Abstract
In preparation for a pilot clinical trial in patients with chronic human immunodeficiency virus type 1 (HIV-1) infection, a novel dendritic cell (DC)-based vaccine is being manufactured. The trial will test the hypothesis that isolated endogenous virus presented by DCs serves as a potent immunogen for activation of CD8+ and CD4+ T cells specific for a broad range of autologous HIV-1 antigens. Production of the vaccine under good manufacture practice conditions involves (i) autologous virus isolation; (ii) superinfection of CD4+ T cells with the virus; (iii) inactivation of the virus in CD4+ T cells, T-cell apoptosis, and coincubation of T cells with autologous DCs; and (iv) product testing and release. Endogenous virus was isolated from peripheral blood-derived CD4+ T cells of three HIV-1-positive subjects by coincubation with autologous OKT-3-stimulated CD4+ T cells. CD4+ T-cell supernatants were tested for p24 levels by enzyme-linked immunosorbent assay (>25 ng/ml) and for the 50% tissue culture infective doses (TCID50; which ranged from 4,642 to 46,416/ml on day 19 of culture). Autologous CD4+ T cells that were separated on immunobeads (>95% purity) and superinfected with virus-expressed p24 (28 to 54%) had TCID50 of >400/ml on days 5 to 10. Virus inactivation with psoralen (20 μg/ml) and UVB irradiation (312 nm) reduced the TCID50 of the supernatants from 199,986 to 11/ml (>99%). 7-Amino-actinomycin D-positive, annexin V-positive CD4+ T cells were fed to autologous DCs generated by using the Elutra cell separation system and the Aastrom system. Flow analysis showed that DC loading was complete in 24 h. On the basis of these translational results and experience with the generation of DCs from HIV-1-infected patients in a previous clinical trial, the Investigational New Drug application for clinical vaccination was submitted and approved by the FDA (application no. BB-IND-13137).
Antiretroviral therapy (ART) has been widely used to suppress human immunodeficiency virus type 1 (HIV-1) replication and increase the number of CD4+ T cells in patients with HIV-1 infection. However, in most of these patients, the recovery of anti-HIV-1-specific T-cell function is incomplete. As the complete restoration of T-cell immune function is considered to be necessary for effective control of the viral infection, additional measures aimed at the bolstering of the HIV-1-specific adaptive immunity in patients treated with ART are being evaluated.
Dendritic cells (DCs) are the most potent antigen-presenting cells that can both prime and sustain memory responses (24, 28). DCs have been used increasingly frequently in vaccines against cancer and viral infections (4, 13, 20). Previous studies from our group showed that DCs derived from the blood of subjects with chronic progressive HIV-1 infection and not receiving ART were able to stimulate anti-HIV-1 reactivity (5). HIV-1-reactive CD8+ T cells are detectable in the peripheral circulation of subjects receiving ART following in vitro activation with many types of HIV-1 antigens, including HIV-1 proteins, HIV-1 peptides, and virus-infected apoptotic cell-loaded matured DCs (6, 10, 14, 22, 23, 31). We hypothesized that it may be possible to reconstitute the reactivity of naïve and memory virus-specific T cells by delivering to patients autologous DCs engineered ex vivo to express and present known immunodominant peptides of HIV-1. To this end, we have recently completed a phase I clinical protocol in which autologous monocyte-derived DCs were pulsed with a mix of three HIV-1 peptides (Gag, Pol, and Env) and one influenza A virus (matrix) major histocompatibility complex class I supertype peptide and delivered as vaccines to 18 HIV-1-infected, ART-treated subjects (5). This vaccination strategy was found to be safe and feasible and resulted in a transient but significant increase in the frequency of CD8+ T cells specific for HIV-1 peptides present in the vaccine (5). On the basis of the results of this trial, we have been considering a strategy of stimulating HIV-1-specific, naïve CD8+ and CD4+ T cells by priming them with DCs engineered to express autologous HIV-1 (19). The rationale for this strategy is that autologous virus represents a large repertoire of the host's diverse HIV-1 antigen pool and offers the potential to elicit the most specific, broadest, and most effective immune responses for each subject's quasispecies of HIV-1, thus increasing vaccine efficacy.
In this report, we provide evidence that the production of an antiviral vaccine containing autologous DCs fed with inactivated HIV-1-infected, autologous, apoptotic CD4+ T cells is feasible, can be successfully accomplished in a good manufacture practice facility, and can be scaled up for therapeutic delivery to HIV-positive (HIV-1+) patients. The production process consists of several steps: (i) isolation of autologous virus from the peripheral blood of HIV-1-infected subjects; (ii) superinfection of autologous enriched CD4+ CD8− T cells with viral supernatants; (iii) virus inactivation by psoralen and UVB irradiation; (iv) testing for p24 levels and the residual HIV-1 load by determining the 50% tissue culture infective doses (TCID50) for apoptotic CD4+ T cells; and (v) loading of autologous DCs with apoptotic, HIV-1-infected CD4+ T cells. Although this process is complex, it has been successfully scaled up for therapeutic vaccine production.
MATERIALS AND METHODS
Patients and healthy donors.
Four HIV-1+ subjects not treated with ART and with high plasma HIV-1 RNA levels (∼50,000 copies/ml) were recruited as peripheral blood mononuclear cell (PBMC) donors for virus isolation after they signed an informed consent approved by the Institutional Review Board (IRB) at the University of Pittsburgh. These subjects were seen at the HIV clinic and donated venous blood weekly, so that autologous feeder PBMCs were available for autologous virus isolation. For development of the assays and to serve as control cells, PBMCs were also obtained from healthy donors in the form of buffy coats purchased from the Central Blood Bank of Pittsburgh, PA. For the scale up of vaccine production, two of the three patients underwent leukapheresis at the Hillman Cancer Center Pheresis Unit to provide monocytes for DC generation. IRB approval was obtained, and the patients consented to the procedure.
Cell lines.
The TZM-b1 and 8E5 cell lines were obtained from the NIH AIDS Research and Reference Reagent Program. The 8E5 cells were originally generated by Thomas Folks by infecting parent cell line A03.01 with HIV strain LAV (later called strain IIIb). The infected cells were treated in such a way (i) that one clone that had a mutation in the integrated copy of the polymerase gene so that no infectious virus was produced (only replication-incompetent particles were released in the supernatant) and (ii) that cells with only one copy of HIV integrated per cell genome were derived (8). The 8E5 cell line was further subcloned to obtain 2A9 cells, which stably express high levels of HIV-1 p24. The 2A9 subclone was used as a positive control for intracellular HIV p24 staining. Uninfected A03.01 cells were used as a negative control. The cell lines were subcultured in RPMI 1640 medium supplemented with 10% (vol/vol) human type AB serum.
