Abstract
Meningococcal outer membrane vesicle (OMV) vaccines, which are treated with detergents to decrease endotoxin activity, are safe and effective in humans. However, the vaccines elicit serum bactericidal antibody responses largely directed against PorA, which is antigenically variable. We previously prepared a native (non-detergent-treated) OMV vaccine from a mutant of group B strain H44/76 in which the lpxL1 gene was inactivated, which resulted in penta-acylated lipid A with attenuated endotoxin activity. To enhance protection, we overexpressed factor H binding protein (fHbp) from the antigenic variant 1 group. The vaccine elicited broad serum bactericidal antibody responses in mice against strains with fHbp variant 1 (∼70% of group B isolates) but not against strains with variant 2 or 3. In the present study, we constructed a mutant of group B strain NZ98/254 with attenuated endotoxin that expressed both endogenous variant 1 and heterologous fHbp variant 2. A mixture of the two native OMV vaccines from the H44/76 and NZ98/254 mutants stimulated proinflammatory cytokine responses by human peripheral blood mononuclear cells similar to those stimulated by control, detergent-treated OMV vaccines from the wild-type strains. In mice, the mixture of the two native OMV vaccines elicited broad serum bactericidal antibody responses against strains with heterologous PorA and fHbp in the variant 1, 2, or 3 group. By adsorption studies, the principal bactericidal antibody target was determined to be fHbp. Thus, native OMV vaccines from mutants expressing fHbp variants have the potential to be safe for humans and to confer broad protection against meningococcal disease from strains expressing fHbp from each of the antigenic variant groups.
Neisseria meningitidis is a gram-negative pathogen that causes meningitis and sepsis in humans. Conjugate vaccines based on the capsular polysaccharide are available against strains with capsular groups A, C, W-135, and Y. No broadly protective vaccine is available against strains with capsular group B, in part because of safety concerns about cross-reactivities of anticapsular antibodies with glycoproteins in human tissues (10, 15). Meningococcal outer membrane vesicle (OMV) vaccines are safe and efficacious in humans (reviewed in reference 20). However, OMV vaccines elicit serum bactericidal antibodies mainly against a major outer membrane porin, PorA (37), which is antigenically variable (31). OMV vaccines therefore are most suitable for the control of epidemics caused by predominantly one strain (17, 36). Wide-scale use of an OMV vaccine in New Zealand recently controlled a long-standing group B epidemic (21).
In recent years, three principal strategies have been pursued to expand vaccine protection against genetically diverse N. meningitidis group B strains (16). One uses detergent-treated OMV vaccines prepared from mutant strains engineered to express more than one PorA (9). A second combines a detergent-treated OMV vaccine with three recombinant proteins containing five novel antigens that were identified as vaccine candidates by “reverse vaccinology” (14). One of these new antigens is factor H binding protein (fHbp), which was previously referred to as GNA 1870 (26) or LP2086 (11). This antigen is a surface-exposed lipoprotein that binds human fH, a downregulator of the alternative complement pathway (25). Expression of fHbp and binding of complement fH enable N. meningitidis to evade innate host defenses (14a, 25, 32, 40). The third vaccine approach uses recombinant fHbps from two antigenic groups (11, 45). In humans, all three vaccine approaches elicited serum bactericidal antibodies (6, 8; P. Richmond, H. Marshall, M. D. Nissen, S. Lambert, T. Jones, W. Gruber, and A. Arora, presented at the 16th International Pathogenic Neisseria Conference, Rotterdam, The Netherlands, 7 to 12 September 2008; M. D. Snape, T. Dawson, A. Morant, B. John, R. Ohene-Kena, R. Borrow, P. Oster, and A. J. Pollard, presented at the 16th International Pathogenic Neisseria Conference, Rotterdam, The Netherlands, 7 to 12 September 2008). However, with the OMV vaccine from mutants with more than one PorA protein, coverage was incomplete for strains with certain PorA types (6, 8, 9). For the vaccines with recombinant fHbp, coverage was incomplete against some strains with antigenic variants and/or with low expression of fHbp (K. U. Jansen, L. K. McNeil, V. Dragalin, A. S. Anderson, S. K. Hoiseth, A. Arora, E. E. Emini, G. W. Zlotnick, and T. Jones, presented at the 16th International Pathogenic Neisseria Conference, Rotterdam, The Netherlands, 7 to 12 September 2008; M. D. Snape et al., presented at the 16th International Pathogenic Neisseria Conference, Rotterdam, The Netherlands, 7 to 12 September 2008).
