Abstract
Groups of 15 laboratory-bred beagles were vaccinated and boosted with either a placebo or adjuvanted bivalent bacterin comprised of a traditional Borrelia burgdorferi strain and a unique ospA- and ospB-negative B. burgdorferi strain that expressed high levels of OspC and then challenged with B. burgdorferi-infected Ixodes scapularis ticks. The vaccinated dogs produced high titers of anti-OspA and anti-OspC borreliacidal antibodies, including borreliacidal antibodies specific for an epitope within the last seven amino acids at the OspC carboxy terminus (termed OspC7) that was conserved among pathogenic Borrelia genospecies. In addition, spirochetes were eliminated from the infected ticks that fed on the bacterin recipients, B. burgdorferi was not isolated from the skin or joints, and antibody responses associated specifically with canine infection with B. burgdorferi were not produced. In contrast, B. burgdorferi was recovered from engorged ticks that fed on 13 (87%) placebo-vaccinated dogs (P < 0.0001), skin biopsy specimens from 14 (93%) dogs (P < 0.0001), and joint tissue specimens from 8 (53%) dogs (P = 0.0022). In addition, 14 (93%) dogs developed specific antibody responses against B. burgdorferi proteins, including 11 (73%) with C6 peptide antibodies (P < 0.0001). Moreover, 10 (67%) dogs developed Lyme disease-associated joint abnormalities (P < 0.0001), including 4 (27%) dogs that developed joint stiffness or lameness and 6 (40%) that developed chronic joint inflammation (synovitis). The results therefore confirmed that the bacterin provided a high level of protection against Lyme disease shortly after immunization.
Dogs with Lyme disease rarely develop acute illness (26); but the infection reliably causes chronic subclinical polyarthritis and/or periarteritis (43) and occasionally causes frank recurrent arthritis with myalgia, fever, anorexia, and lethargy (41, 42); renal failure (11); heart block (24); or neurologic disease (9). In addition, the severity of the illness appears to be influenced by the species and the age of the dog. For example, beagle puppies are prone to oligoarthropathy (2, 39), while adults are more likely to develop asymptomatic synovitis (2, 7, 43). Moreover, Labrador retrievers, golden retrievers, and Shetland sheepdogs appear to be more susceptible to kidney nephropathy (11).
Several commercial dog vaccines are currently available, and each provides protection primarily by inducing the production of anti-OspA borreliacidal antibodies that stimulate complement to form a membrane attack complex (33) that kills Borrelia burgdorferi in the tick midgut as the infected vectors ingest blood (12, 16). The approach has been effective (8, 10, 31, 40), but the vaccines may also fail (23) because the expression of OspA is downregulated immediately after the infected tick begins acquiring a blood meal (36), borreliacidal antibodies specific for OspA are genospecies specific (28, 49), and ticks can be infected with variant OspA-negative Lyme disease spirochetes (15).
Another viable target for antibody-mediated immunity is OspC (18), especially since, in contrast to OspA, the Lyme disease spirochetes upregulate the expression of OspC as the tick begins feeding (36) and express OspC during the early stages of a mammalian infection (45). However, vaccines that provide protection by inducing anti-OspC antibodies have not been pursued aggressively, likely because the extreme heterogeneity, even among B. burgdorferi isolates from the same geographic area (44, 47), suggested that the protection afforded by anti-OspC antibodies would not be comprehensive.
However, researchers (21) recently identified an epitope within the surface-exposed 7 amino acids of the carboxy terminus of OspC (hereafter referred to as OspC7) recognized by anti-OspC borreliacidal antibodies. More significantly, the epitope within the OspC7 region is conserved among the pathogenic Borrelia genospecies, including B. afzelii and B. garinii. Therefore, anti-OspC borreliacidal antibodies formed against the OspC7 region should provide comprehensive protection and should be effective against spirochetes in the tick and during the early stages of mammalian infection. Moreover, in contrast to the use of vaccination to induce anti-OspA borreliacidal antibodies, vaccination with proteins such as OspC that are also expressed during mammalian infection provides the possibility of producing an effective anamnestic immunologic memory response. We therefore developed a bivalent bacterin that induced both anti-OspA and anti-OspC borreliacidal antibodies, including borreliacidal antibodies specific for the conserved epitope within the OspC7 region, and evaluated the ability of immunization to provide protection against challenge from B. burgdorferi-infected ticks.
(This study was presented in part at the 25th American College of Veterinary Internal Medicine Forum, Seattle, WA, 6 to 9 June 2007 [22a, 22b].)
MATERIALS AND METHODS
Spirochetes.
B. burgdorferi S-1-10 is a pathogenic sensu stricto isolate recovered from the kidney of a white-footed mouse captured near La Crosse, WI, that is currently incorporated into a commercially available dog Lyme disease vaccine (Galaxy Lyme). The spirochete contains ospA, ospB, and ospC and expresses each gene when it is cultured at 35°C in the laboratory. B. burgdorferi 50772 is a unique noninfectious ospA- and ospB-negative sensu stricto isolate (1) that expresses high levels of OspC on the surface when it is cultured at 35°C in Barbour-Stoenner-Kelly (BSK) medium (35).
Animals.
Eight-week-old laboratory-bred beagle puppies were obtained from Ridglan Farms (Mount Horeb, WI), randomized without regard to sex or vaccination status, and housed communally on the floor in groups of up to 15 animals each. Five days before the tick challenge, the dogs were housed individually in cages and were kept segregated until the ticks were removed. Thereafter, the dogs were again housed communally on the floor in randomized groups of four to six animals each for the duration of the study. The dogs were housed at ambient temperature (21°C) and were provided food and water ad libitum. The experiments were reviewed and approved by the Schering Plough Animal Care and Use Committee.
Vaccines.
B. burgdorferi S-1-10 or 50772 was cultured in BSK medium at 35°C until it reached logarithmic growth and was then inactivated by adding 10 mM binary ethylenimine, which was subsequently neutralized with sodium thiosulfate. Following inactivation, the spirochetes were concentrated by continuous-flow centrifugation (model RC-5B/26Plus centrifuge; Sorvall) at 15,000 rpm and a flow rate of 40 to 60 ml/min. The antigen was then resuspended alone or in combination in balanced salt solution containing 30 μg of gentamicin/ml and 30 U of nystatin/ml and blended with 5% Emulsigen solution (MVP Laboratories, Inc., Omaha, NE) and 1% HEPES so that a 1-ml dose contained at least 2.5 × 107 spirochetes of each isolate. An antibiotic and an antimycotic agent were added to comply with Schering-Plough's manufacturing procedures for licensed products.
