Abstract
Traditional confirmation procedures for the identification of a pneumococcal serotype require an isolate. Non-culture-based confirmation protocols are available. Some of these confirm only the presence of pneumococci, and others are capable of identifying a limited number of serotypes. The increased use of pneumococcal polysaccharide and conjugate vaccines, especially in high-risk patient groups, and the likely increase in the number of serotypes included in future versions of the conjugate vaccines have necessitated the need for improved enhanced surveillance in order to assess their impact on public health. Since 2006, a multiplexed assay has been used at the Health Protection Agency of the United Kingdom for the detection of 14 pneumococcal serotypes which requires pneumococcal serotype-specific monoclonal antibodies (MAbs). We have developed a microsphere competitive inhibition method capable of detecting 23 pneumococcal capsular polysaccharide serotypes in cerebrospinal fluid (CSF) and urine and serotyping pneumococcal suspensions, utilizing an international reference serum, 89-SF. The assay was shown to be reproducible and specific for homologous polysaccharide. Validation of the assay was performed with a selection of MAbs specific for pneumococcal capsular polysaccharide serotypes, which confirmed the specificity of the assay. Analysis of pneumolysin PCR-positive CSF samples in the competitive inhibition assay determined a serotype for 89% of the samples. The assay developed here is well suited to large-scale epidemiologic studies because the assay is simple, robust, and rapid and utilizes readily available resources.
Streptococcus pneumoniae is a well-known human pathogen and a major etiologic agent of pneumonia, meningitis, and otitis media, as well as sepsis, primarily among young children and older adults (8). The pneumococcus is classified into 91 pneumococcal serotypes that are immunologically distinguishable by their polysaccharide capsules (11, 23). There are 46 serogroups, some of which comprise multiple serotypes and some of which are immunologically cross-reactive (11).
Prior to 2000, the only pneumococcal vaccines available were polysaccharide vaccines containing multiple pneumococcal polysaccharide serotypes which were effective against invasive pneumococcal disease (IPD) in older children and adults (9). In 2000, a pneumococcal seven-valent conjugate vaccine, Prevenar, was introduced into the U.S. infant immunization schedule and was followed by a decline in the incidence of IPD due to the serotypes covered by the vaccine (32, 33). There have now been reports of serotype replacement by serotypes not targeted by the seven-valent conjugate vaccine (1, 20, 21, 26, 29, 33), but overall, a dramatic reduction in the incidence of IPD has been observed.
The seven-valent conjugate vaccine was introduced into the United Kingdom immunization schedule in September 2006 and is given at 2, 4, and 13 months of age (3); and an enhanced surveillance program is ongoing to understand the disease epidemiology in the postvaccination era (www.hpa.org.uk). Serotype determination in those cases of IPD will be valuable for epidemiologic purposes and assessment of the extent of postvaccination serotype replacement among pneumococci causing invasive infections. Serotype information is available for those cases diagnosed by culture; however, information on serotypes from cases confirmed by non-culture-based methods will also be essential. Postvaccination serotype information is currently being generated by the Health Protection Agency (Centre for Infections, Colindale, London, United Kingdom) by a Bio-Plex method with serotype-specific monoclonal antibodies (MAbs), but this methodology is currently limited to the identification of 14 pneumococcal serotypes and the cell wall polysaccharide (C-PS) (27).
Bacterial culture is still considered the “gold standard” method for case confirmation, but non-culture-based methods are of increasing importance in the diagnosis of pneumococcal disease. Due to the large size of the serotype-specific gene region, molecular typing methods based on the polymorphism of pneumococcal capsular genes are still being investigated (15, 16, 22, 25) and identify limited numbers of serotypes. Therefore, PCR confirmation is based on the amplification of repetitive regions and genes encoding products such as pneumococcal surface adhesion molecules, autolysisn (lytA gene) (19), and, most frequently, pneumolysin (ply) (30).
Due to the need for serotype-specific determination, the pneumococcus, with over 90 different serotypes, is a challenging target for serotype confirmation by non-culture-based methods. Following culture, isolates are serotyped, with the gold standard method being the Quellung reaction (13).
During infection, bacteria shed large quantities of capsular polysaccharides into their environment, and this may continue for weeks following infection (31). This allows the detection of these polysaccharides in order to ascertain serotype information. Popular non-culture-based antigen detection methods include latex agglutination, radioimmunoassay, and countercurrent immunoelectrophoresis, all of which require large sample volumes due to the need to perform separate assays for each serotype/serogroup, require some kind of antisera generated by the immunization of animals with whole bacteria, and are slow and tedious to perform. Rapid screening assays which detect the C-PS antigen present in all pneumococci, for which a Food and Drug Administration-approved commercial kit, NOW S. pneumoniae (Binax, Portland, ME), is available, have proven to be clinically useful (6, 7); but these assays do not give information on the capsular serotype of the causative organisms.
Flow cytometric methods for the serotyping of pneumococci have recently been reported (18, 24), and Sheppard et al. (27) reported on a Bio-Plex method for the detection of 14 capsular polysaccharide serotypes and C-PS in cerebrospinal fluid (CSF) and urine samples, but these methods require MAbs against the pneumococcal serotype-specific polysaccharide and such MAbs are not commercially available.
We report here on a sensitive non-culture-based microsphere assay which utilizes small quantities of sample and is capable of serotyping pneumococcal suspensions and distinguishing serotypes for the detection of pneumococcal polysaccharide in CSF and urine without the need for MAbs.
(Part of this work was presented by P. Balmer at the 5th International Symposium on Pneumococci and Pnuemococcal Disease, Alice Springs, Australia, 2006.)
MATERIALS AND METHODS
Samples used.
CSF samples were obtained from children aged 2 to 16 years from Malawi (2) with confirmed cases of pneumococcal meningitis. Confirmed pneumococcal meningitis was defined as an abnormal CSF cell count (>10 cells/μl) plus one or more of the following: CSF positive for pneumococci by culture, CSF Gram stain findings consistent with pneumococci, CSF positive for pneumococcal polysaccharide antigen (by latex agglutination assay), and CSF positive for pneumococcal DNA (2).
For those patients from whom pneumococcal isolates were isolated, pneumococcal suspensions were prepared as follows. Pneumococcal isolates were retrieved from storage by subculture on blood agar plates (tryptic soy agar base supplement with 5% sheep blood), and the plates were incubated overnight at 37°C in 5% CO2. Bacterial cells were suspended in 250 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0), and the turbidity was adjusted to that of a McFarland 1 standard. The suspension was heated at 100°C for 5 min.
There were 63 patients from whom paired CSF and pneumococcal suspensions were obtained.