Conventional OMV vaccines are prepared by detergent treatment of bacterial cells to extract lipooligosaccharide (LOS), which decreases endotoxin activity (12). This treatment also extracts potentially desirable vaccine antigens such as fHbp (22) and GNA 2132 (39). We previously prepared a native (not treated with detergents) OMV vaccine from a mutant of group B strain H44/76 in which we inactivated the gene encoding LpxL1 (22), which is a late-functioning acetyltransferase (38). The mutation resulted in penta-acylated instead of hexa-acylated LOS, which was known to have substantially less endotoxin activity than that of wild-type LOS (38). To enhance protection, we engineered the mutant to overexpress an fHbp in the variant 1 (v.1) group. In mice, a native OMV vaccine from the mutant elicited broad serum bactericidal antibody responses against genetically diverse N. meningitidis strains that expressed fHbp in the v.1 group. However, protection was incomplete against strains expressing fHbp in the v.2 or v.3 group, which in the United States together accounted for ∼30% of disease-causing group B strains (3). The purpose of the present study was to determine whether a mixture of two native OMV vaccines prepared from LpxL1 knockout (KO) mutants engineered to express different fHbp antigenic variants would increase the breadth of protective antibodies against N. meningitidis strains with fHbp from each of the three antigenic groups.
(These data were presented at the 16th International Pathogenic Neisseria Conference, September 2008, Rotterdam, The Netherlands.)
MATERIALS AND METHODS
Neisseria meningitidis strains.
To prepare the OMV vaccines, we used group B strains H44/76 (P1.7,16) and NZ98/254 (P1.7-2,4) and their respective mutants described below. For testing bactericidal activity, we used a panel of invasive isolates from the United States and Europe with PorA proteins heterologous to those of the vaccine strains (n = 14) (Table 1). Eight of these isolates expressed subvariants of fHbp v.1 (small numbers of amino acid differences from the sequence of the fHbp v.1 vaccine), and six strains expressed fHbp in the v.2 (n = 4) or v.3 (n = 2) group (Table 1). One of these strains, 8047, provided the gene to express fHbp v.2 in the mutant of NZ98/254, which was used to prepare the native OMV vaccine. The gene also encoded the protein used as the control recombinant fHbp v.2 vaccine. The fHbps from the other v.2 or v.3 strains differed in their respective amino acid sequences and/or monoclonal antibody (MAb) reactivities from those of fHbp v.2 from strain 8047.
TABLE 1.
Neisseria meningitidis strains from Europe, the United States, and New Zealandg
| Strain | Country of origin | Capsular group | fHbp variant group (peptide ID)d | PorA VR sequence typee | Sequence type complex | Reactivity with anti-fHbp MAbf:
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| JAR 1 | JAR 5 | JAR 10 | JAR 11 | JAR 13 | ||||||
| H44/76a | Norway | B | 1 (1) | 1.7,16 | 32 | + | + | − | − | − |
| NZ98/254a | New Zealand | B | 1 (14) | 1.7-2,4 | 41/44 | − | + | + | − | − |
| GB200 | United Kingdom | B | 1 (13) | 1.22,9 | 269 | + | + | + | − | − |
| SK66 | United States | B | 1 (ND) | 1.22,14 | 269 | − | + | − | − | − |
| SK94 | United States | B | 1 (13) | 1.5-1,10-1 | 1157 | + | + | + | − | − |
| MD1412 | United States | B | 1 (ND) | 1.22,14 | 213 | − | + | + | − | − |
| GB101 | United Kingdom | B | 1 (15) | 1.19,15 | 269 | − | + | − | − | − |
| Z1092 | Germany | A | 1 (4) | 1.5-2,10 | 1 | − | + | − | − | − |
| 4243 | United States | C | 1 (3) | 1.5,2 | 11 | − | + | − | − | − |
| M2197 | United States | C | 1 (2) | 1.7,1 | 11 | − | + | − | − | − |
| 8047b | United States | B | 2 (77) | 1.5-1,2-2 | 8 | − | − | + | + | + |
| 2996c | United Kingdom | B | 2 (16) | 1.19,15 | 8 | − | − | +/− | +/− | +/− |
| GB013 | United Kingdom | B | 2 (19) | 1.22,9 | 269 | − | − | + | + | − |
| MD1248 | United States | B | 2 (ND) | 1.22,26 | 44 | − | − | + | + | − |
| M1239 | United States | B | 3 (28) | 1.23,14 | 41/44 | − | − | − | − | + |
| 03S-0451 | United States | B | 3 (76) | 1.22-1,14 | 32 | − | − | − | − | + |
Strains used to prepare mutants for preparation of native OMV vaccines.