Ticks.
Adult Ixodes scapularis ticks were collected from wooded areas in the endemic focus (20) near Ettrick, WI, by flagging the underbrush. The ticks were immediately transported to the laboratory and were stored at 8°C in 95% humidity until they were used. To confirm infection with Lyme disease spirochetes, the midguts from 50 ticks were examined, and B. burgdorferi was detected in 13 (26%) ticks.
Detection of B. burgdorferi in tick midguts.
The tick midguts were smeared onto glass slides and dried overnight at room temperature. The slides were then fixed in acetone for 10 min and air dried. B. burgdorferi-specific rabbit polyclonal antibodies diluted 1:500 in phosphate-buffered saline (PBS; pH 7.2) and goat anti-rabbit fluorescein isothiocyanate-labeled immunoglobulin G (IgG) antibodies (Sigma-Aldrich, St. Louis, MO) diluted 1:200 in PBS were overlaid sequentially, and the slides were examined by fluorescence microscopy. The rabbit antibodies were obtained by vaccinating and boosting a New Zealand White rabbit with a bacterin comprised of inactivated B. burgdorferi and aluminum hydroxide. The slides prepared from the ticks used to challenge the dogs were masked prior to examination and were scored independently by two experienced individuals.
ELISA.
The OspA or OspC enzyme-linked immunosorbent assay (ELISA) was performed as described previously (21). Briefly, the wells of microtiter plates (Immunolon 2 HB; Thermo Labsystems, Franklin, MA) were coated with 100 μl of recombinant OspA (rOspA) or rOspC (1 μg/ml) contained in carbonate buffer (90 mM NaHCO3, 60 mM Na2CO3, pH 9.6) and incubated overnight at 4°C. Following incubation, the plates were washed three times with PBS (0.05 M, pH 7.2) containing 0.05% Tween 20 (PBS-T). After the plates were washed, 200 μl of blocking buffer comprised of PBS-T and 1% bovine serum albumin (Sigma-Aldrich) was added to each well and the plates were incubated with rotation (150 rpm) for 1 h at room temperature. The plates were washed three times with PBS-T and were incubated for 1 h at room temperature with 100 μl of serum diluted 1:80 to 1:40,960 in blocking buffer. Peroxidase-conjugated goat anti-dog IgG antibody (Kirkegaard & Perry Laboratories, Gaithersburg, MD) diluted 1:10,000 in blocking buffer was then added. After incubation for 1 h, the plates were again washed with TBS-T, 100 μl of o-phenylenediamine substrate (Sigma) was added, and the optical density at 490 nm was determined with a SpectraMax 250 spectrophotometer (Molecular Devices, Sunnyvale, CA). The last dilution to yield an optical density value ≥0.300 above that for control serum from a healthy dog was considered the titer endpoint.
Detection of C6 peptide antibodies.
B. burgdorferi antibodies specific for C6 were detected by using a commercially available test (SNAP 4Dx; IDEXX Laboratories, Westbrook, ME), according to the manufacturer's directions.
Western blotting.
Western blotting was performed by standard techniques. Briefly, B. burgdorferi was boiled for 5 min and 225 μg of protein was loaded onto a 10% to 20% gradient polyacrylamide gel (Bio-Rad, Hercules, CA). After electrophoresis, the proteins were transferred to a polyvinylidene difluoride membrane and blocked with 1% bovine serum albumin in PBS-0.1% Tween 20. The strips were then incubated with diluted serum (1:100) and washed with PBS-0.1% Tween 20. Horseradish peroxidase-labeled anti-dog IgG antibody (Kirkegaard & Perry) diluted 1:8,000 was then added, and the strips were developed with a TMB membrane peroxidase substrate system (Kirkegaard & Perry).
Detection of borreliacidal antibodies.
Anti-OspA or anti-OspC borreliacidal antibodies were detected by a flow cytometric procedure (5). A culture of B. burgdorferi S-1-10 (OspA) or 50772 (OspC) was diluted with BSK medium to a concentration of 5 × 106 organisms/ml. Concomitantly, serum samples were diluted 1:40 with BSK medium and sterilized by passage through a 0.2-μm-pore-size microcentrifuge filter. A 200-μl aliquot was then transferred to a sterile 1.5-ml screw-cap microcentrifuge tube and serially diluted from 1:80 to 1:20,480 with BSK medium. The serum samples were then heat inactivated at 56°C for 10 min, and a 100-μl aliquot of the spirochete suspension (5 × 105 spirochetes) and 5 μl of sterile guinea pig complement (Rockland Immunochemical, Gilbertsville, PA) were added to each dilution of serum. The assay suspensions were mixed thoroughly and incubated at 35°C for 16 to 24 h.
Following incubation, 100 μl of each assay suspension was transferred to a polystyrene tube containing 400 μl of PBS and 1 μg/ml of acridine orange. A FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) was then used to detect borreliacidal activity. Spirochetes were isolated by gating (CellQuest software; Becton Dickinson) and were analyzed with the flow rate set at low. The borreliacidal antibodies were detected indirectly by monitoring the increased fluorescence intensity that occurs when the acridine orange intercalates into blebbed, nonviable spirochetes. A ≥13% shift in the mean fluorescence intensity compared to that for control serum from a healthy dog was considered positive (5). The presence of blebbed, nonmotile B. burgdorferi was confirmed by dark-field microscopy. Serum from a healthy dog was included with each assay as a negative control. A positive control was also included to minimize interassay variability. In addition, serum samples from individual animals were assayed concurrently.