Reagents and antibodies.
RPMI 1640 medium, phosphate-buffered saline (PBS), and Hanks' balanced salt solution were purchased from Invitrogen (Carlsbad, CA); and PermiFlow solution was purchased from Invirion, Inc. (Oakbrook, IL). Ficoll-Hypaque and psoralen were from Sigma-Aldrich (St. Louis, MO). X-Vivo 10 medium was from Cambrex (Walkersville, MD), and DC medium was from CellGenix (Freiburg, Germany). Human type AB serum was purchased from Gemini BioProducts (West Sacramento, CA). Anti-CD3/anti-CD28 and anti-CD8 antibody-charged microbeads were from Miltenyi Biotec Inc. (Auburn, CA). OKT3 monoclonal antibody (MAb) was from OrthoBiotech (Bridgewater, NJ). HIV-1 core antigen (clone KC57) was from Beckman Coulter (Fullerton, CA), and paraformaldehyde was from EMS (Hatfield, PA). Retro-Tek HIV-1 p24-antigen enzyme-linked immunosorbent assay (ELISA) kits were purchased from Zeptometrix (Buffalo, NY). Polybrene was from Fluka Biochemika (Buchs, Switzerland), and chlorophenolred β-d-galactopyronoside was from Roche Diagnostics (Indianapolis, IN). Poly(I-C) was obtained from Amersham (Piscataway, NJ). The following cytokines were purchased from the indicated vendors: interleukin-2 (IL-2) was from Roche Diagnostics; IL-4, IL-1β, and tumor necrosis factor alpha were from CellGenix; granulocyte-macrophage colony-stimulating factor was from Berlex (Seattle, WA); gamma interferon (IFN-γ) was from InterMune (Brisbane, CA); and IFN-α was from Schering Corp. (Kenilworth, NJ). The following antibodies directly labeled with various chromogens for flow cytometry were all purchased from Beckman Coulter (Miami, FL): anti-CD3, -CD4, -CD8, -CD14, -CD80, -CD83, -CD86, -CD11c, -CD40, -CCR7, and -HLA-DR. Antibody (Ab) to p24 was also purchased from Beckman Coulter. Cy3-conjugated goat anti-mouse Ab was from Jackson Immuno-Research Laboratories (West Grove, PA). The fluorochrome DiOC6 was from Molecular Probes (Eugene, OR). Sodium azide was purchased from Fischer Scientific (Waltham, MA). The annexin V and 7-amino-actinomycin D (7-AAD) reagents were purchased from Beckman Coulter.
Endogenous virus isolation.
Peripheral blood obtained from each of the HIV-1+ subjects was centrifuged on Ficoll-Hypaque gradients to recover the PBMCs. Following a wash in Hanks' balanced salt solution, the cells were treated with trypan blue dye, counted, and resuspended at a concentration of 1 × 106 cells/ml in complete RPMI 1640 medium supplemented with 20% (vol/vol) human type AB serum. Depletion of CD8+ T cells was performed with magnetic cell separation (MACS) columns by using microbeads charged with anti-CD8 antibodies. CD8-depleted PBMCs (i.e., PBMCs enriched in CD4+ T cells) were resuspended at a final concentration of 1 × 106 cells/ml in a complete RPMI 1640 medium containing 1 μg/ml MAb OKT3. The cells were plated in T25 vented flasks (Corning, Lowell, MA) set upright with no more than 20 ml of medium per flask. The flasks were incubated for 24 h at 37°C in an atmosphere of 5% CO2 in air. Following incubation, the MAb OKT3-containing medium was removed by centrifugation, and the cells were resuspended at 1 × 106 cells/ml in fresh RPMI 1640 medium supplemented with 10 IU/ml of IL-2 in T25 flasks. The flasks were incubated as described above for 3 to 5 days, at which time (day 6 or 7), half the volume of the culture medium was removed and frozen at −20°C in 15-ml conical tubes. The medium that was removed was replaced with an aliquot of fresh IL-2-containing medium plus freshly prepared autologous feeder cells. These were PBMCs obtained from 20 ml of freshly drawn heparinized blood enriched in CD4+ T cells by MACS and stimulated with OKT3, as described above. In the initial experiments, anti-CD3/CD28 Ab-charged beads were used in place of MAb OKT3 as an alternative T-cell stimulus. However, the addition of anti-CD28 Ab did not maximize the virus yields, and subsequent isolations were performed only with OKT3-charged beads. The cycles of medium harvesting and addition of fresh autologous feeder cells on every 8th day continued for at least 4 weeks. Culture aliquots (viral supernatants) were tested for the presence of virus by measuring the p24 levels.
Testing of T cells and culture supernatants for virus.
Cells in cultures were monitored for p24 expression by flow cytometry every 3 to 5 days. A total of 2 × 105 cells were microcentrifuged for 30 s. The cells were resuspended in 200 μl wash medium (PBS [pH 7.4], 2% [vol/vol] heat-inactivated human type AB serum, 0.1% sodium azide), transferred to a well of the 96-well plate (Becton Dickinson, Franklin Lanes, NJ), centrifuged, and resuspended in 200 μl of 1× PermiFlow solution to permeabilize the cell membrane. The plate was incubated for 40 min in the dark at room temperature and then centrifuged, and the cells were again washed with 200 μl of wash medium. The cells were resuspended at a 1:240 dilution of antibody to HIV-1 core antigen and incubated in the dark at 4°C for 1 h. After one more wash, the cells were fixed in 1% (wt/vol) paraformaldehyde and analyzed on a Coulter Epics XL-MCL flow cytometer. The expression of p24 was routinely determined: it served as a screen because the optimal time for virus production varied with T cells from different donors.
Once the cultures were positive for p24 expression, the titers in the reserved supernatants were determined by using a Retro-Tek HIV-1 p24 antigen ELISA kit. Culture supernatants containing p24 levels of 100 pg/ml or greater were considered positive and were tested for viral infectivity in a TCID50 assay before they were used for subsequent superinfection of autologous CD4+ cells.
TCID50 titer measurements.