Source of the gene for expression of fHbp v.2 in the NZ98/254 mutant and in E. coli for recombinant fHbp v.2 (GenBank accession number FJ422922).
Low expresser of fHbp (GenBank accession number FJ422921) with an amino acid sequence that differed by 1 amino acid from that of strain 8047.
ID, identification number assigned in the fHbp peptide database (http://neisseria.org/perl/agdbnet/agdbnet.pl?file=nm_fhbp.xml&page=browse&locus=public_FHBP_p).
VR, PorA variable region sequence type (determined at http://neisseria.org).
Reactivity with anti-fHbp JAR MAbs was determined by bacterial-cell ELISA as described in the work of Beernink et al. (3) or by Western blotting (41). JAR 1 and JAR 5 were raised against recombinant fHbp v.1 (41), and JAR 10, JAR 11, and JAR 13 were raised against fHbp v.2 (2, 3). JAR 10 cross-reacts with some strains that express fHbp in the v.1 group.
ND, not done; +, an optical density 10- to 5-fold above background in a whole-bacterial-cell, anti-fHbp MAb ELISA; +/−, 3- to 5-fold above background; −, no reactivity. Strains tested by Western blotting were scored as positive (+) or negative (−).
Growth conditions.
N. meningitidis strains were grown at 37°C in an atmosphere containing 5% CO2 on gonococcal agar or in Mueller-Hinton broth supplemented with 0.25% (wt/vol) glucose and 5 μg/ml chloramphenicol or 80 μg/ml kanamycin as required.
Expression of heterologous fHbp.
To express fHbp v.2 in N. meningitidis strain NZ98/254, we used plasmid pComPind, which allowed integration of the gene of interest into the chromosome of N. meningitidis (19). The tac promoter was replaced with the strong promoter from gene nmb1523, and the resulting plasmid was designated pComP1523. The gene encoding fHbp v.2 was amplified by PCR from chromosomal DNA from strain 8047 using primers OK1NdeI (5′-GGAATTCCATATGAATCGAACTGCCTTCTGC-3′) and OK2NsiI (5′-TGCTACGTACTGTTTGCCGGCGATGCCG-3′) and ligated to plasmid pComP1523. The resulting plasmid was designated pComP1523-fHbp8047. Transformation of N. meningitidis was performed as described elsewhere (29), and transformants were selected on gonococcal agar plates containing 5 μg/ml chloramphenicol.
Vaccines.
The native OMV vaccines consisted of membrane blebs that were released by the bacteria into the broth and were prepared as described previously (28). The strains for the OMV vaccines consisted of the previously described LpxL1 KO mutant of H44/76 engineered to overexpress fHbp in the v.1 group (22) and a new LpxL1 KO mutant of strain NZ98/254, which was engineered to express both endogenous fHbp v.1 and a heterologous fHbp in the v.2 group. The NZ98/254 mutant was prepared from a clinical isolate from a group B epidemic in New Zealand. The strain was chosen for the second vaccine because its genetic lineage (as defined by multilocus sequencing type) (4) and PorA protein differed from those of the H44/76 vaccine strain (Table 1).
Control vaccines consisted of OMV vaccines prepared by detergent extraction of bacterial cells of the wild-type strains H44/76 and NZ98/254 by following the procedure described by the Norwegian Institute of Public Health, Oslo (13). Control recombinant protein vaccines consisted of fHbp v.1 and v.2, which were expressed with N-terminal six-His tags in Escherichia coli BL21(DE3) (Invitrogen, Carlsbad, CA) and purified on Ni-Sepharose columns (GE Healthcare, Piscataway, NJ).
Cytokine assay.
Cytokine release by human peripheral blood mononuclear cells (PBMCs) exposed to mixtures of the two native OMV vaccines was measured as described previously using the human 17-plex panel (Bio-Rad, Hercules, CA) (22).
Immunization.
Groups of 4- to 6-week-old female CD-1 mice (Charles River Breeding Laboratories, Calco, Italy) were immunized intraperitoneally (10 to 15 mice per group). For each injection, the mice received a total dose of 2.5 μg (based on protein content) of OMV vaccine (the mixtures contained 1.25 μg of each OMV preparation) or 20 μg of each recombinant protein. The OMV or recombinant protein vaccines were adsorbed with 3 mg of aluminum hydroxide per milliliter of sterile water containing 10 mmol/liter histidine and 9 mg/ml NaCl as previously described (22). The total dose of aluminum hydroxide per injection was 600 μg. Three injections of vaccine were given, each separated by 3 weeks. Blood was collected 3 weeks after the third injection. The experiments complied with the relevant guidelines of Italy and the institutional policies of Novartis vaccines.