Removal of anti-OspA antibodies.
rOspA was recovered from Escherichia coli DH5α expressing an OspA-glutathione-S-transferase fusion protein, as described previously (6), and was then bound to Sepharose 4B by cyanogen bromide (CNBr) activation. Specifically, 0.8 g of CNBr-activated Sepharose 4B (Sigma-Aldrich) was washed with coupling buffer (0.1 M NaHCO3, 0.5 M NaCl, pH 8.3), 2 mg of rOspA in coupling buffer was added to the gel, and the mixture was gently shaken at room temperature for 2 h. After the gel was washed twice with coupling buffer, 4 ml of ethanolamine (pH 9) was added, and the gel was incubated for 2 h to block unbound sites. The gel was washed three times with 50 ml of 0.1 M sodium acetate-0.5 M NaCl (pH 4.0), equilibrated in PBS, and then poured into a column (10 by 105 mm; (Pierce, Rockford, IL). One-milliliter volumes of immune serum diluted 10-fold in PBS were passed over the column four times.
Removal of anti-OspC or anti-OspC7 antibodies.
rOspC and rOspC7 were recovered from Escherichia coli JM109 containing pX3-22 and E. coli JM109 containing pXT7, respectively, and were bound to Tetralink tetrameric avidin resin (Promega, Madison, WI) contained within a column, as described previously (29, 35). One-milliliter volumes of immune serum diluted 10-fold in PBS were passed over the columns four times.
Vaccination and collection of sera.
The dogs were vaccinated subcutaneously in the neck with a 1-ml dose of bacterin or placebo and boosted after 21 days with an additional 1-ml dose. Whole blood was collected by venipuncture of the jugular vein prior to the initial vaccination (day −3), 7 (day 28) or 21 (day 42) days after the booster vaccination, and 90 days after the tick challenge. Serum was separated by centrifugation and was stored at −20°C until it was tested.
Tick challenge.
Three weeks after the booster vaccination, the dogs were shaved on the right side of the thoracic cavity, and each dog was challenged with 10 female and 10 male I. scapularis ticks that were placed in a rubber cup that was secured to the shaved area with bandage wrap and tape. The ticks were allowed to feed for 7 days. After they fed, the ticks were removed and the midguts from the female ticks that had fed to repletion were examined for B. burgdorferi.
Detection of Lyme disease-associated limb disorder.
The dogs were observed daily for 8 months after the tick challenge for joint stiffness (reluctance to bear weight) or lameness (an inability to bear weight). Dogs that developed stiffness or lameness that persisted for 3 consecutive days were euthanized and immediately necropsied. Because of additional causes of joint abnormalities, stiffness or lameness was not considered to be associated with Lyme disease unless spirochetes were also recovered from the joint tissues or the dogs developed antibodies associated specifically with B. burgdorferi exposure or infection. Each observer was blinded to the vaccination status, and the scoring was the consensus of at least two observers.
Immunosuppression.
Dogs that did not develop joint abnormalities within 13 weeks after the tick challenge were administered dexamethasone (0.4 mg/lb of body weight) by intramuscular injection for 5 consecutive days (days 96 to 100 after tick challenge) and were observed for the remainder of the study.
Collection of skin tissue and recovery of B. burgdorferi.
Skin biopsy specimens were collected 1, 3, and 5 months after the tick challenge by injecting a site near the bite sites with 0.5 ml of lidocaine (2%) and removing a biopsy specimen with a disposable 4-mm dermal biopsy punch (Miltex, Inc., York, PA). The biopsy specimens were immediately placed into separate tubes containing 9 ml of BSK medium supplemented with gelatin (20%), rifampin (rifampicin; 40 μg/ml), and kanamycin (8 μg/ml). The cultures were then vortexed, and 1 ml was transferred to an additional tube that also contained 9 ml of BSK (10-fold dilution), and the undiluted and the diluted cultures were incubated at 35°C and examined microscopically for 4 weeks. The ability of the BSK medium to support growth from an inoculum of one organism (4) was confirmed prior to culture.
Collection of joint tissue and recovery of B. burgdorferi.
At necropsy, approximately 1 cm3 of tissue was removed from the joint capsules of the knee and the tarsus of the right hind leg and the elbow and the carpus of the right front leg. The tissues were divided in half, and one portion was combined with 9 ml of fresh BSK medium in a sterile bag and emulsified by passage through a laboratory blender (Stomacher 80; Seward Medical, London, United Kingdom). One milliliter of the suspension was then transferred to a tube containing 9 ml of BSK, and the culture was incubated at 35°C and examined microscopically for 4 weeks. The other portion was placed into a tube containing 9 ml of 10% formalin in saline and forwarded for histopathology studies.
Histopathology of joint capsules.
Formalin-fixed tissues were processed by routine methods, stained with hematoxylin-eosin, and examined for cellular infiltrates associated with dog Lyme disease (43).
Statistics.
The data were analyzed by using Fisher's exact test and mitigated fraction. P values of ≤0.05 were considered significant.
RESULTS
Induction of anti-OspA and anti-OspC borreliacidal antibodies by vaccination.
To determine a formulation that induced anti-OspA and anti-OspC borreliacidal antibodies, we first vaccinated and boosted separate groups of dogs (n = 9) with bacterins that contained either B. burgdorferi S-1-10 or B. burgdorferi 50772 and then characterized the anti-OspA or anti-OspC antibody responses. Vaccination with the S-1-10 bacterin induced significant amounts of anti-OspA antibodies (titer range, 1:5,120 to 1:20,480) and anti-OspC antibodies (titer range, 1:160 to 1:1,280), and vaccination with the 50772 bacterin induced significant amounts of anti-OspC antibodies (titer range, 1:1,280 to 1:10,240). However, borreliacidal antibodies were detected only by using the homologous isolate bacterin (Table 1). In addition, removal of the anti-OspA antibodies significantly abrogated the borreliacidal activity detected by using isolate S-1-10, and removal of the anti-OspC antibodies (fourfold or greater reduction) or anti-OspC7 antibodies (twofold or greater reduction) reduced the 50772-specific activity (Table 2). It was therefore necessary to vaccinate the dogs with a bacterin comprised of a combination of B. burgdorferi S-1-10 and 50772 to induce both anti-OspA and anti-OspC borreliacidal antibodies, including borreliacidal antibodies specific for the conserved epitope within the C-terminal OspC7 region.
TABLE 1.