Cells of the TZM-bl cell line, which carry a copy of the HIV promoter long terminal repeat in tandem with the β-galactosidase (β-Gal) enzyme gene, were used as indicator cells. If TZM-bl cells become infected with HIV-1, the β-Gal gene is also transcribed and is detected as a color reaction. The TZM-bl cells were dispensed into the wells of a 96-well assay plate at a concentration of 2 × 105 cells/ml in the assay medium containing 10 μg/ml Polybrene. Culture supernatants were titrated into the wells, and the plate was incubated for 40 to 48 h at 37°C in an atmosphere of 5% CO2 in air. Fixative solution was then added, followed by the addition of a substrate, chlorophenolred β-d-galactopyranoside, and incubation for 4 h to develop the color. The optical density of each well was measured at 570 nm with a microplate reader. The HIV titer was calculated as the TCID50 by the Spearman-Karber method (11).
Superinfection of CD4+-enriched T cells with autologous HIV-1.
PBMCs were obtained from the venous blood of each patient by Ficoll-Hypaque centrifugation or elutriation (for a large-scale production). Enrichment in CD4+ T cells was performed by MACS, as described above, or with the CliniMACS system (Miltenyi Biotec).
CD4+ T cells were cultured at 1 × 106 cells/ml in RPMI 1640 complete medium containing 20% (vol/vol) heat-inactivated human type AB serum and supplemented with 10 IU/ml human IL-2 and 1 μg/ml MAb OKT3 in upright T75 flasks (Corning) at 37°C in a 5% CO2 incubator for 24 to 72 h. The cells were then washed with RPMI 1640 complete medium to remove MAb OKT3, and HIV-1 superinfection was initiated.
Supernatants with high titers of autologous HIV-1 previously generated and frozen were used for the superinfection of CD4+ cells. Autologous CD4+ cells (5 × 106 to 10 × 106) were resuspended in 1 to 5 ml viral supernatant plus 5 μg/ml Polybrene in conical tubes, which were placed, with the caps loosened, in a 5% CO2 incubator at 37°C for 2 h and gently shaken every 15 to 30 min to evenly distribute the cells. RPMI 1640 complete medium plus 10 IU/ml human IL-2 was added to adjust the cell density to 1 × 106 cells/ml. The cells were then cultured in upright T25 flasks at 37°C in a 5% CO2 incubator. Aliquots of cells were taken for analysis of p24 levels on days 4 to 10, as described above. The cultures were continued until at least 40 to 65% of the cells became positive for p24.
Psoralen and UVB irradiation treatment of superinfected CD4+ cells.
The HIV-1-superinfected CD4+ cells were treated by psoralen and UVB irradiation to induce apoptosis and inactivate the virus. The cells were collected and washed several times with RPMI 1640 medium and were then resuspended in X-Vivo 10 medium containing 20 μg/ml psoralen at 1 × 106 to 10 × 106 cells/ml. The cells were aliquoted and placed into the wells of six-well tissue culture plates at 0.5 × 106 cells/well. With the lid off, the plates were placed 1 in. below a UVB light source (Spectronics, Westbury, NY) inside a biosafety cabinet for 30 min. The plates were rotated every 5 to 10 min. At the end of the irradiation, the cells were combined and washed in RPMI 1640 complete medium three times to remove the psoralen. Next, the cells were incubated in medium at 37°C in a 5% CO2 incubator for 12 to 24 h to allow them to undergo apoptosis. Apoptosis of CD4+ T cells was confirmed by comparing the annexin V binding of untreated and psoralen- and UVB irradiation-treated cells by flow cytometry. Aliquots of psoralen- and UVB irradiation-treated and untreated cells were also analyzed by the TCID50 assay to ensure that the virus had been inactivated.
Generation of DCs.
PBMCs were obtained from each of the patients whose virus was isolated as described above by using heparinized venous blood (90 ml) or leukapheresis products (for large-scale production). Monocytes were separated by adherence to plastic or elutriation by using the Elutra cell separation system (Gambro BCT, Lakewood, CO). Monocytes were recovered and plated in T162 flasks or cartridges, which are a component of the Aastrom Biosciences, Inc. (Ann Arbor, MI), Replicell system. In this closed computer-monitored system used for the large-scale manufacture of DCs, from 0.5 × 109 to 2.5 × 109 monocytes suspended in CellGenix DC medium supplemented with 1,000 U/ml IL-4 and 1,000 U/ml of recombinant human granulocyte-macrophage colony-stimulating factor were cultured for 6 days in a sealed cartridge. The cartridge was placed in a computer-monitored chamber of an incubator, and fresh medium was automatically added as needed. Immature DCs (iDCs) were harvested on day 6 or 7 with the Aastrom Replicell processor; washed in medium; counted; evaluated for sterility, viability, recovery, and phenotype; and either aliquoted for cryopreservation or directly used for coculture with superinfected CD4+ cells.
Coculture of DCs with autologous apoptotic CD4+ T cells.
To prepare the vaccine, HIV-1-superinfected apoptotic CD4+ T cells were coincubated with autologous iDCs at an iDC/CD4+ T-cell ratio of 1.5:1. The cultures were set up in T25 flasks at a concentration of 1 × 106 to 1.5 × 106 cells/ml of CellGenix DC medium. Maturation cytokines consisting of a mix of 50 ng/ml tumor necrosis factor alpha, 25 ng/ml IL-1β, 1,000 U/ml IFN-γ, 3,000 U/ml IFN-α, and 20 μg/ml poly(I-C) were added, as described by Mailliard et al. (16); and the cultures were incubated at 37°C in a 5% CO2 incubator for 12 to 24 h. The matured DCs were then examined microscopically and by flow cytometry to verify that apoptotic bodies (ApBs) were indeed ingested during coculture. Prior to psoralen and UVB irradiation treatment, an aliquot of superinfected CD4+ T cells was stained with a lipophilic cationic fluorochrome, DiOC6. Cells were stained with 2 μg/ml of DiOC6 in PBS for 30 min at 37°C and were then washed three times in PBS. They were resuspended in irradiation medium and subjected to the psoralen and UVB irradiation treatment. These labeled CD4+ T cells were cocultured with autologous iDCs as described above and were then stained with anti-CD11c Ab (Beckman Coulter) for determination of the uptake of labeled ApBs by DCs by flow cytometry. An aliquot of the cocultured cells was also placed in a Lab-Tek II chamber slide system (Nalgene Nunc International Corp., Naperville, IL), stained with anti-CD11c Ab, and counterstained with a secondary Cy3-conjugated goat anti-mouse Ab for examination by confocal microscopy.
Cell staining and flow cytometry.