Serology.
Serum samples from four to five mice were pooled (two to three pools per vaccine treatment group). Total immunoglobulin G (IgG) antibody titers to fHbp v.1, v.2, or LOS were measured by enzyme-linked immunosorbent assay (ELISA), which was performed as described elsewhere (22). The antigen on the plate consisted of purified His-tagged fHbp v.1 or v.2 or purified LOS prepared from the LpxL1 KO mutant of strain H44/76 as described previously (42). Complement-mediated serum bactericidal antibody titers were measured using washed, log-phase bacteria grown in Mueller-Hinton broth supplemented with 0.25% glucose and 0.02 mmol/liter CMP-NANA (22, 40). The human complement consisted of sera from healthy adults lacking intrinsic bactericidal activity.
Adsorption of antibodies.
For defining the antigenic targets of bactericidal antibodies, we adsorbed serum pools on columns containing purified recombinant fHbp v.1 and v.2 or purified LOS, each covalently bound to cyanogen bromide-activated agarose (Sigma, St. Louis, MO). In brief, recombinant fHbp or LOS was diluted in coupling buffer (0.1 mol/liter NaHCO3, 0.5 mol/liter NaCl; pH 8.4) and incubated with the matrix overnight at 4°C. The column was washed with coupling buffer to remove the unbound ligand. Reactive sites on the matrix were blocked with 0.1 M diethanolamine (diluted in coupling buffer), and additional nonspecific binding sites were blocked with normal mouse serum. After being washed, the matrix was incubated with the test serum sample overnight at 4°C. The adsorbed serum was eluted with Dulbecco's buffer, and the samples were concentrated to the original volumes.
RESULTS
The NZ98/254 mutant expresses fHbp in the v.1 and v.2 groups.
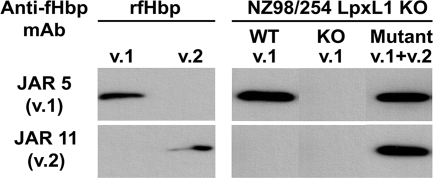
We transformed group B strain NZ98/254 with plasmid pComP1523-fHbp8047, containing a gene that encodes fHbp in the v.2 group. The strain also was engineered to have attenuated endotoxin by inactivation of the lpxL1 gene. The proteins were visualized in the OMV preparations by Western blotting with anti-fHbp MAbs JAR 5 and JAR 11, which are specific for fHbp in the v.1 and v.2 groups, respectively (2, 3, 41). Since the endogenous fHbp v.1 gene was not inactivated, the OMV vaccine prepared from the mutant expressed both fHbp v.1 and v.2 (Fig. 1, far right). The OMV vaccine prepared from the control LpxL1 KO mutant of strain NZ98/254 that expressed only endogenous fHbp v.1 reacted only with the anti-fHbp v.1 MAb (left side of right panel).
FIG. 1.
Expression of fHbp v.1 and v.2 in OMV vaccines prepared from LpxL1 KO mutants of strain NZ98/254. Primary antibodies were anti-fHbp MAbs JAR 5, which is specific for fHbp v.1 (2, 3, 41), and JAR 11, which is specific for fHbp v.2 (2, 3). NZ98/254 LpxL1 KO, native OMV from mutants of NZ98/254 with inactivated LpxL1; WT v.1, mutant expressing only endogenous wild-type fHbp v.1; KO v.1, mutant with inactivated gene encoding fHbp v.1; Mutant v.1+v.2, mutant with both endogenous fHbp v.1 and heterologous fHbp v.2; rfHbp, recombinant fHbp v.1 or v.2.
The native OMV vaccine from the NZ98/254 mutant elicits predominantly anti-fHbp v.2 antibody responses.