Urine samples obtained from healthy adults were spiked with pneumococcal polysaccharide serotypes and were used to assess the suitability of urine as a sample matrix in the competitive inhibition assay.
Conjugation of pneumococcal polysaccharide antigens to carboxylated microspheres.
Pneumococcal polysaccharides were conjugated to carboxylated microspheres by the method previously described by Lal et al. (12), except that 23 bead sets were prepared by the conjugation of serotype 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F (American Type Culture Collection [ATCC], Manassas, VA) and serotype 6A (donated by Wyeth Vaccines, Pearl River, NY) purified pneumococcal polysaccharides to the carboxylated microspheres (Luminex, Austin, TX). Following the conjugation of polysaccharide to poly-l-lysine, the bead sets were incubated for 1.5 h (serotypes 1, 2, 3, 4, 5, 6A, 7F, 8, 9N, 9V, 11A, 12F, 14, 15B, 17F, 19A, 20, 22F, and 33F), 2 h (serotype 18C), or 3 h (serotypes 6B, 19F, and 23F) at room temperature in the dark.
Competitive inhibition microsphere assay for detection of pneumococcal capsular polysaccharide serotype.
Diluent buffer was prepared by adding 2 μg/ml of pneumococcal C-PS (Statens Serum Institute, Copenhagen, Denmark) and 8 μg/ml of pneumococcal serotype 10A polysaccharide (ATCC) to phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-T; Sigma-Aldrich, Dorset, United Kingdom) and 0.02% newborn bovine serum (MP Biomedicals, Basingstoke, United Kingdom). Samples whose serotypes were unknown (CSF or urine samples or pneumococcal suspensions) were diluted 1/5 in diluent buffer, and 25 μl of each sample with an unknown serotype was added in duplicate to a 96-well MV Multiscreen filter plate (Fisher Scientific, Loughborough, United Kingdom) along with 25 μl of a cocktail of all 23 pneumococcal polysaccharide-conjugated microspheres at a concentration of 5,000 beads per region per well. Standard reference serum 89-SF (Food and Drug Administration) was prepared at a 1/35 dilution, 25 μl was added to each well except blank wells, and the plates were incubated for 20 min at room temperature on a plate shaker (WVR International, United Kingdom) at 500 rpm in the dark. A control well which contained 89-SF only was included on each plate. The beads were collected by vacuum filtration with a vacuum manifold (Millipore, Watford, United Kingdom) and were washed twice with 100 μl of PBS-T. An aliquot (100 μl) of a 1/200 dilution of R-phycoerythrin-conjugated anti-human immunoglobulin G (IgG; Jackson ImmunoResearch Laboratories, West Grove, PA) in PBS was added to each well, and the plate was incubated for 20 min as described above. After the beads were washed, they were resuspended in 125 μl PBS-T and the mixture was shaken for 10 s before the results were read on a Bio-Plex instrument (Bio-Rad, Hertfordshire, United Kingdom). Data were acquired in real time by using the Bio-Plex Manager (version 4.1.1) computer software package (Bio-Rad).
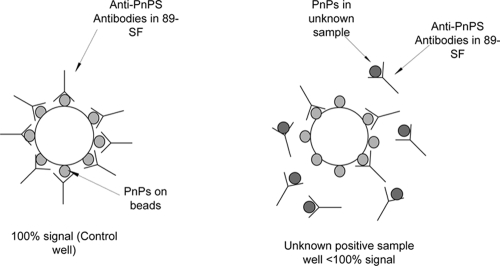
The percent reduction in the mean fluorescent intensity (MFI) in the test wells was calculated from the value for the control well, which contained 89-SF only and which gave 100% binding (Fig. 1).
FIG. 1.
Principle of the competitive inhibition microsphere assay for the detection of pneumococcal capsular polysaccharide (PnPs) serotypes. The MFI of the test well is compared to that of the control well in which 89-SF binds to the pneumococcal capsular polysaccharide on the bead, resulting in a 100% signal. Capture antibody (89-SF) binds to any pneumococcal capsular polysaccharide present in samples whose serotypes are unknown and not to the pneumococcal capsular polysaccharide on the bead, resulting in a reduction in the MFI in the test well.
Assay sensitivity.
The sensitivity of reference serum 89-SF as a capture antibody was assessed by assaying a cocktail of the 23 pneumococcal polysaccharide serotypes over a range of concentrations (8,000 ng/ml to 1 ng/ml) in PBS and urine.
Assay specificity.
The ability of the assay to detect the correct pneumococcal polysaccharide serotype was determined by spiking PBS or urine with the individual pneumococcal polysaccharide serotypes at a concentration of 250 ng/ml and testing by the assay described above.
Assay reproducibility.
The reproducibility of the assay developed was determined by the multiple testing of a panel of 42 CSF samples. From the mean percent inhibition given with each sample, the overall assay variation was calculated.
Correlation with PCR results.
A pneumolysin PCR was performed as described previously (2, 4). The results for the 42 CSF samples assayed in the competitive inhibition microsphere assay were compared to the cycle threshold (CT) number obtained in the pneumolysin PCR (4). A CT number of 45 indicated a negative cycle.
DNA was extracted from the pneumococcal suspensions (prepared as described above), and sequential multiplex PCRs were performed with the 64 pneumococcal suspensions and CSF pairs to determine the capsular serotype present, as described previously (22).
The serotypes determined by PCR were compared to those determined by the competitive inhibition microsphere assay.
Assay validation with MAbs.
The detection of the correct pneumococcal polysaccharide serotype in the CSF or pneumococcal suspension samples was validated by the use of MAbs. The MAbs used were those previously described by Yu et al. (34): MAb Hyp1G4, directed against the serotype 1 polysaccharide; MAb Hyp6AM3, directed against the serotype 6A polysaccharide; MAb Hyp6BM7, directed against the serotype 6B polysaccharide; MAb Hyp14M11, directed against the serotype 14 polysaccharide; MAb Hyp19FM3, directed against the serotype 19F polysaccharide; and MAb Dob9, directed against the serotype 19A/19F polysaccharide (all MAbs kindly supplied by Moon Nahm, University of Alabama, Birmingham). The assay was performed as described above, with the exception that a 1/100 dilution of MAbs was used in place of 89-SF, followed by R-phycoerythrin-conjugated goat anti-mouse IgG and IgM (Jackson ImmunoResearch Laboratories, West Grove, PA).
RESULTS
Development of competitive inhibition microsphere assay for detection of pneumococcal capsular polysaccharide serotypes.