To determine the percentages of CD3+ CD8+ and CD3+ CD4+ T cells and to confirm the purity of the cultured DCs, cells were stained with appropriate panels of labeled MAbs for 15 min at 4°C. Isotype control Abs were included in all instances. The cells were then washed and examined by multicolor flow cytometry. Intracellular staining for p24 was performed as follows. Cells were first incubated with MAbs against surface markers CD3 and CD4. After the cells were washed, they were fixed with 4% (vol/vol) paraformaldehyde in PBS for 20 min at room temperature, washed once in PBS containing 0.5% (vol/vol) bovine serum albumin (BSA) and 2 mM EDTA, permeabilized with PBS containing 0.5% BSA and 0.1% (vol/vol) saponin, and stained with the anti-p24 Ab or isotype control Ab whose titers were predetermined. After a further wash with PBS containing BSA and saponin, the cells were resuspended in fluorescent-activated cell sorter analysis flow solution and were analyzed by flow cytometry.
Statistical analyses.
Differences between the levels of expression of surface markers, the levels of growth, the levels of annexin V binding, and the viral titers in cells prior to and after infection with the virus were examined by paired Student's t test. Differences were considered significant at a P value of <0.05.
RESULTS
Cultivation and isolation of autologous HIV-1 from PBMCs.
Four HIV-1+ subjects not treated with ART were recruited for this study. The subjects donated blood weekly, so that fresh autologous feeder PBMCs were available for culture of enriched CD4+ T cells. The isolation of endogenous virus from the PBMCs of these subjects involved the depletion of CD8+ T cells by negative selection of CD4+ T cells on immune magnetic beads. The CD4+ T-cell-enriched fractions contained from 90 to 95% CD3+ CD4+ T cells, as determined by flow cytometry. In the initial experiments, the CD4+ T cells selected were stimulated with immunobeads coated with anti-CD3 and anti-CD28 MAbs or anti-CD3 MAb overnight, centrifuged, washed, and resuspended in medium supplemented with IL-2. They were fed fresh autologous CD4+ T cells, and this process of feeding was repeated at weekly intervals for 4 to 6 weeks. Endogenous virus was successfully isolated from these CD4+ T cells from three of four subjects, as judged by the p24 levels in culture supernatants measured by ELISA (Table 1). We failed to isolate virus from one subject (subject 4), possibly because of the learning curve: this was the very first subject that we recruited. Stimulation with MAb OKT3 alone in the presence of autologous feeder cells gave better results than stimulation with anti-CD3/CD28 beads (Table 1). Allogeneic feeder cells obtained from healthy donors were not as effective in promoting viral isolation as fresh autologous feeder cells (Table 1).
TABLE 1.
Viral isolation in supernatants of CD4+ T-cell cultures determined by measurement of p24 levelsa
| HIV-1+ subject no. | p24 concn (ng/ml) in the following feeder cells:
|
||
|---|---|---|---|
| Allogeneic cells (healthy donor) | Autologous cells + anti-CD3 beads | Autologous cells + anti-CD3/ CD28 beads | |
| 1 | 38.0 | >50 | >50 |
| 2 | 2.7 | >50 | 4.0 |
| 3 | 7.0 | >50 | 6.3 |
The supernatants of cultures established with various feeder cells (allogeneic cells, autologous cells plus anti-CD3 immunobeads, or autologous cells plus anti-CD3/CD28 immunobeads) were harvested on day 29 and tested for p24 levels by ELISA.
In subsequent experiments, the viral infectivity of the cultures was determined in TCID50 assays, in addition to by determination of p24 levels (Table 2). The culture supernatants were repeatedly tested on various days (days 4 to 37), and the highest titers were obtained on days 10 to 19 (Table 2). One subject's CD4+ T-cell cultures (subject 4) were negative for p24 and infectious virus. On the basis of the data obtained with the cells of subject 3 (Table 2), the supernatant of which had a p24 level of 39 ng/ml and over 14,000 TCID50/ml, it was arbitrarily determined that a level of >25 ng/ml p24 in the supernatant likely reflected successful viral isolation and, therefore, that the culture supernatant could be used for the superinfection of autologous CD4+ CD8− T cells. Consequently, the viral supernatants of cell cultures for subjects 1, 2, and 3 were aliquoted and frozen for use in the superinfection experiments.
TABLE 2.
Viral isolation in supernatants of autologous CD4+ T-cell cultures determined by measurement of TCID50a
| HIV-1+ subject no. | p24 level (ng/ml) | No. of TCID50/ml |
|---|---|---|
| 1 | 337 | 31,623 |
| 2 | 227 | 215 |
| 3 | 39 | 14,678 |
| 4 | 0.2 | 3 |
The supernatants of cultures established with autologous feeder cells were harvested on days 10 to 19. The TCID50 was measured by colorimetric assays with the TZM-bl indicator cell line, as described in Materials and Methods.
Superinfection of CD4+ CD8− T cells with autologous virus.
Experiments were initially performed with the preparations of enriched CD4+ T cells obtained from the PBMCs of a healthy donor by negative selection on immunobeads. These cells were incubated in the presence of the cryopreserved and thawed viral culture supernatants. The results indicated that superinfection of CD4+ T cells from healthy subjects with the viral supernatants of HIV-1+ subjects was successful. For example, on day 5 of culture with the viral supernatant of healthy subject 1, 28% of CD4+ T cells were p24 positive, as indicated by intracytoplasmic staining for p24 expression by flow cytometry. The level of p24 in the supernatant was 20 ng/ml for this subject. Therefore, the conditions established for superinfection of CD4+ T cells from healthy donors were next used for infection of freshly harvested CD4+ CD8− T cells from the three HIV-1+ subjects who had donated PBMCs for viral isolation and whose culture supernatants were positive for HIV-1, as shown in Table 1. The subjects' enriched CD4+ T cells (98% purity) were cultured with the individual viral culture supernatants, which were thawed and added to the cells. Superinfection was evaluated by intracytoplasmic p24 staining and flow cytometry and by testing lysed CD4+ T cells and their supernatants for p24 by ELISA daily for 4 to 10 days after infection. Table 3 presents the highest values obtained for the cells and infectious supernatant of all three HIV-1+ subjects on the indicated day. In aggregate, the results of these experiments confirmed that substantial levels of p24 can be detected in superinfected CD4+ T cells and their supernatants following 5 to 10 days of incubation with cryopreserved and thawed autologous viral culture supernatants.
TABLE 3.
