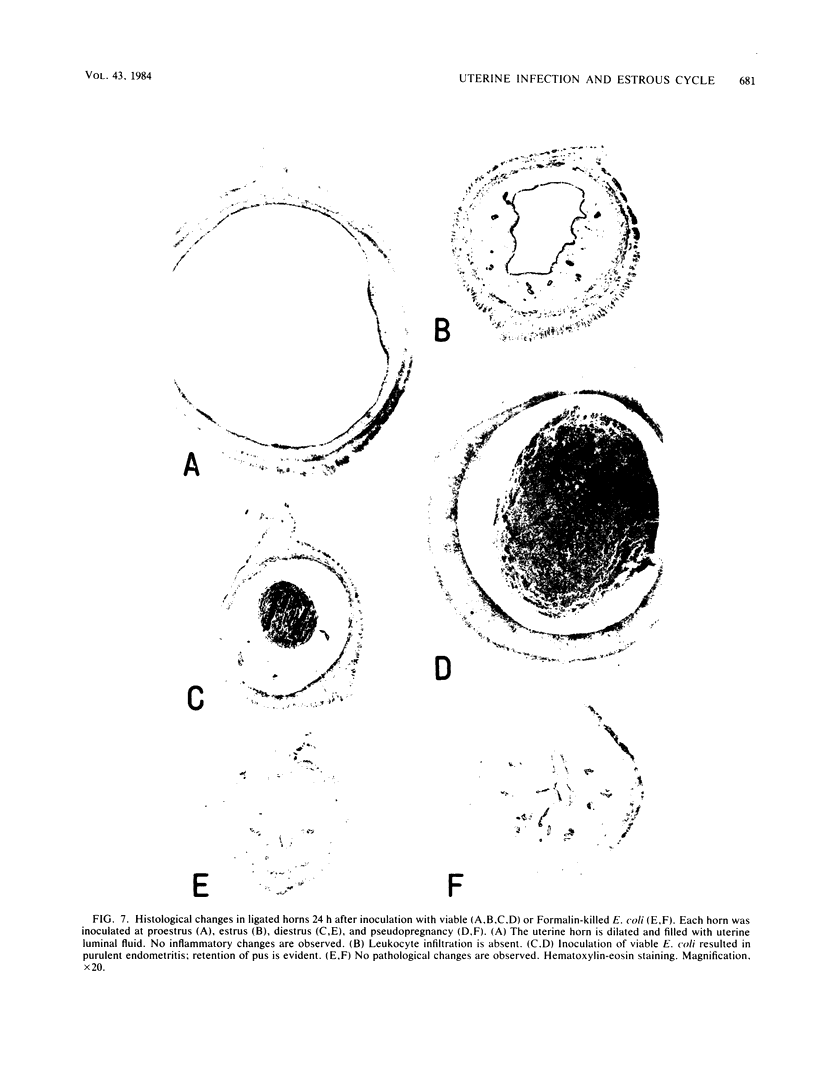
Escherichia coli was inoculated into the uterine lumen of rats and rabbits at different estrous stages; one uterine horn of each animal was ligated at the cervical end. In rats, a large number of E. coli were retained in the ligated horns regardless of the estrous stage. E. coli inoculated at diestrus or pseudopregnancy induced purulent endometritis, but when inoculated at proestrus-estrus the organism caused asymptomatic infection. In nonligated horns, few E. coli were recovered, and marked histopathological changes were not observed. Large numbers of E. coli were retained in the nonligated horn at proestrus as a result of physiological constriction of the cervix. E. coli inoculated at proestrus never caused purulent endometritis in either the ligated horn or the nonligated horn. In rabbits, E. coli infused into ligated horns brought about purulent inflammation irrespective of ovarian states. The number of recoverable E. coli was reduced rapidly at the follicular phase as compared with the luteal phase. These results suggest that the stage of the estrous cycle when animals are inoculated with E. coli influences the course of the uterine infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK W. G., SIMON J., KIDDER H. E., WILTBANK J. N. Bactericidal activity of the uterus in the rabbit and the cow. Am J Vet Res. 1954 Apr;15(55):247–251. [PubMed] [Google Scholar]
- BLACK W. G., SIMON J., MCNUTT S. H., CASIDA L. E. Investigations on the physiological basis for the differential response of estrous and pseudopregnant rabbit uteri to induced infection. Am J Vet Res. 1953 Apr;14(51):318–323. [PubMed] [Google Scholar]
- BLACK W. G., ULBERG L. C., KIDDER H. E., SIMON J., MCNUTT S. H., CASIDA L. E. Inflammatory response of the bovine endometrium. Am J Vet Res. 1953 Apr;14(51):179–183. [PubMed] [Google Scholar]
- CASIDA L. E., HAWK H. W., MCNUTT S. H., SIMON J. Investigations on the endocrine-controlled defense mechanism of estrous and pseudopregnant rabbit uteri. Am J Vet Res. 1957 Jan;18(66):171–173. [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Effect of Escherichia coli alpha-hemolysin on human peripheral leukocyte function in vitro. Infect Immun. 1982 Sep;37(3):966–974. doi: 10.1128/iai.37.3.966-974.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Effect of Escherichia coli alpha-hemolysin on human peripheral leukocyte viability in vitro. Infect Immun. 1982 May;36(2):455–461. doi: 10.1128/iai.36.2.455-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE FEO V. J. Temporal aspect of uterine sensitivity in the pseudopregnant or pregnant rat. Endocrinology. 1963 Feb;72:305–316. doi: 10.1210/endo-72-2-305. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Hanson L. A., Jodal U., Lindberg U., Akerlund A. S. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet. 1976 Sep 4;1(7984):490–492. [PubMed] [Google Scholar]
- Edén C. S., Larsson P., Lomberg H. Attachment of Proteus mirabilis to human urinary sediment epithelial cells in vitro is different from that of Escherichia coli. Infect Immun. 1980 Mar;27(3):804–807. doi: 10.1128/iai.27.3.804-807.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emödy L., Pál T., Safonova N. V., Kuch B., Golutva N. K. alpha-Haemolysin: an additive virulence factor in Escherichia coli. Acta Microbiol Acad Sci Hung. 1980;27(4):333–342. [PubMed] [Google Scholar]