All assay parameters were optimized empirically in preliminary studies to obtain optimal assay conditions, including the optimal capture antibody (89-SF) and the dilutions of the samples whose serotypes were unknown. It was shown that the proposed methodology is feasible and that 89-SF is a suitable antibody source, with a reduction in the MFI seen with a particular serotype bead set when the homologous serotype polysaccharide was added to the assay. The inclusion of two adsorbents, C-PS and serotype 10A capsular polysaccharide, to the diluent buffer to neutralize nonspecific antibodies was found to improve the specificity of the assay.
Assay sensitivity.
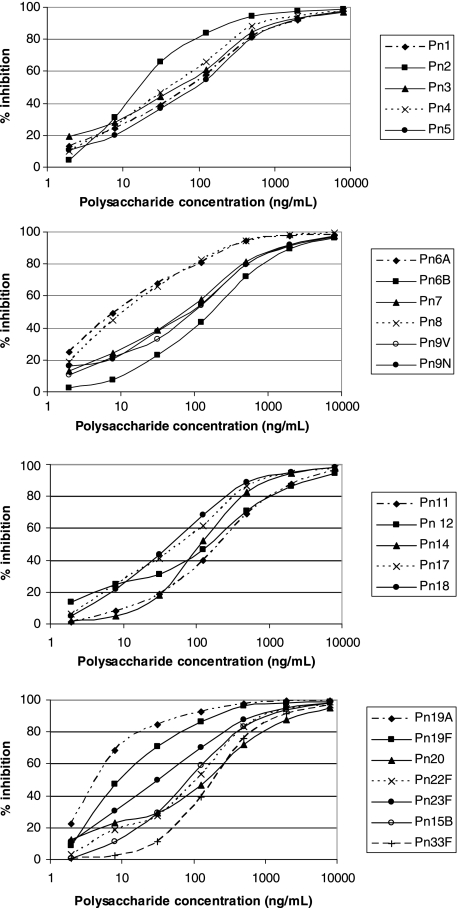
The sensitivity of the assay was assessed by the assay of PBS spiked with the pneumococcal polysaccharide serotypes at concentrations ranging from 1 to 8,000 ng/ml. The assay was shown to be sensitive, with inhibition seen over a range of polysaccharide concentrations. The sensitivity of the assay varied for each serotype, with 50% inhibition seen at polysaccharide concentrations that varied from 5 ng/ml for serotype 19A to 200 ng/ml for serotypes 6B, 11A, and 33F (Fig. 2). Similar curves were produced when urine was spiked with various concentrations of polysaccharide, demonstrating that the assay can also detect polysaccharide and the specific serotype in urine (data not shown).
FIG. 2.
Competitive inhibition with various concentrations of polysaccharide. The competitive inhibition assay is shown to be sensitive, with an increase in inhibition being seen with increasing polysaccharide concentrations.
Assay specificity.
In order to confirm that the 23-plex competitive inhibition assay was detecting only serotype-specific capsular polysaccharide, the specificities of the assays were determined. Following the addition of 1 of the 23 pneumococcal polysaccharides at a concentration of 250 ng/ml to the reaction mixture, inhibition of the MFI of the bead set with the homologous serotype was compared to that of the bead sets with heterologous serotypes. High levels of inhibition of the bead sets with the homologous serotypes was seen, and low levels of inhibition of the bead sets with the heterologous serotypes was seen for all serotypes (Table 1) except between those pneumococcal serotypes within a serogroup, e.g., serotypes 6A and 6B. The effects of related serotypes in the assay varied depending upon the serogroup. Cross-reactions did not limit the ability of the assay to discriminate between serotypes 19A and 19F, the serotype 19A polysaccharide inhibited the MFI signals of both serotypes 19A and 19F, but the serotype 19F polysaccharide inhibited the MFI signal of only serotype 19F; this was also true for serotypes 6A and 6B, with the serotype 6B polysaccharide inhibiting the MFI signals of both serotypes 6A and 6B but with the serotype 6A polysaccharide inhibiting the MFI signal of only serotype 6A.
TABLE 1.
Specificity of pneumococcal 23-plex competitive inhibition assay by determination of percent inhibition of MFI on addition of 2.5 μg/ml polysaccharide compared to that for control containing no polysaccharide
| Inhibitor | % Inhibition of MFI for serotypea:
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6A | 6B | 7 | 8 | 9V | 9N | 11A | 12F | 14 | 15B | 17F | 18C | 19A | 19F | 20 | 22F | 23F | 33F | |
| 1 | 83.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 94.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 78.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.8 | 0 | 0 | 0 |
| 4 | 0.2 | 0 | 0 | 86.8 | 0 | 0 | 0 | 0 | 0 | 0.2 | 0 | 0 | 0.3 | 0 | 1.8 | 0 | 0 | 0 | 2.4 | 0 | 0 | 1.9 | 0 |
| 5 | 0 | 0 | 0 | 0 | 77.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6A | 1.1 | 0 | 0 | 0 | 0 | 95.2 | 8.9 | 0.7 | 0 | 1.6 | 0.2 | 0 | 1.3 | 0 | 0.6 | 5.0 | 0.4 | 0.1 | 5.7 | 0 | 1.3 | 1.5 | 0 |
| 6B | 4.8 | 1.4 | 2.2 | 4.6 | 4.8 | 44.1 | 67.9 | 3.8 | 2.5 | 5.2 | 4.5 | 4.9 | 6.7 | 0 | 1.4 | 4.0 | 4.0 | 0.7 | 6.3 | 1.1 | 2.0 | 4.3 | 0 |
| 7 | 8.9 | 3.6 | 7.9 | 10.5 | 9.0 | 8.7 | 1.6 | 63.0 | 4.5 | 7.8 | 10.7 | 7.1 | 10.0 | 0 | 6.6 | 6.7 | 10.0 | 3.5 | 3.9 | 0.5 | 5.7 | 8.4 | 1.2 |