Superinfection of autologous CD4+ CD8− T cells with viral supernatants obtained from three HIV-1+ subjectsa
| Subject (day) | % p24+ cells | p24 concn
|
No. of TCID50s/ml | |
|---|---|---|---|---|
| ng/105 cells | ng/ml | |||
| 1 (5) | 30 | 1.7 | 55 | 31,623 |
| 2 (10) | 5 | 0.8 | 15 | 464 |
| 3 (6) | 18 | 2.3 | 0.1 | 10,000 |
| Positive controls | 52 | 3.6 | 17 | 3,162 |
| Negative controls | 0 | 0 | 0 | 0 |
Autologous CD4+ T cells were cultured in the presence of viral supernatants for 5 to 10 days, and the percentage of p24-positive (p24+) cells as well as p24 levels in cells and culture supernatants were measured each day. The highest values obtained are presented. CD4+ T cells were separated from the PBMCs of each subject by negative selection on immunobeads. Their own viral supernatants were used for superinfection. The percentage of p24+ cells was determined by flow cytometry, cell-associated p24 was assayed with lysed CD4+ T cells, and ELISA was used to measure p24 levels in CD4+ T-cell supernatants. The TCID50 was measured as described in Materials and Methods. Negative controls were uninfected autologous CD4+ T cells, and positive controls were cells of a CD4+ T-cell line infected with HIV-1 (2A9 subclone) in the laboratory.
Psoralen and UVB light inactivation of autologous virus.
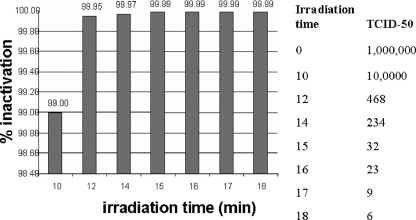
To inactivate the virus, we used UVB light (3 mW/m2) in the presence of psoralen (20 μg/ml) and monitored viral infectivity by use of the TCID50 assay. The experiments were first performed with primary CD4+ T cells expressing high titers of HIV IIIb (Fig. 1). Having established the conditions for virus inactivation that resulted in a 99.99% decrease in the viral titer (4 log10 units), we next applied the procedure to HIV-1-superinfected CD4+ T cells from the three subjects included in the study. Table 4 summarizes the results of these experiments and shows that the treatment consistently reduced the viral titers in the treated CD4+ T cells to essentially undetectable levels of infectious virus.
FIG. 1.
Inactivation of cell-associated HIV IIIb by UVB (312-nm) irradiation in the presence of psoralen (20 μg/ml). Infected CD4+ T cells were treated with UVB light and psoralen for various periods of time. Following inactivation, the infectivity of the virus was determined in TCID50 assays.
TABLE 4.
HIV-1 inactivation in CD4+ CD8− T cells by UVB light and psoralena
| Cell treatment | TCID50/ml for subject no.:
|
||
|---|---|---|---|
| 1 | 2 | 3 | |
| Untreated | 31,623 | 2,154 | 14,678 |
| Treated | 22 | 2 | 3 |
| Positive control (not treated) | 681 | 3,162 | 3,162 |
CD4+ CD8− T cells obtained from all three subjects were superinfected with the autologous virus supernatants and were treated with UVB light (3 mW/m2) and psoralen (20 μg/ml) for 30 min to inactivate the virus. Supernatants were tested for infectivity in TCID50 assays prior to and after treatment. HIV IIIb-infected cells were used as a positive control.
Apoptosis of CD4+ CD8− T cells after psoralen and UVB light treatment.
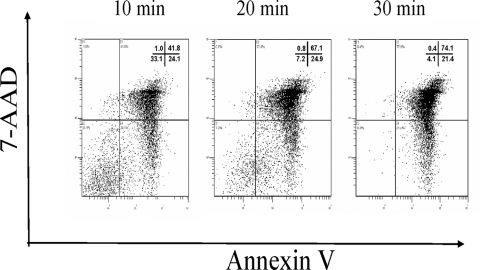
We have previously shown that psoralen and UV light treatment result in the apoptosis of HIV-1-infected CD4+ T cells that can serve as a source of antigen (30). In the present experiments, we determined the effects of psoralen and UVB light treatment on CD4+ T-cell viability and apoptosis. As illustrated in Fig. 2, after only 30 min of irradiation in the presence of psoralen, 95% of the cells were annexin V positive and almost 75% were also 7-AAD positive, suggesting apoptosis and/or necrosis of the virally infected CD4+ T cells. This was a desirable result, because the vaccine was to be prepared with DC-fed ApBs of autologous CD4+ T cells containing inactivated endogenous HIV-1.
FIG. 2.
Effects of psoralen and UVB light treatment on CD4+ T-cell viability: 30 min of treatment is sufficient to induce apoptosis and necrosis (annexin V and 7-AAD positivity) in >75% of the cells. Annexin V and 7-AAD staining were performed 4 h after completing irradiation.
Loading of DCs with endogenous virus-infected, inactivated CD4+ CD8− T cells.
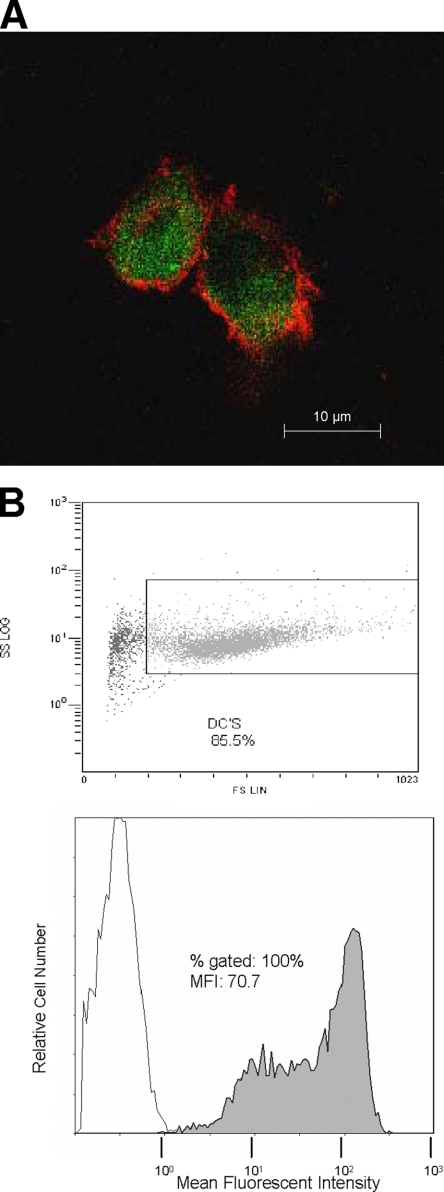
After the psoralen and UVB light treatment, ApBs of CD4+ CD8− T cells were fed to autologous iDCs. The uptake of ApB by iDCs was monitored by flow cytometry by using aliquots of superinfected CD4+ CD8− T cells labeled with DiOC6 prior to the psoralen and UVB light treatment. As shown in Fig. 3, coincubation of the iDCs with labeled autologous CD4+ T cells in the presence of maturation cytokines for 12 h resulted in the uptake of ApBs by the majority of DCs.