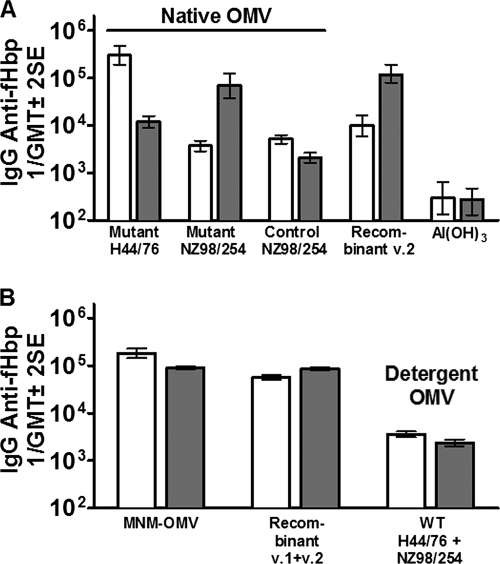
As expected, mice immunized with a native OMV vaccine prepared from the LpxL1 KO mutant of H44/76, which was engineered to overexpress fHbp in the antigenic v.1 group, developed high serum IgG antibody responses predominantly against fHbp v.1 (Fig. 2A). However, the native OMV vaccine from the NZ98/254 mutant that expressed both endogenous fHbp v.1 and heterologous fHbp v.2 elicited IgG antibody responses predominantly to fHbp v.2. The magnitude of the IgG response to fHbp v.2 was similar to that of control mice immunized with a recombinant fHbp v.2 vaccine (Fig. 2A). The control native OMV vaccine from the LpxL1 KO mutant of NZ98/254 that expressed only endogenous fHbp v.1 stimulated relatively low IgG titers against both fHbp v.1 and fHbp v.2.
FIG. 2.
IgG antibody responses to fHbp v.1 (white bars) or fHbp v.2 (gray bars) as measured by ELISA. All native OMV vaccines were from LpxL1 KO mutants. (A) Responses to individual vaccines. Mutant H44/76, OMV vaccine with overexpressed fHbp in the v.1 group; Mutant NZ98/254, OMV vaccine with endogenous fHbp v.1 and heterologous fHbp v.2; Control NZ98/254, OMV vaccine with endogenous fHbp in the v.1 group; Recombinant v.2, recombinant fHbp v.2 encoded by the gene from strain 8047; Al(OH)3, control (used as the adjuvant for OMV and recombinant protein vaccines). (B) Responses to vaccine mixtures. MNM-OMV, native OMV from H44/76 and NZ98/254 mutants with fHbp v.1 and v.2; Recombinant v.1+v.2, recombinant fHbp v.1 and v.2; Detergent OMV WT H44/76 + NZ98/254, detergent-treated OMV from the respective wild-type strains. SE, standard error.
A mixture of two OMV vaccines from mutants elicits serum IgG responses to both fHbp v.1 and fHbp v.2 and broad bactericidal activity.
We immunized mice with a mixture of two native OMV vaccines prepared from the mutants of strains H44/76 and NZ98/254. For simplicity, we designated the mixture of the two native mutant OMV vaccines the “MNM-OMV vaccine.” Mice immunized with this vaccine developed high serum IgG antibody responses to both fHbp v.1 and fHbp v.2 (Fig. 2B). Control mice immunized with a mixture of the two detergent-treated OMV vaccines from the respective wild-type strains developed 50-fold-lower IgG anti-fHbp antibody responses than mice immunized with the MNM-OMV or recombinant fHbp v.1 and v.2 vaccines.
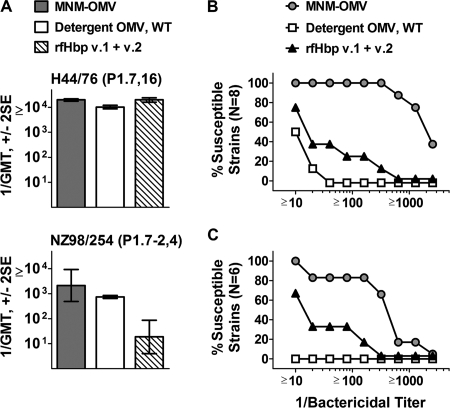
Mice immunized with either the MNM-OMV vaccine or a mixture of the two detergent-treated OMV vaccines from the wild-type strains developed high serum bactericidal titers against the parental H44/76 or NZ98/254 strain used to prepare the vaccines (Fig. 3A). The mixture of the two recombinant fHbp vaccines (v.1 and v.2) elicited high serum bactericidal titers against strain H44/76 (Fig. 3A, upper panel), which expressed fHbp v.1 with an amino acid sequence identical to that of recombinant fHbp v.1. The serum bactericidal antibody titers of the recombinant fHbp vaccine group were lower against strain NZ98/254 (Fig. 3A, lower panel), which is known to be a relatively low expresser of a subvariant of fHbp in the v.1 group (26).
FIG. 3.