| 8 | 7.0 | 2.7 | 9.7 | 7.7 | 3.9 | 6.3 | 0.7 | 5.7 | 98.3 | 7.1 | 8.0 | 8.8 | 7.7 | 0 | 2.2 | 6.4 | 4.1 | 2.3 | 8.4 | 0 | 5.5 | 8.5 | 0.5 |
| 9V | 5.3 | 3.4 | 6.5 | 7.0 | 7.7 | 6.0 | 1.5 | 2.4 | 7.5 | 81.6 | 34.2 | 7.4 | 10.6 | 0.5 | 6.1 | 9.2 | 7.3 | 4.0 | 8.9 | 0 | 5.0 | 5.3 | 0.4 |
| 9N | 7.1 | 3.0 | 6.9 | 8.4 | 4.6 | 7.7 | 2.0 | 6.4 | 4.8 | 24.8 | 75.9 | 2.0 | 8.6 | 0 | 2.8 | 5.1 | 2.9 | 2.3 | 9.5 | 0 | 2.2 | 9.5 | 1.0 |
| 11A | 10.6 | 3.2 | 9.3 | 7.3 | 8.1 | 10.5 | 2.5 | 9.5 | 7.4 | 7.2 | 7.9 | 73.3 | 10.7 | 1.4 | 6.2 | 11.5 | 7.7 | 3.7 | 10.1 | 0 | 4.5 | 9.1 | 2.3 |
| 12F | 9.7 | 4.5 | 6.9 | 9.3 | 6.8 | 6.2 | 4.2 | 8.7 | 7.4 | 9.2 | 8.1 | 10.5 | 69.5 | 3.8 | 3.8 | 7.9 | 5.0 | 6.0 | 8.1 | 0 | 7.2 | 6.8 | 3.2 |
| 14 | 6.0 | 3.8 | 1.2 | 2.7 | 2.4 | 4.5 | 3.7 | 4.2 | 2.2 | 3.5 | 2.9 | 2.6 | 4.7 | 84.0 | 7.0 | 4.5 | 2.0 | 3.7 | 5.2 | 0 | 6.4 | 4.7 | 2.8 |
| 15 | 7.8 | 4.4 | 8.6 | 9.1 | 6.6 | 6.8 | 3.6 | 9.2 | 5.4 | 7.7 | 7.7 | 6.4 | 9.0 | 3.8 | 84.3 | 8.2 | 6.3 | 2.3 | 7.5 | 1.6 | 6.4 | 7.2 | 3.0 |
| 17F | 13.1 | 5.0 | 13.2 | 12.8 | 11.7 | 10.9 | 4.5 | 10.4 | 10.8 | 12.0 | 7.6 | 14.3 | 14.5 | 4.3 | 7.1 | 89.6 | 10.2 | 6.0 | 13.4 | 3.0 | 8.0 | 10.9 | 4.1 |
| 18C | 8.7 | 4.0 | 7.7 | 8.4 | 7.7 | 7.6 | 3.3 | 7.4 | 9.4 | 7.3 | 7.5 | 9.6 | 11.1 | 3.0 | 3.9 | 9.9 | 87.6 | 6.5 | 11.1 | 0 | 8.1 | 11.3 | 3.0 |
| 19A | 11.5 | 4.1 | 11.3 | 8.4 | 9.3 | 8.3 | 3.8 | 7.4 | 10.5 | 17.2 | 24.8 | 8.6 | 12.0 | 3.3 | 4.9 | 10.6 | 15.2 | 98.3 | 33.9 | 0 | 8.1 | 9.8 | 2.5 |
| 19F | 8.1 | 4.5 | 7.6 | 8.4 | 8.8 | 8.9 | 3.7 | 6.0 | 8.2 | 8.7 | 8.4 | 9.7 | 8.6 | 3.5 | 3.1 | 7.4 | 1.5 | 2.8 | 92.4 | 0 | 1.7 | 8.5 | 3.4 |
| 20 | 17.3 | 4.4 | 8.5 | 10.7 | 8.0 | 8.3 | 4.5 | 6.9 | 9.8 | 7.5 | 6.2 | 1.9 | 11.0 | 3.0 | 7.5 | 7.6 | 6.6 | 3.1 | 5.7 | 69.8 | 5.9 | 8.8 | 2.8 |
| 22F | 7.1 | 3.7 | 6.7 | 10.9 | 7.0 | 6.2 | 3.9 | 7.2 | 7.7 | 10.2 | 10.0 | 10.1 | 6.5 | 3.6 | 3.8 | 6.2 | 5.1 | 4.6 | 7.3 | 0 | 90.8 | 9.2 | 3.3 |
| 23F | 7.6 | 4.2 | 7.4 | 7.0 | 6.5 | 7.0 | 3.5 | 4.1 | 4.5 | 7.3 | 8.4 | 7.6 | 10.3 | 2.8 | 2.9 | 7.7 | 7.1 | 3.8 | 5.9 | 7.0 | 7.4 | 92.0 | 3.1 |
| 33F | 10.4 | 4.4 | 9.5 | 11.3 | 10.5 | 11.0 | 4.6 | 7.0 | 12.3 | 10.0 | 9.3 | 5.4 | 11.4 | 4.2 | 3.2 | 13.7 | 7.7 | 3.4 | 9.4 | 5.6 | 8.9 | 14.6 | 86.7 |
Boldface data indicate the levels of homologous inhibition.
The assay was able to discriminate between serotypes 9V and 9N, with a high level inhibition seen with the homologous polysaccharide at the concentration used, although the level of interference by the polysaccharide with a related heterologous serotype seen was greater than that by the unrelated serotypes seen, indicating that a higher inhibition threshold may be required for the determination of these serotypes.
The effect of cross-reacting serotypes on the assay outcome was also determined with low levels of polysaccharide (5 to 50 ng/ml). The same effect was seen when the serotype 6B polysaccharide was included in the assay, with a reduction in the MFI signals for serotypes 6A and 6B being seen but with the reduction in the signal for serotype 6B being greater. No cross-reaction effect was seen for serotypes 9V and 9N and serotypes 19A and 19F with this low level of polysaccharide, with clear distinctions between serotypes being attained.
Assay reproducibility.
In order to confirm that the 23-plex competitive inhibition assay was reproducible, CSF samples from known cases of pneumococcal disease (n = 42) were assayed six times. For each sample, the coefficient of variance was calculated, and the coefficients of variance for all samples were <25%; from these values, the average assay variation was calculated to be 15%, from which an inhibition cutoff of 30% was determined. Examination of the assay sensitivity data (Fig. 2) reflects the suitability of the 30% cutoff. Inhibition of the MFI signals of greater than 30% was determined to be a positive result for that serotype, with the exception of serotypes for which a cross-reacting serotype existed in the assay. The levels of inhibition for cross-reacting serotypes must be analyzed together to determine if inhibition is for only one serotype or for both serotypes. In cases in which inhibition of both serotypes was observed, only a serogroup could be determined.
Correlation to PCR results.