FIG. 3.
DCs loaded with endogenous virus-infected, inactivated CD4+ T cells. (A) Confocal microscopy image illustrating the uptake of DiOC6-labeled ApBs by autologous iDCs surface stained with fluorescein isothiocyanate-labeled anti-CD11c Ab; (B) flow cytometry analysis of the uptake of DiOC6-labeled ApBs after 12 h of their coculture with iDCs.
Generation of monocyte-derived DCs from HIV-1+ subjects.
We have previously reported that monocyte-derived DCs can be successfully obtained from subjects with HIV-1 infection (7). However, those studies used different techniques for the generation of DCs or the subjects had undergone ART and their viral titers were reduced (3). As it was not certain that DCs can be reliably generated by our new procedures from HIV-1+ subjects who were not on ART, we proceeded to generate DCs from two of the three patients on a therapeutic scale, using leukapheresis as a source of peripheral blood monocytes. The subjects signed informed consent and agreed to undergo leukapheresis. Elutriation was used for the isolation of monocytes from PBMCs, and separation of CD4+ CD8− T cells was performed on a CliniMACS instrument. The method selected for DC maturation involved the mix of cytokines made up to induce the polarization of DC to αDC1, as reported previously (16). The phenotypic characteristics of DCs of one of the patients are summarized in Table 5. In aggregate, the data from two scale-up experiments showed that the DCs generated from the monocytes of untreated subjects with chronic HIV-1 infection have the phenotypic and functional properties (i.e., IL-12 p70 production) similar to those previously generated from healthy donors or HIV-1-infected subjects receiving ART therapy (3, 5).
TABLE 5.
Characteristics of DCs generated from monocytes of an HIV-1+ subject
| Phenotype | % Positive cells
|
||
|---|---|---|---|
| iDCs | αDC1 cells alonea | αDC1 cells + ApBs | |
| HLA-DR | 86 | 94 | 97 |
| CD80 | 50 | 94 | 93 |
| CD83 | 4 | 60 | 49 |
| CD86 | 19 | 86 | 91 |
| CD11c | 97 | 96 | 96 |
| CD40 | 86 | 96 | 97 |
| CCR7 | 10 | 45 | 42 |
DC characteristics before and after they were loaded with ApBs of autologous CD4+ CD8− T cells superinfected with endogenous virus.
The ApB-loaded autologous DCs were shown to meet the release criteria established by our laboratory for therapeutic DC product release (i.e., 14-day sterility, negative results; gram stain, negative for mycoplasmas and endotoxin; viability, >75%; purity, >75%; stability, >4 h at room temperature) (Table 6).
TABLE 6.
Criteria established for therapeutic DC product releasea
| Parameter | Cell count | % Purityb |
|---|---|---|
| Initial white blood cell count | 3.9 × 1010 | |
| Elutra fraction 2 (CD45+ CD14−) | 1.2 × 1010 | 99 |
| Elutra fraction 5 (CD45+ CD14+) | 5.6 × 109 | 77 |
| Post-CliniMACS CD4+ CD8− T cells | 9.8 × 108 | 95 |
The criteria established by our laboratory for therapeutic DC product release are as follows: recovery, 57%; viability, 87% after coincubation with apoptotic CD4+ CD8− T cells; sterility, mycoplasma and endotoxin negative at 14 days; level of IL-12 p70 production, 473 pg/ml; TCID50/ml for untreated CD4+ CD8− T cells, 1,467,799; TCID50/ml for mDCs plus ApBs, 3; and stability at room temperature (on the basis of the DC viability and phenotype), 4 h.
The purity of DCs was determined by flow cytometry, as described in Materials and Methods.
A summary of the process of manufacturing a vaccine containing autologous DCs loaded with ApBs of inactivated endogenous HIV-1 is shown in Table 7.
TABLE 7.
Manufacturing process for a vaccine containing autologous DCs loaded with ApB of inactivated endogenous HIV-1
| Step | Comment |
|---|---|
| Find HIV-1+ subjects not on ART therapy | |
| Isolate endogenous virus from the subject's PBMCs | p24 titer of CD4+ CD8− T-cell supernatants of ≥25 ng/ml |
| Freeze virus-positive supernatants | |
| Proceed with leukapheresis of the subject | |
| Separate and recover monocytes and T cells by using the Elutra system | |
| Separate CD4+ T cells by negative selection on immunobeads (CliniMACS) and cryopreserve CD4+ T cells | |
| Generate iDCs in the Aastrom Replicell closed system | Yield, 2 × 108 DCs |
| Characterize iDCs, aliquot the iDCs, and cryopreserve the iDCs for vaccine formulation pending superinfection of autologous CD4+ T cells (4 to 10 days) | |
| Superinfect autologous CD4+ CD8− T cells with the viral supernatants | 10 to 20 ml per 5 × 107 T cells |
| Inactivate virus in CD4+ T cells with UVB light and psoralen (30 min) | |
| Coincubate thawed iDCs with ApBs of T cells for 12 to 24 h | Ratio, 2 DCs to 1 CD4+ CD8− T cell or 4 × 107 DCs to 2 × 107 T cells |
| Determine sterility, p24 titer, DC phenotype, and viability | Sterile, endotoxin and mycoplasma negative, >70% purity, >80% viability |
| Establish vaccine release criteria |
DISCUSSION
The ex vivo loading of DCs with ApBs of autologous CD4+ CD8− T cells which had been superinfected with endogenous inactivated HIV-1 could potentially produce an anti-HIV-1 vaccine that would be effective in the control of chronic HIV-1 infection. The rationale for selecting this strategy is based on evidence that DCs loaded with HIV-1-infected apoptotic cells can stimulate both anti-HIV-1 CD8+ and anti-HIV-1 CD4+ T-cell responses in vitro (12, 31). Furthermore, there is well-documented evidence for the existence of HIV-1 variants or quasispecies even in subjects treated with ART (2). Moreover, it has been established that infectious viruses can be isolated from PBMCs for several years while the individuals are on ART, even when virus RNA is not detectable in plasma (25). The diversity of viral quasispecies is very high due to rapid viral replication. The presence and extent of the diversity of quasispecies is expected to have a strong impact on antiviral immune responses (2). Immune responses are broadly targeted and are likely to be less effective in the presence of rapidly arising and highly diverse viral quasispecies (29). The recognition of viral proteins by T cells is highly specific, and the diversity of the quasispecies requires the continuous production of new effector T cells in a futile attempt to control infection. Immunotherapy as an adjunct to ART is expected to help the immune system control HIV-1; and DC-based vaccines targeting endogenous HIV-1, presumably including its multiple variants, might be particularly efficacious. The advantage of targeting autologous virus representing a large repertoire of the host's diverse HIV-1 antigen pool to elicit naïve, effector, and memory virus-specific T cells provides a strong incentive for the implementation of this approach in the clinic. Furthermore, the diversity of HIV-1 diversity appears to be diminished after ART (27), and this finding further supports the use of autologous HIV as an antigen during ART. In the ongoing clinical trial implementing this strategy of vaccination of HIV-1+ subjects, plans are to test the HIV antigen pool for the diversity of the reisolated virus.