Borreliacidal antibody titers in immune seraa collected 7 days after vaccination and boost with bacterins containing B. burgdorferi S-1-10 or 50772
| Bacterin | No. (%) of serum samples containing borreliacidal antibodies detected by using:
|
Titerb range | |
|---|---|---|---|
| B. burgdorferi 50772 | B. burgdorferi S-1-10 | ||
| Placebo | 0 | 0 | |
| S-1-10 | 0 | 9 (100) | 5,120-20,480 |
| 50772 | 9 (100) | 0 | 1,280-10,240 |
Nine serum samples were tested.
Reciprocal dilution.
TABLE 2.
Effect of removing anti-OspA, anti-OspC, or anti-OspC7 antibodies on borreliacidal activity in immune seraa collected 7 days after vaccination and boost with the bivalent (isolate S-1-10 and 50772) bacterin
| Serum sample | Borreliacidal antibody titerb detected by using:
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
B. burgdorferi S-1-10
|
B. burgdorferi 50772
|
|||||||
| Untreated | rOspA adsorbed | rOspC adsorbed | rOspC7 adsorbed | Untreated | rOspA adsorbed | rOspC adsorbed | rOspC7 adsorbed | |
| OspA controlc | 2,560 | NDd | 2,560 | 2,560 | NTe | NT | NT | NT |
| OspC controlc | NT | NT | NT | NT | 2,560 | 2,560 | ND | ND |
| 1 | 20,480 | 160 | 20,480 | NT | 10,240 | 10,240 | 1,280 | 2,560 |
| 2 | 10,240 | 160 | 10,240 | NT | 10,240 | 10,240 | 640 | 5,120 |
| 3 | 10,240 | ND | 10,240 | NT | 10,240 | 10,240 | 640 | 2,560 |
| 4 | 5,120 | ND | 5,120 | NT | 5,120 | 5,120 | 640 | 1,280 |
| 5 | 5,120 | 80 | 5,120 | NT | 5,120 | 5,120 | 640 | 1,280 |
Five immune serum samples were tested.
Reciprocal dilution. rOspA adsorbed, anti-OspA antibodies removed; rOspC adsorbed, anti-OspC antibodies removed; rOspC7 adsorbed, anti-OspC7 antibodies removed.
Human Lyme disease serum containing anti-OspA or anti-OspC borreliacidal antibodies.
ND, none detected.
NT, not tested.
Ability of the bivalent bacterin to prevent infection with B. burgdorferi.
On the basis of the previous findings, we then vaccinated an additional group of dogs (n = 15) with a bivalent (S-1-10 and 50772) bacterin and challenged them with B. burgdorferi-infected ticks. Immediately prior to the tick challenge (3 weeks after the booster vaccination), the immune sera contained antibodies against several B. burgdorferi proteins, including significant levels of antibodies specific for OspA and OspC (Fig. 1). In addition, a significant amount of the anti-OspA antibodies (titers, ≥1:5,120) and anti-OspC antibodies (titers, ≥1:640) were borreliacidal, and a significant proportion of the anti-OspC borreliacidal antibodies were specific for the conserved OspC7 epitope (titers, ≥1:320). Subsequently, B. burgdorferi was detected in the midguts from 34 (32%) of 106 partially or fully engorged ticks recovered from the placebo recipients, and the total included at least 1 positive tick from 13 (87%) dogs (Table 3). In addition, spirochetes were recovered from the skin biopsy specimens from 14 (93%; P < 0.0001) control dogs and also from the elbow (n = 6), carpus (n = 3), or tarsus (n = 2) from 8 (53%) placebo recipients. In contrast, B. burgdorferi was not detected in the engorged ticks (n = 99) that fed on the bacterin recipients (P < 0.0001), and spirochetes were not recovered from the skin biopsy specimens (P < 0.0001) or joint tissues (P = 0.0022) from the vaccinated dogs.
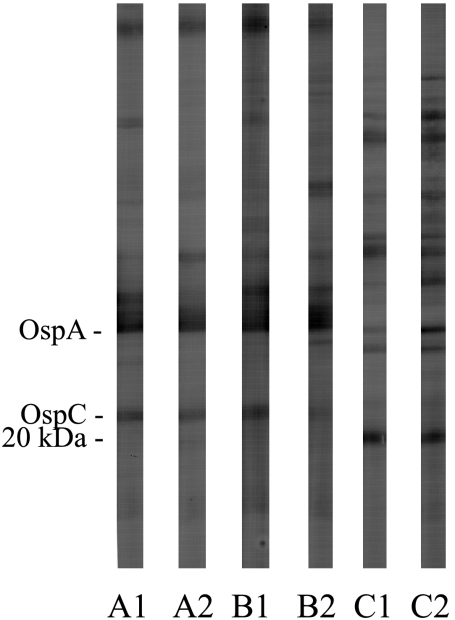
FIG. 1.
Examples of the antibody responses detected by Western blotting after vaccination with the bivalent bacterin or infection with B. burgdorferi. Strips A and B, immune sera from two bacterin recipients collected immediately prior to tick challenge (strips A1 and A2) or 90 days after tick challenge (strips B1 and B2); strips C1 and C2, immune sera from two placebo recipients collected 90 days after tick challenge. Monoclonal anti-OspA and anti-OspC antibodies were run concurrently to identify their locations on the strips.
TABLE 3.
Ability of the bivalent bacterin to sterilize feeding ticks and prevent infection with B. burgdorferi
| Parameter | No. (%) positive
|
P | |
|---|---|---|---|
| Controls (n = 15) | Bacterin (n = 15) | ||
| Dogs with B. burgdorferi- infected ticks | 13 (87) | 0 | <0.0001 |
| B. burgdorferi from skin at: | |||
| 1 mo | 12 (80) | 0 | <0.0001 |
| 3 mo | 11 (73) | 0 | <0.0001 |
| 5 mo | 9 (75)a | 0 | <0.0001 |
| Total dogs infected | 14 (93) | 0 | <0.0001 |
| B. burgdorferi from joints | |||
| Elbow | 6 (40)b | 0 | 0.017 |
| Carpus | 3 (20)c | 0 | 0.224 |
| Knee | 0 | 0 | |
| Tarsus | 2 (13) | 0 | 0.483 |
| Total dogs infected | 8 (53) | 0 | 0.0022 |
n = 12; three dogs were necropsied prior to the end of study due to joint stiffness or lameness.