Serum bactericidal antibody responses of mice immunized with vaccine mixtures. The vaccine groups were native OMVs from LpxL1 KO mutants of H44/76 and NZ98/254 expressing fHbp v.1 and v.2 (MNM-OMV), detergent-treated OMVs from the indicated wild-type strains (Detergent OMV, WT), and recombinant fHbp v.1 and 2 (rfHbp v.1 + v.2). (A) GMTs against wild-type strains used to prepare mutants for the native OMV vaccines. P1.7-2,4 and P1.7,16 refer to the variable region sequence types of the respective PorA proteins of the test strains. (B and C) Reverse cumulative distributions of bactericidal titers against strains expressing PorA proteins heterologous to those of the vaccine strains and fHbp in the antigenic v.1 group (eight strains) (B) or v.2 and v.3 groups (six strains) (C). The eight v.1 strains and six v.2 or v.3 strains are described in Table 1. SE, standard error.
Against a panel of 14 N. meningitidis strains with PorA proteins heterologous to those of the vaccine strains, the MNM-OMV vaccine elicited broad serum bactericidal antibody responses. At a 1:100 dilution, the sera were bactericidal against all eight strains tested with fHbp in the v.1 group (Fig. 3B). The geometric mean titers (GMTs) were 1:2,074, 1:28, and 1:11 for mice immunized with the MNM-OMV vaccine, the recombinant fHbp v.1 and v.2 vaccines, or the two detergent-treated OMV vaccines from the wild-type strains (P < 0.0001 by analysis of variance), respectively. Similar results were observed against the six test strains that expressed fHbp of the antigenic v.2 group (four strains) or v.3 group (two strains) (Fig. 3C). The GMTs against these strains were 1:279, 1:20, and <1:10 (P < 0.025 by analysis of variance), respectively. Although the MNM-OMV vaccine did not contain fHbp v.3, the magnitudes of the bactericidal antibody responses against the strains with fHbp v.2 or v.3 were similar (reciprocal GMTs of 235 and 359, respectively).
Serum bactericidal antibodies elicited by the MNM-OMV vaccine are directed largely against fHbp.
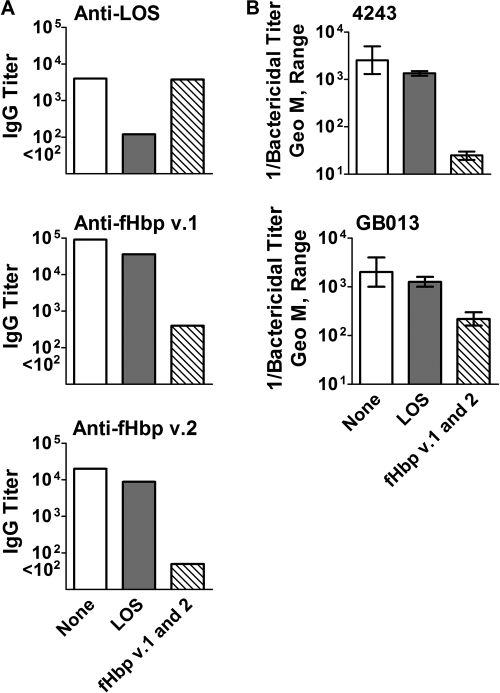
We used a solid-phase matrix with covalently bound LOS or recombinant fHbp v.1 and v.2 to adsorb antibodies from a serum pool from mice immunized with the MNM-OMV vaccine. In an ELISA, adsorption on the LOS column removed 95% of the anti-LOS antibody, and adsorption on the fHbp v.1/v.2 column removed ∼99% of the anti-fHbp antibodies (anti-v.1 reciprocal GMT of 90,000 to 400 after absorption and anti-v.2 reciprocal GMT of 20,000 to <100 after absorption) (Fig. 4A). The adsorption was specific, as there was no significant decrease in anti-LOS titers after adsorption of the serum pool on the fHbp column and there was only a twofold decrease of anti-fHbp titers after adsorption on the LOS column.
FIG. 4.
Effect of adsorption of anti-LOS or anti-fHbp antibodies from a serum pool from mice immunized with the MNM-OMV vaccine. (A) IgG antibody titers to LOS, fHbp v.1, or fHbp v.2 as measured by ELISA. (B) Serum bactericidal titers against strains 4243 (fHbp v.1) and GB013 (fHbp v.2). None, unadsorbed serum; LOS, serum adsorbed on a LOS column; fHbp v.1 and v.2, serum adsorbed on a fHbp v.1 plus fHbp v.2 column. The error bars in panel B represent the range in results of replicate assays performed on different days. Geo M, geometric mean.
Against both group B strains tested, the respective bactericidal GMTs of the unadsorbed and LOS-adsorbed serum pools were similar to each other (Fig. 4B). In contrast, adsorption of the anti-fHbp antibodies removed nearly all of the bactericidal activity against strain 4243, which expressed fHbp in the v.1 group (Fig. 4B, upper panel), while the titer of the fHbp-adsorbed serum decreased by approximately 10-fold against a second strain, GB013, which expressed fHbp in the v.2 group (Fig. 4B, lower panel). The residual bactericidal activity against GB013 indicated that antibodies in addition to those directed at fHbp contributed to the bactericidal activity.