DC-based vaccines for chronic HIV-1 infection have been shown to be safe and feasible (5, 9, 13, 15). Our own phase I clinical trial with HIV-1 peptide-based autologous DC vaccines for subjects receiving ART documented the safety of the vaccine and the development of a transient but significant presence of anti-HIV-1 peptide-specific T cells in the patients' blood following vaccination (5). A higher dose of DCs (5 × 106 to 10 × 106) was more effective than a lower dose of DCs (1 × 106 to 3 × 106). This transient and limited response to the vaccine indicated that the generation of T cells targeted to recognize and eliminate autologous HIV-1 species might be a more effective way of immunization. Our experience with vaccines containing DCs loaded with ApBs of cancer cells in three different clinical trials indicates that tetramer-positive tumor-specific T cells are generated in response to the vaccines (unpublished data). However, the prospect of producing a vaccine comprised of autologous virus is not as easily implemented as strategies that use tumor cells, as it requires isolation of the virus and its inactivation prior to vaccination. To confirm the feasibility of generating autologous virus from untreated patients with HIV-1 infection, we have completed a series of preclinical studies, which are described here.
The initial experiments reported in the present study were performed on a small scale, to demonstrate that viral isolation, inactivation of the virus, and loading of the inactivated virus into autologous DCs can be accomplished in a research laboratory. With the successful production of viral supernatants in three of four HIV-1+ subjects, the procedure was scaled up for clinical use. Large-scale, clinical-grade DC preparation is routine and has been broadly used with peripheral blood products obtained from patients with cancer or HIV-1 infection in our and other laboratories (5, 17, 18, 21, 26, 30). Although the entire HIV-1 vaccine production process is complex, it was shown to be feasible and applicable to the controlled good manufacturing practice setting. The multiple steps involved in the production of the vaccine can be timed to accommodate the anticipated therapy with ART, followed by treatment interruption prior to DC generation and therapeutic vaccine delivery.
The preclinical feasibility studies described here indicate that the autologous virus-based vaccine can now be routinely produced for a clinical trial. The numbers, viability, phenotypic characteristics, and stability of the DC-fed ApBs of CD4+ T cells infected with endogenous virus will be considered in defining the release criteria for the vaccine. On the basis of the reported data, we have initiated a phase I clinical vaccination trial under Investigational New Drug application no. 13137 and Institutional Review Board approval no. 0702006 for patients with chronic HIV-1 infection at the University of Pittsburgh Medical Center. It remains to be determined whether this strategy will prove to be clinically effective.
Acknowledgments
This study was supported in part by Production Assistance for Cellular Therapies (PACT) under contract N01-HB-37165 from the National Heart, Lung, and Blood Institute to T.L.W. and by NIH grant U19 AI055794 to C.R.R.
Footnotes
Published ahead of print on 26 November 2008.
REFERENCES
- 1.Blankson, J. N., D. Persaud, and R. F. Siliciano. 2002. The challenge of viral reservoirs in HIV-1 infection. Annu. Rev. Med. 53557-593. [DOI] [PubMed] [Google Scholar]
- 2.Brander, C., N. Frahm, and B. D. Walker. 2006. The challenges of host and viral diversity in HIV vaccine design. Curr. Opin. Immunol. 18430-437. [DOI] [PubMed] [Google Scholar]
- 3.Connolly, N., S. Riddler, J. Stanson, W. Gooding, C. R. Rinaldo, S. Ferrone, and T. L. Whiteside. 2007. Levels of antigen processing machinery components in dendritic cells generated for vaccination of HIV-1+ subjects. AIDS 211683-1692. [DOI] [PubMed] [Google Scholar]
- 4.Connolly, N. C., B. A. Colleton, and C. R. Rinaldo. 2007. Treating HIV-1 infection with dendritic cells. Curr. Opin. Mol. Ther. 9353-363. [PubMed] [Google Scholar]
- 5.Connolly, N. C., T. L. Whiteside, C. Wilson, V. Kondragunta, C. R. Rinaldo, and S. A. Riddler. 2008. Therapeutic immunization with HIV-1 peptide-loaded dendritic cells is safe and immunogenic in HIV-1-infected individuals. Clin. Vaccine Immunol. 15284-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan, Z., X. L. Huang, L. Borowski, J. W. Mellors, and C. R. Rinaldo, Jr. 2001. Restoration of anti-human immunodeficiency virus type 1 (HIV-1) responses in CD8+ T cells from late-stage patients on prolonged antiretroviral therapy by stimulation in vitro with HIV-1 protein-loaded dendritic cells. J. Virol. 754413-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan, Z., X. L. Huang, L. Zheng, C. Wilson, L. Borowski, J. Liebmann, P. Gupta, J. Margolick, and C. Rinaldo. 1997. Cultured blood dendritic cells retain HIV-1 antigen-presenting capacity for memory CTL during progressive HIV-1 infection. J. Immunol. 1594973-4982. [PubMed] [Google Scholar]
- 8.Folks, T. M., D. Powell, M. Lightfoote, S. Koenig, A. S. Fauci, S. Benn, A. Rabson, D. Daugherty, H. E. Gendelman, M. D. Hoggan, S. Venkatesan, and M. A. Martin. 1986. Biological and biochemical characterization of a cloned Leu-3-cell surviving infection with the acquired immune deficiency syndrome retrovirus. J. Exp. Med. 164280-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia, F., M. Lejeune, N. Climent, C. Gil, J. Alcami, V. Morente, L. Alos, A. Ruiz, J. Setoain, E. Fumero, P. Castro, A. Lopez, A. Cruceta, C. Piera, E. Florence, A. Pereira, A. Libois, N. Gonzalez, M. Guila, M. Caballero, F. Lomena, J. Joseph, J. M. Miro, T. Pumarola, M. Plana, J. M. Gatell, and T. Gallart. 2005. Therapeutic immunization with dendritic cells loaded with heat-inactivated autologous HIV-1 in patients with chronic HIV-1 infection. J. Infect. Dis. 1911680-1685. [DOI] [PubMed] [Google Scholar]
- 10.Huang, X. L., Z. Fan, B. A. Colleton, R. Buchli, H. Li, W. H. Hildebrand, and C. R. Rinaldo, Jr. 2005. Processing and presentation of exogenous HLA class I peptides by dendritic cells from human immunodeficiency virus type 1-infected persons. J. Virol. 793052-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubert, J. 1984. Spearman-Karber method, p. 65-66. In Bioassays, 2nd ed. Hunt Publishing, Dubuque, IA.