Includes two dogs that developed joint stiffness or lameness.
Includes one dog that developed joint stiffness or lameness.
Ability of the bivalent bacterin to prevent seroconversion.
Immune serum samples collected 90 days after the tick challenge from 14 (93%) placebo recipients contained antibodies against numerous B. burgdorferi proteins (Fig. 1), and the banding patterns from each seropositive serum specimen were similar to those described in a report of a previous study (30) that used immune sera from culture-confirmed B. burgdorferi-infected beagles. In addition, each seropositive serum contained antibodies that bound to an uncharacterized approximately 20-kDa protein previously found (30) to be strongly associated with B. burgdorferi infection in beagles, and 11 (79%) serum samples also contained B. burgdorferi infection-specific (27) C6 antibodies. In contrast, the immune sera from the bacterin recipients remained seropositive, but no significant additional reactive bands, including antibodies against a 20-kDa protein or C6 antibodies, were detected (P < 0.0001).
Ability of the bivalent bacterin to prevent Lyme disease-associated joint abnormalities.
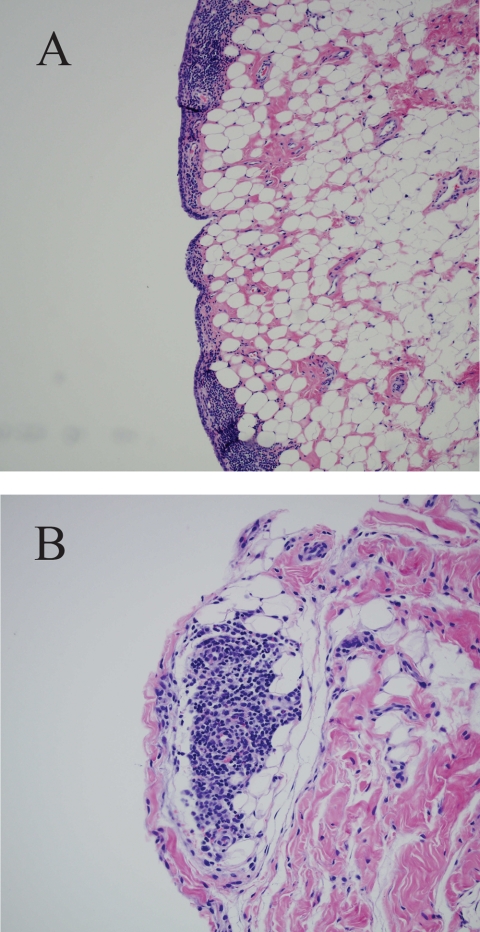
Prior to immunosuppression of the dogs with dexamethasone (13 weeks after the booster vaccination), one placebo-vaccinated dog developed joint lameness or stiffness, and B. burgdorferi was recovered from the affected joint (Table 4). Within the 2 weeks immediately after immunosuppression, two additional placebo recipients developed lameness, and spirochetes were also recovered from the affected joints. An additional placebo recipient developed lameness 7 weeks after the dogs were immunosuppressed, but spirochetes were not detected. However, the immune serum from the animal contained antibodies against numerous B. burgdorferi proteins in a pattern consistent with that in the other seropositive placebo recipients, and the antibody response included antibodies specific for the 20-kDa protein and the C6 peptide. In addition, 6 (55%) of the 11 placebo recipients that failed to develop lame or stiff joints had mild to moderate mono- or polyarthritis characterized by infiltrations of lymphocytes and/or plasma cells in the synovial or subsynovial joint tissues (Fig. 2). In contrast, two bacterin recipients developed stiff rear legs prior to immunosuppression (≤7 weeks after the booster vaccination), but spirochetes were not recovered from the affected joints and C6 antibodies or antibodies specific for a 20-kDa protein were not present in the immune serum. Therefore, the lameness was likely attributable to causes other than infection with B. burgdorferi. In addition, the joint tissues from the 13 remaining bacterin recipients had normal cellular morphologies (P = 0.0034).
TABLE 4.
Ability of the bivalent bacterin to prevent Lyme disease-associated joint abnormalities
| Abnormality | No. (%) positive
|
P | |
|---|---|---|---|
| Controls (n = 15) | Bacterin (n = 15) | ||
| Stiffness or lameness | 4 (27) | 0a | 0.0996 |
| Synovitis | 6 (55)b | 0c | 0.0034 |
| Total | 10 (67) | 0 | <0.0001 |
Two stiff or lame dogs that received bacterin were not included because spirochetes were not recovered and serologic evidence of B. burgdorferi infection or exposure was not detected.
n = 11; the joints from four dogs that developed joint stiffness or lameness were not examined.
n = 13; the joints from two dogs that developed joint stiffness or lameness were not examined.
FIG. 2.
Example of joint lesions caused by infection with B. burgdorferi. (A) Deep lymphocyte infiltration of synovial connective tissue of knee joint capsule; (B) focal plasma cell infiltrations in synovial and subsynovial connective tissue from carpus.
DISCUSSION
There are currently several commercial vaccines that prevent canine infection with B. burgdorferi by inducing the production of anti-OspA borreliacidal antibodies that kill the spirochetes in the tick as the parasites obtain a blood meal. While the strategy has largely proven successful, the approach can be ineffective and the numbers of vaccine failures can be significant. For example, an OspA vaccine (25) or whole-cell bacterin (10) failed to prevent B. burgdorferi infection in 40% and 22% of recipients, respectively. However, the recent finding (21) that borreliacidal antibodies also recognize a conserved surface-exposed region (OspC7) within the C terminus of OspC suggested that a bacterin that stimulated both anti-OspA and anti-OspC borreliacidal antibodies specific for the OspC7 region would provide more comprehensive and long-lasting protection. We therefore evaluated the ability of a bacterin that induced both anti-OspA and anti-OspC borreliacidal antibodies to protect dogs against B. burgdorferi-infected ticks.