The results of testing of the 42 CSF samples from known cases of pneumococcal disease in the competitive inhibition assay for detection of pneumococcal capsular polysaccharide were compared to the numbers of CTs obtained in the pneumolysin PCR. Of the 42 samples tested, 24 had a pneumococcal polysaccharide serotype detected in the competitive inhibition assay and also had a pneumolysin PCR CT number indicating a positive result. Six samples were negative for pneumococcal polysaccharide in the competitive inhibition assay but had pneumolysin PCR CT numbers that indicated that these samples were positive; for two of these six samples, pneumococci were detected in the corresponding suspension. For the remaining four samples, the cases were due to pneumococci expressing a serotype capsular polysaccharide not included in this assay; the serotype PCR determined that the serotypes of the capsular polysaccharide present in these samples were 35F and 15A in one sample each and 16F in two samples. Twelve samples were negative by both the competitive inhibition assay and had pneumolysin PCR CT numbers of 45 (indicating a negative pneumolysin PCR result). No samples were positive for pneumococcal polysaccharide in the competitive inhibition assay and pneumolysin PCR negative.
The pneumococcal serotypes in 63 CSF samples and pneumococcal suspensions from known cases of IPD were determined by sequential multiplexed PCR (21) and were compared to the pneumococcal polysaccharide serotypes determined in these samples by the competitive inhibition assay, the results of which are shown in Table 2. There were four samples in which the serotype determined by PCR was different from that determined by the competitive inhibition assay. For one sample, the serotypes in the CSF and the pneumococcal suspension determined by PCR agreed but different serotypes in the CSF and pneumococcal suspension were determined by the competitive inhibition assay. For three samples, each assay detected the same serotype in each of the two sample types, but the serotypes that each methodology determined were different. For these three samples, the serotype determined by the competitive inhibition assay with 89-SF was confirmed with serotype-specific MAbs.
TABLE 2.
Pneumococcal serotypes identified by assay of CSF and pneumococcal suspensions in the competitive inhibition microsphere assay and PCR
| Sample identifier | Serotype identifieda
|
|||||
|---|---|---|---|---|---|---|
| CSF
|
Pneumococcal suspension
|
|||||
| PCR | Antigen capture
|
PCR | Antigen capture
|
|||
| 89-SF | MAbs | 89-SF | MAbs | |||
| 1 | ||||||
| 5b | 6A/6B | 1 | 1 | 6A/6B | 22F | |
| 7 | 19A | 19A | 1 | 1 | ||
| 9 | 6A/6B | 1 | Not tested | 6A/6B | 1 | 1 |
| 10 | 12F | 12F | 12F | 12F | ||
| 12 | 12F | 12F | 12F | 12F | ||
| 17 | 14 | No sample | No sample | 14 | 14 | 14 |
| 19 | 35B | 35B | ||||
| 21 | 6A/6B | 6A | 6A | 6A/6B | 6A | 6A |
| 22 | 19F | 19F | 19F | 19F | 19F | 19F |
| 25 | 6A/6B | 6B | 6B | 6A/6B | 6B | 6B |
| 26 | 8 | 8 | 8 | 8 | ||
| 28c | 5 | 5 | ||||
| 30 | 6A/6B | 1 | 1 | 6A/6B | 1 | 1 |
| 32 | 14 | 14 | 14 | 14 | No sample | No sample |
| 33 | 14 | 14 | 14 | 14 | ||
| 38 | 14 | 14 | 14 | 14 | No sample | No sample |
| 45 | 6A/6B | 6B | 6B | 6A/6B | 6B | 6B |
| 46 | 7F | 7F | 7F | 7F | ||
| 47 | 23F | 23F | 23F | |||
| 51 | 6A/6B | 6B | 6B | 6A/6B | No sample | No sample |
| 52 | 1 | 1 | 1 | 1 | 1 | 1 |
| 54 | 16F | 16F | ||||
| 55 | 23F | 23F | 23F | 23F | ||
| 59 | 1 | 1 | 1 | 1 | 1 | 1 |
| 60 | 6A/6B | 6B | 6B | 6A/6B | 6B | 6B |
| 65 | 6A/6B | 6B | 6B | 6A/6B | 6B | 6B |
| 66 | 23F | 23F | 23F | 23F | ||
| 69 | 23F | 23F | 23F | 23F | ||
| 72 | 23F | 23F | 23F | 23F | ||
| 77 | 1 | No sample | No sample | 1 | 1 | 1 |
| 78 | 23F | 23F | 23F | 23F | ||
| 79c,d | 6A/6B | 6A/6B | 6A/6B | 6A/6B | ||
| 80 | 23F | 23F | 23F | 23F | ||
| 81 | No growth | 9V/9N | No growth | 9V/9N | ||
| 82 | 1 | 1 | 1 | 1 | 1 | 1 |
| 90e | sg18 | 18C | sg18 | 18C | ||
| 93 | No growth | 6B | 6B | No growth | No sample | No sample |
| 95 | 1 | 1 | 1 | 1 | 1 | 1 |
| 97 | 1 | 1 | 1 | 1 | 1 | 1 |
| 104b | 12F | 12F | 12F | 5 | ||
| 105 | No growth | 14 | 14 | No growth | 14 | 14 |
| 107 | 15A | 15A | ||||
| 110 | No growth | 14 | 14 | No growth | 14 | 14 |
| 111 | 1 | 1 | 1 | 1 | 1 | 1 |
| 112 | sg18 | 18c | sg18 | 18C | ||
| 113 | 17F | 17F | 17F | 17F | ||
| 116 | No growth | 23F | No growth | No sample | ||
| 118 | ||||||
| 119 | 1 | 1 | 1 | 1 | 1 | 1 |
| 122 | No growth | 1 | Not tested | No growth | 1 | 1 |
| 126 | 35F | 35F | ||||
| 127 | No growth | 1 | Not tested | No growth | 1 | 1 |
| 138 | 16F | 16F | ||||
| 139 | ||||||
| 140 | 14 | 14 | 14 | 14 | 14 | 14 |
| 148 | 10A | 10A | ||||
| 150 | 6A/6B | 6A | 6A | 6A/6B | 6A | 6A |
| 153 | 6B | 6B | No sample | |||
| 155 | No sample | 23F | No growth | 23F | ||
| 159 | 4 | 4 | 4 | 4 | ||
| 161 | No growth | No growth | No sample | |||
| 162 | 1 | 1 | 1 | 1 | 1 | 1 |
Blank cell, no serotype detected.
Discrepancy between the serotype identified in CSF and that identified in the suspension.
No serotype was identified in the CSF.
Serotype 6A inhibition was greater than that of serotype 6B inhibition; no discrimination was achieved with the serotype 6A- and serotype 6B-specific MAbs.
The PCR assay determined only serogroup 18.
Assay of CSF and pneumococcal suspensions from cases of pneumococcal disease.