PBMCs have similar cytokine responses when exposed to the MNM-OMV vaccine or detergent-treated OMV vaccines from the wild-type strains.
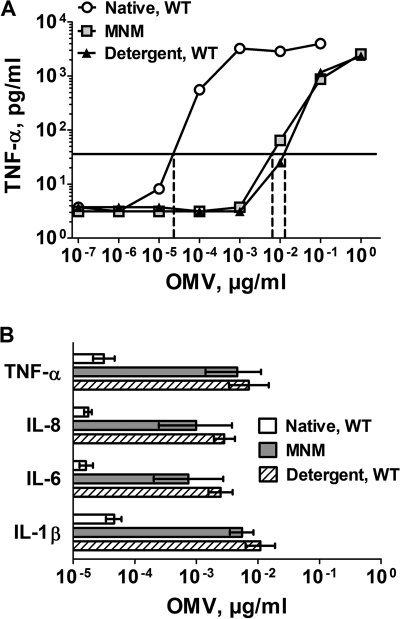
In our previous study, the native OMV vaccine from a mutant of H44/76 in which the LpxL1 gene had been inactivated had substantially decreased proinflammatory activity as measured by the release of the cytokines interleukin 1β (IL-1β), IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) by OMV-stimulated human PBMCs. For the present study, we analyzed the release of these proinflammatory cytokines by human PBMCs exposed to the MNM-OMV vaccine. The TNF-α responses from a representative experiment are shown in Fig. 5A. The concentrations of the MNM-OMV vaccine and the mixture of the two detergent-treated OMV vaccines, which stimulated cytokine responses 10-fold higher than the background level, were similar for each of the vaccines (∼10−2 μg/ml). A much lower concentration of the control native OMV vaccine from the NZ98/254 wild-type strain (∼5 × 10−4 μg/ml) stimulated the release of the same concentration of TNF-α as that elicited by the two other vaccines (Fig. 5A). The OMV concentrations required for release of IL-1β, IL-6, IL-8, and TNF-α were measured in three experiments, each with PBMCs from different donors (Fig. 5B). For each cytokine, the geometric mean concentrations of the native wild-type OMV that elicited responses 10-fold above background were 50- to 100-fold lower than those of the detergent-treated OMV or MNM-OMV vaccines (P < 0.005). The differences between the respective geometric mean OMV concentrations of the mixture of the detergent-treated OMV and MNM-OMV vaccines were not significant (P > 0.15).
FIG. 5.
(A) Release of TNF-α after incubation of human PBMCs for 4 hours with OMV vaccines. The OMV concentrations that resulted in a 10-fold-increased release of TNF-α concentrations above background are shown on the x intercepts. Native, WT, native OMV from wild-type NZ98/254; MNM, MNM-OMV vaccine; Detergent, WT, mixture of two detergent-extracted OMV vaccines from wild-type strains of H44/76 and NZ98/254. (B) Concentrations of OMV that resulted in a 10-fold-increased release of the proinflammatory cytokines TNF-α, IL-1β, IL-6, and IL-8. The bars represent the geometric mean concentrations determined in three experiments with different blood donors. Error bars indicate two times the standard error. Native, WT, native OMV from wild-type NZ98/254; MNM, MNM-OMV vaccine; Detergent, WT, mixture of two detergent-extracted OMV vaccines from wild-type strains of H44/76 and NZ98/254. Differences between the GMTs of MNM-OMV vaccine and detergent-treated OMV from the wild-type strain were not significant (P > 0.15).
DISCUSSION
As a vaccine antigen, fHbp is unique in that antibodies to fHbp both activate classical complement pathway bacteriolysis and block the binding of fH, which increases the susceptibility of the bacteria to bacteriolysis by the alternative complement pathway (2, 40). However, in previous studies, coverage by anti-fHbp antibodies elicited by recombinant fHbp vaccines was incomplete against strains with low levels of fHbp expression. One approach to extend coverage is to include additional antigens in the vaccine, such as recombinant NadA, GNA 2132 (14), and a detergent-treated OMV vaccine (M. D. Snape et al., presented at the 16th International Pathogenic Neisseria Conference, Rotterdam, The Netherlands, 7 to 12 September 2008). Another approach is to prepare native OMV vaccines from mutants in which endotoxin is attenuated and that overexpress fHbp, such as described in our previous study (22). However, the vaccine in that study expressed only fHbp v.1, and coverage was incomplete against strains with fHbp in the v.2 or v.3 group.