Notably, vaccination with a bacterin that contained only a typical (32) infectious B. burgdorferi isolate (isolate S-1-10) recovered from a wild white-footed mouse induced detectable levels of anti-OspA and anti-OspC antibodies, but only borreliacidal antibodies specific for OspA were detected by the borreliacidal antibody test. In contrast, vaccination with ospA- and ospB-negative isolate 50772 induced anti-OspC borreliacidal antibodies that were readily detectable. This is significant, because the expression of OspC is upregulated in laboratory B. burgdorferi isolates when the spirochetes are incubated at 35° to 37°C (37, 38) and immunoblots prepared from the spirochetes readily detect anti-OspC antibodies (32, 35). One could therefore have expected vaccination with isolate S-1-10 to also induce high levels of anti-OspC borreliacidal antibodies. Additional studies to understand this discrepancy remain necessary, but the most likely explanation is that the 50772 spirochetes express significantly more OspC per organism, and therefore, the organisms stimulate a significantly more robust anti-OspC antibody response that results in the greatly increased production of anti-OspC borreliacidal antibodies. The fact that researchers have previously shown (35) that isolate 50772 expresses significantly more OspC than B. burgdorferi 297, another typical ospA- and ospB-positive isolate, supports this explanation.
Since nonborreliacidal anti-OspC antibodies are unlikely to provide protection (14, 19, 34, 46) and significant levels of anti-OspC borreliacidal antibodies were not produced when the dogs were vaccinated with isolate S-1-10, we combined isolate S-1-10 with isolate 50772 and confirmed that vaccination with the combination induced high levels of both anti-OspA and anti-OspC borreliacidal antibodies, including a significant proportion of borreliacidal antibodies specific for the OspC7 region. We then showed that dogs that received a placebo became reliably infected with B. burgdorferi, while spirochetes were not recovered from the bacterin recipients. In addition, anti-20-kDa-protein antibodies strongly associated with active B. burgdorferi infection in beagles (30) were not detected in the bacterin recipients, but the response was detected in 14 (93%) dogs that received the placebo. Moreover, B. burgdorferi-specific C6 antibodies were consistently (73%) produced by the placebo recipients, and the response was also not detected in the bacterin recipients. The latter finding is also consistent with previous findings (3) that the level of expression of the VlsE surface protein by infectious isolates such as S-1-10 becomes negligible during laboratory cultivation, and noninfectious isolate 50772 may have also lost plasmid lp28-1, which encodes VlsE (22). Finally, the placebo recipients commonly (n = 10) developed joint stiffness or lameness (n = 4) or subclinical joint synovitis (n = 6) characteristic (43) of Lyme disease in beagles. In contrast, two bacterin recipients limped after the tick challenge, but the response was not likely due to B. burgdorferi. It should be noted, however, that additional studies to determine whether the stiffness or lameness in these animals was caused by the vaccination are warranted. However, the possibility seems remote, since the limping occurred several weeks after the vaccinations and significant side effects attributable to vaccination have not been detected in several current ongoing experiments with the same bacterin.
The collective findings therefore confirmed that the anti-OspA and anti-OspC borreliacidal antibodies induced by the bacterin provided a high level of protection against challenge with B. burgdorferi-infected ticks. Moreover, the addition of B. burgdorferi 50772 to induce significant levels of anti-OspC borreliacidal antibodies, including borreliacidal antibodies specific for the conserved epitope within the OspC7 region (29), also offers the possibility the bacterin will provide protection against other pathogenic Borrelia genospecies. In addition, the initial borreliacidal antibody response to OspC may prime the recipients to elicit a protective anamnestic immune response during subsequent challenges. This possibility could be considered unlikely in light of the findings of a study presented in a previous report (17) in which the researchers failed to detect a protective anamnestic immune response in mice after vaccination with a rOspC and subsequent challenge with B. burgdorferi by tick transmission. However, dismissing the possibility on the basis of those findings may be premature for several reasons. For example, Lovrich et al. (29) showed that mice were inappropriate subjects for use in the assessment of the effectiveness of the OspC7 region as a Lyme disease vaccine because mice fail to develop anti-OspC7-specific borreliacidal antibodies, despite heavy infection with host-adapted spirochetes. In addition, the leader sequence of the rOspC used by Gilmore et al. (17) was truncated, so the protein was likely nonlipidated. This is significant, because the lipid moiety is essential for the ability of B. burgdorferi lipoproteins to generate a strong humoral immune response after vaccination (13, 48). Moreover, differences in the conformation between the native OspC expressed by B. burgdorferi 50772 and the rOspC generated in E. coli may also cause significant differences in immunogenicity.
In summary, bacterins and recombinant vaccines that protect dogs by inducing borreliacidal anti-OspA antibodies have been used commercially for over a decade, and while each provides a significant level of protection, recent findings have suggested that a combination of anti-OspA and anti-OspC borreliacidal antibodies would provide an improvement. Therefore, additional studies to evaluate the ability of the bivalent bacterin to provide protection against other pathogenic Borrelia genospecies and induce an effective memory immune response are warranted and ongoing.
Footnotes
Published ahead of print on 3 December 2008.