CSF samples and pneumococcal suspensions from known cases of IPD were assayed with 89-SF, the results of which are shown in Table 2. For five subjects, a pneumococcal serotype was determined in the suspension but no serotype was determined by the use of CSF. CSF samples from four of these five subjects had pneumolysin PCR CTs which were indicative of being pneumolysin positive; the fifth CSF sample was not assayed by the pneumolysin PCR. For two subjects, the serotype identified in the suspension differed from the serotype identified in the CSF.
For six subjects, a serotype was determined by the serotype-specific PCR but no serotype was determined by the competitive inhibition assay because the assay did not contain these serotypes.
Assay validation with MAbs.
Once the assay was developed and the sensitivity, specificity, and reproducibility were determined, the assay was validated with MAbs specific for pneumococcal capsular serotypes 1, 6A, 6B, 14, and 19F. CSF samples and pneumococcal suspensions were assayed with a cocktail of MAbs Hyp1G4, Hyp6AM3, Hyp6BM7, Hyp14M11, and Hyp19FM3. The serotype determined in each of the sample types and the serotype determined in the assay with 89-SF and the MAbs are shown in Table 2. High-level MAb inhibition was seen for those samples determined to be serotype 1, 14, or 19F in the assay with 89-SF.
Discrimination between serotypes 6A and 6B in samples determined by the assay with 89-SF was confirmed by the assay of these samples with serotype 6A- and 6B-specific MAbs. For CSF samples containing serotype 6A, only the serotype 6A MFI was reduced in the 89-SF assay; this result was confirmed by assays with the MAbs. However, pneumococcal suspensions containing serotype 6A gave reduced serotype 6A- and 6B-specific MFI signals in the 89-SF assay, with the reduction for serotype 6A being greater than that for serotype 6B, indicating the presence of serotype 6A, which was confirmed in the assay with the MAbs. Pneumococcal suspensions and CSF samples containing serotype 6B reduced both the serotype 6A and the serotype 6B MFIs in the assay with 89-SF, but the inhibition of serotype 6B was greater than that of serotype 6A, indicating the presence of serotype 6B, which was confirmed in the assay with MAbs. One pneumococcal suspension showed a reduction in both the serotype 6A and the serotype 6B MFIs in the 89-SF assay, with the reduction for serotype 6A being greater than that for serotype 6B, indicating the presence of serotype 6A. However, in the assay with the MAbs, the MFIs for both the serotype 6A- and serotype 6B-specific MAbs were reduced, indicating the presence of both serotype 6A and serotype 6B.
DISCUSSION
We report here on a competitive inhibition flow analysis assay which is able to detect pneumococcal serotype-specific polysaccharide in CSF and urine and determine the serotype of pneumococcal suspensions, utilizing as a capture antibody a standard reference serum which is available internationally. Standard human serum pool 89-SF was investigated as a source of capture antibody due to its high levels of pneumococcal serotype-specific antibodies. It was shown that following adsorbance of nonspecific antibodies with C-PS and the serotype 10A capsular polysaccharide, it was possible to detect pneumococcal polysaccharide in both CSF and urine without antibody interference. On titration of the polysaccharide, the assay was able to detect pneumococcal polysaccharide over seven fourfold dilutions for all serotypes when the polysaccharide was diluted in PBS and urine.
Previous studies have utilized the percent inhibition of binding to assess assay sensitivity (14). By using a cutoff of 50% inhibition, it was observed that a minimum polysaccharide concentration of between 5 ng/ml (serotype 19A) and 200 ng/ml (serotypes 6B, 11A, and 33F) could be detected. Such a level of sensitivity is similar to or greater than the levels of sensitivity of other assays (34).
The assay developed was shown to be specific for each of the 23 serotypes and was able to discriminate between cross-reactive serotypes. The effect of related serotypes in the assay was investigated for serotypes 6A and 6B with the use of MAbs against serotypes 6A and 6B. The assay could clearly detect serotype 6A in CSF, PBS, and urine. However, suspensions containing serotype 6A pneumococci gave reductions in both the serotype 6A and the serotype 6B signals, with the reduction in the signal for serotype 6A being greater than that for serotype 6B, indicating the presence of serotype 6A pneumococci, which was confirmed by the use of MAbs specific for serotype 6A and 6B polysaccharides. The reason for this difference between sample types could be due to the differences in the conformation of the polysaccharide within the two sample types caused by the heat treatment of the suspensions during preparation.
For PBS and urine spiked with the serotype 6B polysaccharide and for CSF samples and pneumococcal suspensions containing the serotype 6B polysaccharide, inhibition was seen with both the serotype 6A- and serotype 6B-specific bead sets, although the level of inhibition of serotype 6B was always greater than that of serotype 6A. The determination of serotype 6B was proven to be correct by the use of MAbs specific for serotype 6A and 6B polysaccharides.
The inhibition seen with both serotype 6A- and serotype 6B-specific bead sets in the presence of serogroup 6 polysaccharide in the assay with 89-SF may be because 89-SF contains cross-reacting antibody. 89-SF is a pool of human serum derived following immunization with the 23-valent pneumococcal polysaccharide vaccine and does not contain antibodies to serotype 6A. Therefore, the serotype 6A antibodies present in 89-SF are either naturally derived or cross-reacting antibodies produced following vaccination, as immunization with serotype 6B confers protection against serotype 6A. Whitney et al. (33) have shown that immunization with the seven-valent conjugate vaccine is effective against all seven individual serotypes represented in the vaccine, as well as serotype 6A. The cross-reacting antibody theory is supported by the fact that the MAbs do not cross-react and detect only beads conjugated with homologous polysaccharide. Although the assay described here has the ability to discriminate between serotypes 6A and 6B, one pneumococcal suspension showed a reduction in the MFIs for both serotype 6A and serotype 6B in the 89-SF assay, which was confirmed by testing with the MAbs for both serotypes 6A and 6B, indicating a mixed infection. Therefore, samples with which reduced MFIs for serotypes 6A and 6B are seen can be reported only as serogroup 6 in the multiplexed assay with 89-SF, unless further clarification is obtained in monoplex serotype 6A- and serotype 6B-specific assays.
Park et al. (23) have recently discovered a new capsular serotype within serogroup 6, serotype 6C, which has only small structural differences from serotype 6A. No samples which were known to contain the serotype 6C polysaccharide were analyzed in the current study. The results for those samples determined by the competitive inhibition assay to contain serotype 6A were confirmed by the use of MAb Hyp6AM3, which recognizes only serotype 6A. Currently, there is no serotype 6C polysaccharide available for inclusion in this assay to investigate the assay's ability to detect the presence of the serotype 6C polysaccharide. Serotype 6C has only small structural differences from serotype 6A, and therefore, only a determination of serogroup 6 can be made by this assay in its current format.