- Fried F. A., Vermeulen C. W., Ginsburg M. J., Cone C. M. Etiology of pyelonephritis: further evidence associating the production of experimental pyelonephritis with hemolysis in Escherichia coli. J Urol. 1971 Sep;106(3):351–354. doi: 10.1016/s0022-5347(17)61286-2. [DOI] [PubMed] [Google Scholar]
- Fried F. A., Wong R. J. Etiology of pyelonephritis: significance of hemolytic Escherichia coli. J Urol. 1970 Jun;103(6):718–721. doi: 10.1016/s0022-5347(17)62033-0. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Bacterial adherence to mucosal surfaces and its inhibition by secretory antibodies. Adv Exp Med Biol. 1974;45(0):315–325. doi: 10.1007/978-1-4613-4550-3_38. [DOI] [PubMed] [Google Scholar]
- HAWK H. W., BRINSFIELD T. H., TURNER G. D., WHITMORE G. W., NORCROSS M. A. EFFECT OF OVARIAN STATUS ON INDUCED ACUTE INFLAMMATORY RESPONSES IN CATTLE UTERI. Am J Vet Res. 1964 Mar;25:362–366. [PubMed] [Google Scholar]
- HAWK H. W., TURNER G. D., SYKES J. F. The effect of ovarian hormones on the uterine defense mechanism during the early stages of induced infection. Am J Vet Res. 1960 Jul;21:644–648. [PubMed] [Google Scholar]
- HAWK H. W., TURNER G. D., SYKES J. F. The leukotaxic properties of uterine exudates as related to the endocrine-controlled uterine defense mechanism. Am J Vet Res. 1961 Nov;22:1117–1120. [PubMed] [Google Scholar]
- HAWK H. W., TURNER G. D., SYKES J. F. Variation in the inflammatory response and bactericidal activity of the sheep uterus during the estrous cycle. Am J Vet Res. 1961 Jul;22:689–692. [PubMed] [Google Scholar]
- Hawk H. W. Inflammatory responses of the uterus. J Anim Sci. 1971;32 (Suppl 1):55–65. [PubMed] [Google Scholar]
- Ishikawa M., Fuchs A. R. Electrical and mechanical activity of rat uterus in vivo during the estrous cycle. Am J Obstet Gynecol. 1978 Nov 15;132(6):611–619. doi: 10.1016/0002-9378(78)90852-9. [DOI] [PubMed] [Google Scholar]
- KILLINGBECK J., HAYNES N. B., LAMMING G. E. INFLUENCE OF ISOLATED COMPONENTS OF THE UTERINE SECRETION ON PHAGOCYTOSIS. Nature. 1963 Jul 20;199:255–256. doi: 10.1038/199255a0. [DOI] [PubMed] [Google Scholar]
- McDermott M. R., Clark D. A., Bienenstock J. Evidence for a common mucosal immunologic system. II. Influence of the estrous cycle on B immunoblast migration into genital and intestinal tissues. J Immunol. 1980 Jun;124(6):2536–2539. [PubMed] [Google Scholar]
- SMITH H. W. The haemolysins of Escherichia coli. J Pathol Bacteriol. 1963 Jan;85:197–211. doi: 10.1002/path.1700850119. [DOI] [PubMed] [Google Scholar]
- Svanborg-Edén C., Svennerholm A. M. Secretory immunoglobulin A and G antibodies prevent adhesion of Escherichia coli to human urinary tract epithelial cells. Infect Immun. 1978 Dec;22(3):790–797. doi: 10.1128/iai.22.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- Wira C. R., Sandoe C. P. Hormonal regulation of immunoglobulins: influence of estradiol on immunoglobulins A and G in the rat uterus. Endocrinology. 1980 Mar;106(3):1020–1026. doi: 10.1210/endo-106-3-1020. [DOI] [PubMed] [Google Scholar]
- van den Bosch J. F., Postma P., de Graaff J., MacLaren D. M. Haemolysis by urinary Escherichia coli and virulence in mice. J Med Microbiol. 1981 Aug;14(3):321–331. doi: 10.1099/00222615-14-3-321. [DOI] [PubMed] [Google Scholar]