- 12.Larsson, M., J. F. Fonteneau, M. Lirvall, P. Haslett, J. D. Lifson, and N. Bhardwaj. 2002. Activation of HIV-1 specific CD4 and CD8 T cells by human dendritic cells: roles for cross-presentation and non-infectious HIV-1 virus. AIDS 161319-1329. [DOI] [PubMed] [Google Scholar]
- 13.Lisziewicz, J., D. I. Gabrilovich, G. Varga, J. Xu, P. D. Greenberg, S. K. Arya, M. Bosch, J. P. Behr, and F. Lori. 2001. Induction of potent human immunodeficiency virus type 1-specific T-cell-restricted immunity by genetically modified dendritic cells. J. Virol. 757621-7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lore, K., M. R. Betts, J. M. Brenchley, J. Kuruppu, S. Khojasteh, S. Perfetto, M. Roederer, R. A. Seder, and R. A. Koup. 2003. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J. Immunol. 1714320-4328. [DOI] [PubMed] [Google Scholar]
- 15.Lu, W., L. C. Arraes, W. T. Ferreira, and J. M. Andrieu. 2004. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat. Med. 101359-1365. [DOI] [PubMed] [Google Scholar]
- 16.Mailliard, R. B., A. Wankowicz-Kalinska, Q. Cai, A. Wesa, C. M. Hilkens, M. L. Kapsenberg, J. M. Kirkwood, W. J. Storkus, and P. Kalinski. 2004. Alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 645934-5937. [DOI] [PubMed] [Google Scholar]
- 17.Morse, M. A., and H. K. Lyerly. 2000. Clinical applications of dendritic cell vaccines. Curr. Opin. Mol. Ther. 220-28. [PubMed] [Google Scholar]
- 18.Nicolette, C., D. Healey, I. Tcherepanova, P. Whelton, T. Monesmith, L. Coombs, L. H. Finke, T. Whiteside, and F. Miesowicz. 2007. Dendritic cells for active immunotherapy: optimizing design and manufacture in order to develop commercially and clinically viable products. Vaccine 25(Suppl.)B47-B60. [DOI] [PubMed] [Google Scholar]
- 19.Nobile, M., R. Correa, J. A. Borghans, C. D'Agostino, P. Schneider, R. J. De Boer, G. Pantaleo, and Swiss HIV Cohort Study. 2004. De novo T-cell generation in patients at different ages and stages of HIV-1 disease. Blood 104470-477. [DOI] [PubMed] [Google Scholar]
- 20.Osada, T., T. M. Clay, C. Y. Woo, M. A. Morse, and H. K. Lyerly. 2006. Dendritic cell-based immunotherapy. Int. Rev. Immunol. 25377-413. [DOI] [PubMed] [Google Scholar]
- 21.Palucka, A. K., B. Laupeze, C. Aspord, H. Saito, G. Jego, J. Fay, S. Paczesny, V. Pascual, and J. Banchereau. 2005. Immunotherapy via dendritic cells. Adv. Exp. Med. Biol. 560105-114. [DOI] [PubMed] [Google Scholar]
- 22.Pantaleo, G., and R. A. Koup. 2004. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat. Med. 10806-810. [DOI] [PubMed] [Google Scholar]
- 23.Rinaldo, C. R., Jr., X. L. Huang, Z. Fan, J. B. Margolick, L. Borowski, A. Hoji, C. Kalinyak, D. K. McMahon, S. A. Riddler, W. H. Hildebrand, R. B. Day, and J. W. Mellors. 2000. Anti-human immunodeficiency virus type 1 (HIV-1) CD8+ T-lymphocyte reactivity during combination antiretroviral therapy in HIV-1-infected patients with advanced immunodeficiency. J. Virol. 744127-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossi, M., and J. W. Young. 2005. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J. Immunol. 1751373-1381. [DOI] [PubMed] [Google Scholar]
- 25.Schuitemaker, H., M. Koot, N. A. Kootstra, M. W. Dercksen, R. E. de Goede, R. P. van Steenwijk, J. M. Lange, J. K. Schattenkerk, F. Miedema, and M. Tersmette. 1992. Biological phenotype of HIV-1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J. Virol. 661354-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuler, G., B. Schuler-Thorner, and R. M. Steinman. 2003. The use of dendritic cells in cancer immunotherapy. Curr. Opin. Immunol. 15138-147. [DOI] [PubMed] [Google Scholar]
- 27.Siliciano, J. D., and R. F. Siliciano. 2004. A long-term latent reservoir for HIV-1: discovery and clinical implications. J. Antimicrob. Chemother. 546-9. [DOI] [PubMed] [Google Scholar]
- 28.Steinman, R. M. 2003. The control of immunity and tolerance by dendritic cell. Pathol. Biol. (Paris) 5159-60. [DOI] [PubMed] [Google Scholar]
- 29.Walker, B. D., and B. T. Korber. 2001. Immune control of HIV: the obstacles of HLA and viral diversity. Nat. Immunol. 2473-475. [DOI] [PubMed] [Google Scholar]
- 30.Whiteside, T. L. 2008. Evaluation of dendritic cell products generated for human therapy and post-treatment immune monitoring, p. 2-17. In Advances in vaccine development and manufacturing. BioPharm International, Iselin, NJ.
- 31.Zhao, X. Q., X. L. Huang, P. Gupta, L. Borowski, Z. Fan, S. C. Watkins, E. K. Thomas, and C. R. Rinaldo, Jr. 2002. Induction of anti-human immunodeficiency virus type 1 (HIV-1) CD8+ and CD4+ T-cell reactivity by dendritic cells loaded with HIV-1 X4-infected apoptotic cells. J. Virol. 763007-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]