Sequential multiplexed PCR was performed with 63 pairs of CSF and pneumococcal suspensions, the serotype results of which were compared to the serotypes determined by the competitive inhibition assay. Of the 63 pairs, discrepant serotypes determined by the two methodologies were seen for only 4 samples. For one sample the serotype determined in both sample types by PCR were in agreement, but the serotype determined by the competitive inhibition assay in the two sample types differed from each other and from that determined by PCR. For one sample, the serotype determined in the pneumococcal suspension by the competitive inhibition assay differed from the serotype determined by the same method in the CSF and the serotypes determined by PCR. For two samples, serotypes 6A and 6B were determined by PCR, but only one serotype was detected by the competitive inhibition assay, which was confirmed by assays with MAbs. These differences could be due to the selection of a colony of only a single serotype during the preparation of the suspensions or the fact that the disease in these cases was due to multiple serotypes.
Serotype-specific identification assays have previously been reported and include assays based on latex agglutination (28) and enzyme-linked immunosorbent assay (ELISA) (17). Leeming et al. (17) reported on an ELISA capable of detecting serotype-specific polysaccharide in urine, but this assay is dependent upon the availability of MAbs with high degrees of specificity for pneumococcal capsular polysaccharides which are not commercially available. An additional drawback of performing such ELISAs is that they are labor intensive, as an individual ELISA specific for each serotype must be performed.
The assay developed here is limited by the commercial availability of pneumococcal serotype-specific polysaccharides and the inability to quantify the amount of antigen present in samples, which may be of use in distinguishing urine samples from individuals with pneumococcal disease from samples from S. pneumoniae carriers.
The multiplexed assay is a rapid way of serotyping pneumococcal suspensions and detecting pneumococcal polysaccharide in CSF and urine. The detection of pneumococcal polysaccharide in CSF is confirmation of IPD, but the pneumococcal polysaccharide detected in urine may be derived from nasopharyngeal carriage. Further development is required to enable the detection of pneumococcal polysaccharide in other specimen types, such as plasma and pleural fluid. Preliminary studies have shown the ability of the assay to detect pneumococcal polysaccharide in EDTA-anticoagulated blood samples, although caution is required when the presence of pneumococcal polysaccharide in EDTA-anticoagulated blood from those ages 0 to 2 years is used as a means of diagnosis of IPD, as Dagan et al. (5) noted a high rate of detection of pneumococcal DNA in healthy infant and child controls which was found to be associated with nasopharyngeal carriage.
The assay developed in the study described here is well suited to large-scale epidemiologic studies because it is a rapid, simple, and robust procedure and utilizes readily available resources. Although 89-SF is a finite resource, it can be replaced by any serum sample containing pneumococcal serotype-specific IgG antibodies.
The ability to rapidly serotype the pneumococci in samples from cases of IPD is of increasing importance in the postvaccination era and provides vital serotype information in order to assess vaccine efficacy. The assay developed in the study described here is simple, does not require the presence of pneumococcal DNA, and can be performed with serum samples. This is of importance in both developed and developing countries, especially with the introduction of pneumococcal conjugate vaccines planned into the latter through the GAVI Alliance's accelerated development and introduction plans.
Acknowledgments
We thank Moon Nahm and Robert Burkin, University of Alabama, for the donation of pneumococcal serotype 1-, 6A-, 6B-, 14-, and 19F-specific MAbs and Wyeth Vaccines Research for the donation of pneumococcal capsular polysaccharide of serotype 6A.
The initial work on the development of this assay was commenced during a Public Health Laboratory Service (London, United Kingdom)-funded Ph.D. studentship cosupervised by Maureen Dawson (Manchester Metropolitan University, United Kingdom) and Tim Harrison (Health Protection Agency, London, United Kingdom).
Footnotes
Published ahead of print on 17 December 2008.
REFERENCES
- 1.Albrich, W. C., W. Baughman, B. Schmotzer, and M. M. Farley. 2007. Changing characteristics of invasive pneumococcal disease in metropolitan Atlanta, Georgia, after introduction of a 7-valent pneumococcal conjugate vaccine. Clin. Infect. Dis. 441569-1576. [DOI] [PubMed] [Google Scholar]
- 2.Carrol, E. D., M. Guiver, S. Nkhoma, L. A. Mankhambo, Marsh J., P. Balmer, D. L. Banda, G. Jeffers, The IPD Study Group, S. A. White, E. M. Molyneux, M. E. Molyneux, R. L. Smyth, and A. Hart. 2007. High pneumococcal DNA loads are associated with mortality in Malawian children with invasive pneumococcal disease. Pediatr. Infect. Dis. J. 26416-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chief Medical Officer, Chief Nursing Officer, and Chief Pharmaceutical Officer. 2006. Important changes to the childhood immunisation programme. Department of Health, London, United Kingdom.
- 4.Corless, C., M. Guiver, R. Borrow, V. Edward-Jones, A. J. Fox, and E. B. Kaczmarski. 2001. Simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae in suspected cases of meningitis and septicemia using real-time PCR. J. Clin. Microbiol. 391553-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagan, R., O. Shriker, I. Hazan, E. Leibovitz, D. Grennberg, F. Schlaeffer, and R. Levy. 1998. Prospective study to determine clinical relevance of detection of pneumococcal DNA in sera of children by PCR. J. Clin. Microbiol. 36669-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominguez, J., N. Gali, S. Blanco, P. Pedroso, C. Prat, L. Matas, and V. Ausina. 2001. Detection of Streptococcus pneumoniae antigen by a rapid immunochromatographic assay in urine samples. Chest 119243-249. [DOI] [PubMed] [Google Scholar]
- 7.Dominguez, J., S. Blanco, C. Rodrigo, M. Azuara, N. Gali, A. Mainou, A. Esteve, A. Castellvi, C. Prat, L. Matas, and V. Ausina. 2003. Usefulness of urinary antigen detection by an immunochromatographic test for diagnosis of pneumococcal pneumonia in children. J. Clin. Microbiol. 412161-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fedson, D., and D. Musher. 2004. Pneumococcal polysaccharide vaccine, p. 529-588. In S. A. Plotkin and W. A. Orenstein (ed.), Vaccines, 4th ed. Saunders, Philadelphia, PA.
- 9.Fedson, D. S. 1998. Pneumococcal vaccination in the United States and 20 other developed countries, 1981-1996. Clin. Infect. Dis. 261117-1123. [DOI] [PubMed] [Google Scholar]
- 10.Reference deleted.