REFERENCES
- 1.Anderson, J. F., R. A. Flavell, L. A. Magnarelli, S. W. Barthold, F. S. Kantor, R. Wallich, D. H. Persing, D. Mathiesen, and E. Fikrig. 1996. Novel Borrelia burgdorferi isolates from Ixodes scapularis and Ixodes dentatus ticks feeding on humans. J. Clin. Microbiol. 34524-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appel, M. J. G., S. Allen, R. H. Jacobson, T. L. Lauderdale, Y. F. Chang, S. J. Shin, J. W. Thomford, R. J. Todhunter, and B. A. Summers. 1993. Experimental Lyme disease in dogs produces arthritis and persistent infection. J. Infect. Dis. 167651-664. [DOI] [PubMed] [Google Scholar]
- 3.Bykowski, T., K. Babb, K. von Lackum, S. P. Riley, S. J. Norris, and B. Stevenson. 2006. Transcriptional regulation of the Borrelia burgdorferi antigenically variable VlsE surface protein. J. Bacteriol. 1884879-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callister, S. M., K. L. Case, W. A. Agger, R. F. Schell, R. C. Johnson, and J. L. E. Ellingson. 1990. Effects of bovine serum albumin on the ability of Barbour-Stoenner-Kelly medium to detect Borrelia burgdorferi. J. Clin. Microbiol. 28363-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callister, S. M., D. A. Jobe, W. A. Agger, R. F. Schell, T. J. Kowalski, S. D. Lovrich, and J. A. Marks. 2002. Ability of the borreliacidal antibody test to confirm Lyme disease in clinical practice. Clin. Diagn. Lab. Immunol. 9908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callister, S. M., R. F. Schell, K. L. Case, S. D. Lovrich, and S. P. Day. 1993. Characterization of the borreliacidal antibody response to Borrelia burgdorferi in humans: a serodiagnostic test. J. Infect. Dis. 167158-164. [DOI] [PubMed] [Google Scholar]
- 7.Cerri, D., R. Farina, E. Andreani, R. Novuloni, A. Pedrini, and G. Cardini. 1994. Experimental infection of dogs with Borrelia burgdorferi. Res. Vet. Sci. 57256-258. [DOI] [PubMed] [Google Scholar]
- 8.Chang, Y. F., M. J. Appel, R. H. Jacobson, S. J. Shin, P. Harpending, R. Straubinger, L. A. Patrican, H. Mohammed, and B. A. Summers. 1995. Recombinant OspA protects dogs against infection and disease caused by Borrelia burgdorferi. Infect. Immun. 633543-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, Y. F., V. Novosel, C. F. Chang, B. A. Summers, D. P. Ma, Y. W. Chiang, W. M. Acree, H. J. Chu, S. Shin, and D. H. Lein. 2001. Experimental induction of chronic borreliosis in adult dogs exposed to Borrelia burgdorferi-infected ticks and treated with dexamethasone. Am. J. Vet. Res. 621104-1112. [DOI] [PubMed] [Google Scholar]
- 10.Chu, H. J., L. G. Chavez, B. M. Blumer, R. W. Sebring, T. L. Wasmoen, and W. M. Acree. 1992. Immunogenicity and efficacy study of a commercial Borrelia burgdorferi bacterin. J. Am. Vet. Med. Assoc. 201403-411. [PubMed] [Google Scholar]
- 11.Dambach, D. M., C. A. Smith, R. M. Lewis, and T. J. Van Wickle. 1997. Morphologic, immunohistochemical, and ultrastructural characterization of a distinctive renal lesion in dogs putatively associated with Borrelia burgdorferi infection: 49 cases (1987-1992). Vet. Pathol. 3485-96. [DOI] [PubMed] [Google Scholar]
- 12.de Silva, A. M., S. R. Telford III, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 83271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erdile, L. F., and B. Guy. 1997. OspA lipoprotein of Borrelia burgdorferi is a mucosal immunogen and adjuvant. Vaccine 15988-996. [DOI] [PubMed] [Google Scholar]
- 14.Exner, M. M., W. Xiaoyang, D. R. Blanco, J. N. Miller, and M. L. Lovett. 2000. Protection elicited by native outer membrane protein Oms66 (p66) against host-adapted Borrelia burgdorferi: conformational nature of bactericidal epitopes. Infect. Immun. 682647-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fikrig, E., H. Tao, S. W. Barthold, and R. A. Flavell. 1995. Selection of variant Borrelia burgdorferi isolates from mice immunized with outer surface protein A or B. Infect. Immun. 631658-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fikrig, E., S. R. Telford III, S. W. Barthold, F. S. Kantor, A. Spielman, and R. A. Flavell. 1992. Elimination of Borrelia burgdorferi from vector ticks feeding on OspA-immunized mice. Proc. Natl. Acad. Sci. USA 895418-5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilmore, R. D., Jr., R. M. Bacon, A. M. Carpio, J. Piesman, M. C. Dolan, and M. L. Mbow. 2003. Inability of outer-surface protein C (OspC)-primed mice to elicit a protective anamnestic immune response to a tick-transmitted challenge of Borrelia burgdorferi. J. Med. Microbiol. 52551-556. [DOI] [PubMed] [Google Scholar]
- 18.Gilmore, R. D., Jr., K. J. Kappel, M. C. Dolan, T. R. Burkot, and B. J. B. Johnson. 1996. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect. Immun. 642234-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golde, W. T., J. Piesman, M. C. Dolan, M. Kramer, P. Hauser, Y. Lobet, C. Capiau, P. Desmons, P. Voet, D. Dearwester, and J. C. Frantz. 1997. Reactivity with a specific epitope of outer surface protein A predicts protection from infection with the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 65882-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson, C. A., S. D. Lovrich, W. A. Agger, and S. M. Callister. 2002. Reassessment of a midwestern Lyme disease focus for Borrelia burgdorferi and the human granulocytic ehrlichiosis agent. J. Clin. Microbiol. 402070-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jobe, D. A., S. D. Lovrich, R. F. Schell, and S. M. Callister. 2003. C-terminal region of outer surface protein C binds borreliacidal antibodies in sera from patients with Lyme disease. Clin. Diagn. Lab. Immunol. 10573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labandiera-Rey, M., J. Seshu, and J. T. Skare. 2003. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect. Immun. 714608-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.LaFleur, R. L., J. Johnson, S. M. Callister, D. A. Jobe, S. D. Lovrich, D. Abdelmagid, and R. F. Schell. 2007. Abstr. 25th Am. Coll. Vet. Intern. Med. Forum, abstr. 192.
- 22b.LaFleur, R. L., J. Johnson, S. M. Callister, D. A. Jobe, S. D. Lovrich, D. Abdelmagid, and R. F. Schell. 2007. Abstr. 25th Am. Coll. Vet. Intern. Med. Forum, abstr. 193.