- 11.Henrichsen, J. 1995. Six newly recognized types of Streptococcus pneumoniae. J. Clin. Microbiol. 332759-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lal, G., P. Balmer, E. Stanford, S. Martin, R. Warrington, and R. Borrow. 2005. Development and validation of a nonaplex assay for the simultaneous quantitation of antibodies to nine Streptococcus pneumoniae serotypes. J. Immunol. Methods 296135-147. [DOI] [PubMed] [Google Scholar]
- 13.Lalitha, M. K., K. Thomas, R. S. Kumar, and M. C. Steinhoff. 1999. Serotyping of Streptococcus pneumoniae by coagglutination with 12 pooled antisera. J. Clin. Microbiol. 37263-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lankinen, K. S., S. Rintamaki, R. Syrjanen, T. Kilpi, P. Ruutu, and M. Leinonen. 2004. Type-specific enzyme immunoassay for detection of pneumococcal capsular polysaccharide antigens in nasopharyngeal specimens. J. Microbiol. Methods 56193-199. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence, E. R., C. A. Arias, B. Duke, D. Beste, K. Broughton, A. Efstratiou, R. C. George, and L. M. Hall. 2000. Evaluation of serotype predication by cpsA-cpsB gene polymorphism in Streptococcus pneumoniae. J. Clin. Microbiol. 3 81319-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence, E. R., D. B. Griffiths, S. A. Martin, R. C. George, and L. M. Hall. 2003. Evaluation of semiautomated multiplex PCR assay for determination of Streptococcus pneumoniae serotypes and serogroups. J. Clin. Microbiol. 41601-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leeming, J., K. Cartwright, S. A. Morris R. Martin, M. D. Smith, and South-West Pneumococcus Study Group. 2005. Diagnosis of invasive pneumococcal infection by serotype-specific urinary antigen detection. J. Clin. Microbiol. 434972-4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin, J., M. Kaltoft, G. Brandao A Echaniz-Aviles, M. C. Brandileone, S. K. Hollingshead, W. H. Benjamin, and M. H. Nahm. 2006. Validation of a multiplexed pneumococcal serotyping assay with clinical samples. J. Clin. Microbiol. 44383-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McAvin, J. C., and P. A. Reilly. 2003. Sensitive and specific methods for rapid identification of Streptococcus pneumoniae using real-time fluorescence PCR. J. Clin. Microbiol. 393446-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McEllistrem, M. C., J. Adams, E. O. Mason, and E. R. Wald. 2003. Epidemiology of acute otitis media caused by Streptococcus pneumoniae before and after licensure of the 7-valent pneumococcal protein conjugate vaccine. J. Infect. Dis. 1881679-1684. [DOI] [PubMed] [Google Scholar]
- 21.Pai, R., M. R. Moore, T. Pilishvili, R. E. Gertz, C. G. Whitney, B. Beall, and Active Bacterial Core Surveillance Team. 2005. Post vaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J. Infect. Dis. 1921988-1995. [DOI] [PubMed] [Google Scholar]
- 22.Pai, R., R. E. Gertz, and B. Beall. 2006. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J. Clin. Microbiol. 44124-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park, I. H., D. G. Pritchard, R. Cartee, A. Brandao, M. C. Brandileone, and M. H. Nahm. 2007. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 451225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park, M., D. E. Briles, and M. Nahm. 2000. A latex bead-based flow cytometric immunoassay capable of simultaneous typing of multiple pneumococcal serotypes (multibead assay). Clin. Diagn. Lab. Immunol. 7486-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin, L. G., and A. Rizvi. 2004. PCR-based assays for detection of 14 Streptococcus pneumoniae serotypes 3, 19F and 23F in respiratory specimens. J. Med. Microbiol. 53595-602. [DOI] [PubMed] [Google Scholar]
- 26.Schutze, G. E., N. C. Tucker, and E. O. Mason. 2004. Impact of the conjugate pneumococcal vaccine in Arkansas. Pediatr. Infect. Dis. J. 231125-1129. [PubMed] [Google Scholar]
- 27.Sheppard, C. L., K. Brown, T. G. Harrison, T. R. Jones, R. Borrow, and R. C. George. 2008. Non-culture surveillance of Streptococcus pneumoniae serotypes causing pneumococcal empyema in UK children, post conjugate vaccine introduction, abstr. 149. Abstr. 6th Int. Symp. Pneumococci Pneumococcal Dis.
- 28.Singhal, A., M. K. Lalitha, T. J. John, K. Thomas, P. Raghupathy, S. Jacob, and H. C. Steinhoff. 1996. Modified latex agglutination test for rapid detection of Streptococcus pneumoniae and Haemophilus influenzae in cerebrospinal fluid and direct serotyping of Streptococcus pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 15472-477. [DOI] [PubMed] [Google Scholar]
- 29.Singleton, R. J., T. W. Hennessy, L. R. Bulkow, L. L. Hammitt, T. Zulz, D. A. Hurlburt, J. C. Butler, K. Rudolph, and A. Parkinson. 2007. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 2971784-1792. [DOI] [PubMed] [Google Scholar]
- 30.Toikka, P., S. Nikkari, O. Ruuskanen, M. Leinonen, and J. Mertsola. 1999. Pneumolysin PCR-based diagnosis of invasive pneumococcal infection in children. J. Clin. Microbiol. 37633-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tugwell, P., and B. M. Greenwood. 1975. Pneumococcal antigen in lobar pneumonia. J. Clin. Pathol. 28118-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, N. M. Bennett, R. Lynfield, A. Reingold, P. R. Cieslak, T. Pilishvili, D. Jackson, R. R. Facklam, J. H. Jorgensen, A. Schuchat, and Active Bacterial Core Surveillance of Emerging Infections Program Network. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 3481737-1746. [DOI] [PubMed] [Google Scholar]
- 33.Whitney, C. G., T. Pilishvili, M. M. Farley, W. Schaffner, A. S. Craig, R. Lynfield, A. Nyquist, K. A. Gershman, M. Vazquez, M. N. Bennett, A. Reingold, A. Thomas, M. P. Glode, E. R. Zell, J. H. Jorgensen, B. Beall, and A. Schuchat. 2006. Effectiveness of seven-valent pnuemococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 3681495-1502. [DOI] [PubMed] [Google Scholar]
- 34.Yu, J., J. Lin, W. H. Benjamin, K. B. Waites, C. Lee, and M. Nahm. 2005. Rapid multiplex assay for serotyping pneumococci with monoclonal and polyclonal antibodies. J. Clin. Microbiol. 43156-162. [DOI] [PMC free article] [PubMed] [Google Scholar]