- 23.Levy, S. A., K. K. Clark, and L. T. Glickman. 2005. Infection rates in dogs vaccinated and not vaccinated with an OspA Borrelia burgdorferi vaccine in a Lyme disease-endemic area of Connecticut. Int. J. Appl. Res. Vet. Med. 31-5. [Google Scholar]
- 24.Levy, S. A., and P. H. Duray. 1988. Complete heart block in a dog seropositive for Borrelia burgdorferi. Similarity to human Lyme carditis. J. Vet. Intern. Med. 2138-144. [DOI] [PubMed] [Google Scholar]
- 25.Levy, S. A., B. A. Lissman, and C. M. Ficke. 1993. Performance of a Borrelia burgdorferi bacterin in borreliosis-endemic areas. J. Am. Vet. Med. Assoc. 2021834-1838. [PubMed] [Google Scholar]
- 26.Levy, S. A., and L. A. Magnarelli. 1992. Relationship between development of antibodies to Borrelia burgdorferi in dogs and the subsequent development of limb/joint borreliosis. J. Am. Vet. Med. Assoc. 200344-347. [PubMed] [Google Scholar]
- 27.Levy, S. A., T. P. O'Connor, J. L. Hanscom, and P. Shields. 2002. Utility of an in-house C6 ELISA test kit for determination of infection status of dogs naturally exposed to Borrelia burgdorferi. Vet. Ther. 3308-315. [PubMed] [Google Scholar]
- 28.Lovrich, S. D., S. M. Callister, B. K. DuChateau, L. C. L. Lim,. J. Winfrey, S. P. Day, and R. F. Schell. 1995. Abilities of OspA proteins from different seroprotective groups of Borrelia burgdorferi to protect hamsters from infection. Infect. Immun. 632113-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovrich, S. D., D. A. Jobe, R. F. Schell, and S. M. Callister. 2005. Borreliacidal OspC antibodies specific for a highly conserved epitope are immunodominant in human Lyme disease and do not occur in mice or hamsters. Clin. Diagn. Lab. Immunol. 12746-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovrich, S. D., R. L. LaFleur, D. A. Jobe, J. C. Johnson, K. E. Asp, R. F. Schell, and S. M. Callister. 2007. Borreliacidal OspC antibody response of canines with Lyme disease differs significantly from that of humans with Lyme disease. Clin. Vaccine Immunol. 14635-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma, J., P. M. Hine, E. R. Clough, D. Fish, R. T. Coughlin, G. A. Beltz, and M. G. Shew. 1996. Safety, efficacy, and immunogenicity of a recombinant Osp subunit canine Lyme disease vaccine. Vaccine 141366-1374. [DOI] [PubMed] [Google Scholar]
- 32.Magnarelli, L. A., J. F. Anderson, R. C. Johnson, R. B. Nadelman, and G. P. Wormser. 1994. Comparison of different strains of Borrelia burgdorferi sensu lato used as antigens in enzyme-linked immunosorbent assays. J. Clin. Microbiol. 321154-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowling, J. M., and M. T. Philipp. 1999. Killing of Borrelia burgdorferi by antibody elicited by OspA vaccine is inefficient in the absence of complement. Infect. Immun. 67443-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padilla, M. L., S. M. Callister, R. F. Schell, G. L. Bryant, D. A. Jobe, S. D. Lovrich, B. K. DuChateau, and J. R. Jensen. 1996. Characterization of the protective borreliacidal antibody response in humans and hamsters after vaccination with a Borrelia burgdorferi outer surface protein A (OspA) vaccine. J. Infect. Dis. 176739-746. [DOI] [PubMed] [Google Scholar]
- 35.Rousselle, J. C., S. M. Callister, R. F. Schell, S. D. Lovrich, D. A. Jobe, J. A. Marks, and C. A. Wieneke. 1998. Borreliacidal antibody production against outer surface protein C of Borrelia burgdorferi. J. Infect. Dis. 178733-741. [DOI] [PubMed] [Google Scholar]
- 36.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 922909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevenson, B., T. G. Schwan, and P. A. Rosa. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 634535-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Straubinger, R. K. 2000. PCR-based quantification of Borrelia burgdorferi organisms in canine tissues over a 500-day postinfection period. J. Clin. Microbiol. 382192-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Straubinger, R. K., T. Dharma Rao, E. Davison, B. A. Summers, R. H. Jacobson, and A. B. Frey. 2002. Protection against tick-transmitted Lyme disease in dogs vaccinated with a multiantigenic vaccine. Vaccine 20181-193. [DOI] [PubMed] [Google Scholar]
- 41.Straubinger, R. K., A. F. Straubinger, L. Harter, R. H. Jacobson, Y. F. Chang, B. A. Summers, H. N. Erb, and M. J. Appel. 1997. Borrelia burgdorferi migrates into joint capsules and causes an up-regulation of interleukin-8 in synovial membranes of dogs experimentally infected with ticks. Infect. Immun. 651273-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Straubinger, R. K., A. F. Straubinger, B. A. Summers, H. N. Erb, L. Harter, and M. J. Appel. 1998. Borrelia burgdorferi induces the production and release of proinflammatory cytokines in canine synovial explant cultures. Infect. Immun. 66247-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Summers, B. A., A. F. Straubinger, R. H. Jacobson, Y. F. Chang, M. J. Appel, and R. K. Straubinger. 2005. Histopathological studies of experimental Lyme disease in the dog. J. Comp. Pathol. 1331-13. [DOI] [PubMed] [Google Scholar]
- 44.Theisen, M., B. Frederiksen, A. M. Lebech, and J. Vuust. 1993. Polymorphism in ospC gene of Borrelia burgdorferi and immunoreactivity of OspC protein: implications for taxonomy and for use of OspC protein as a diagnostic antigen. J. Clin. Microbiol. 312570-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tilly, K., J. K. Krum, A. Bestor, M. W. Jewett. D. Grimm, D. Bueschel, R. Byram, D. Dorward, M. J. VanRaden, P. Stewart, and P. Rosa. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 743554-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ulbrandt, N. D., D. R. Cassatt, N. K. Patel, W. C. Roberts, C. M. Bachy, C. A. Fazenbaker, and M. S. Hanson. 2001. Conformational nature of the Borrelia burgdorferi decorin binding protein A epitopes that elicit protective antibodies. Infect. Immun. 694799-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, I. N., D. Dykhuizen, W. Qiu, and J. Dunn. 1999. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics 15115-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weis, J. J., Y. Ma, and L. F. Erdile. 1994. Biological activities of native and recombinant Borrelia burgdorferi outer surface protein A: dependence on lipid modification. Infect. Immun. 624632-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilske, B., U. Busch, V. Fingerle, F. Jauris-Heipke, V. Preac-Mursic, D. Rossler, and G. Will. 1996. Immunological and molecular variability of OspA and OspC. Implication for Borrelia vaccine development. Infection 24208-212. [DOI] [PubMed] [Google Scholar]