To expand protection, we constructed a new LpxL1 KO mutant of strain NZ98/254, which expressed a heterologous fHbp in the v.2 group. Since we did not inactivate the endogenous fHbp gene, the mutant also expressed fHbp v.1. The immunized mice had high IgG antibody responses to fHbp v.2 but minimal responses to fHbp v.1 (Fig. 2A). Control mice immunized with a native OMV vaccine prepared from a mutant of NZ98/254 that expressed only endogenous fHbp v.1 had low IgG anti-fHbp antibody responses. These results and our previous data (18, 23) indicated that overexpression of fHbp, or expression of a heterologous fHbp gene, was required to increase OMV anti-fHbp antibody responses. The mechanisms underlying the low anti-fHbp antibody responses to native OMV vaccines containing endogenous fHbp require additional study. Note that most humans recovering from meningococcal disease also showed relatively low anti-fHbp antibody responses (24). Collectively, these observations suggest that fHbp is under relatively weak natural immune selection, which may help to explain why fHbp expressed by genetically diverse strains is relatively well conserved within the major antigenic groups (3, 26).
In mice, the MNM-OMV vaccine elicited high IgG antibody responses against both v.1 and v.2 fHbp (Fig. 2B) and high serum bactericidal antibody activity against genetically diverse N. meningitidis strains with fHbp in each of the three variant groups. For those studies, we tested predominantly group B strains and a few group A and C strains (Table 1). The test strains were selected to have PorA proteins heterologous to those of the strains used to prepare the OMV vaccines. From previous studies, it was well established that OMV vaccines (native or detergent treated) elicited high bactericidal antibody responses against strains with PorA matched to those of the OMV vaccine (17, 18, 28, 37). Based on serum adsorption studies, a large portion of the bactericidal activity elicited by the MNM-OMV vaccine against strains with heterologous PorA was directed at fHbp. This result was similar to our previous results with sera from mice immunized with individual native OMV vaccines from mutants (18, 22, 23). Other investigators have reported that antibodies to meningococcal LOS can have bactericidal activity (7, 30, 46; W. D. Zollinger, M. Donets, B. L. Brandt, B. Ionin, E. E. Moran, D. Schmiel, V. Pinto, M. Fisseha, L. Labrie, R. Marques, and P. Keiser, presented at the 16th International Pathogenic Neisseria Conference, Rotterdam, The Netherlands, 7 to 12 September 2008). However, depletion of anti-LOS antibodies in the immune serum from mice immunized in the present study did not decrease bactericidal activity (Fig. 5B). This result was consistent with previous LOS antibody depletion studies of sera from mice immunized sequentially with native OMV vaccines from three strains, each with heterologous PorA molecules (28).
The anti-fHbp antibodies elicited by the native OMV vaccine had greater bactericidal activity than those elicited in control mice immunized with a recombinant fHbp v.1 and v.2 vaccine. One possibility is that naturally occurring adjuvants in the OMV, such as PorB (5, 27, 33, 43) and LOS (35), contributed to the broader anti-OMV serum bactericidal activity. However, the magnitudes of the respective IgG anti-fHbp titers elicited by the MNM-OMV and recombinant fHbp vaccines were similar to each other. These results suggested that qualitative differences, not quantitative differences in antibody responses, were responsible for the greater antibody functional activity of the sera from the MNM-OMV group. Although not measured in the present study, in a previous study, the IgG subclass distributions of anti-fHbp antibodies elicited by a native OMV vaccine from a mutant with overexpressed fHbp v.1 were similar to those elicited by a control recombinant fHbp vaccine (18). Thus, differences in IgG subclass response did not appear to contribute to the greater serum bactericidal activity elicited by the OMV vaccine.
Three factors may explain the greater serum bactericidal activity elicited by the OMV vaccine than that elicited by the control recombinant fHbp vaccines. First, not all of the epitopes required for eliciting serum bactericidal antibody may have been expressed by the control recombinant fHbp vaccines. Second, the anti-fHbp antibodies elicited by the MNM-OMV vaccine may have targeted surface-exposed epitopes better than the antibodies elicited by the control recombinant fHbp vaccines. A third possible contributing factor may have been antibodies stimulated by other antigens in the MNM-OMV vaccine, such as transferrin binding protein, NspA, or GNA 2123, which could be bactericidal or cooperate with the anti-fHbp antibodies to elicit broader bactericidal activity (44).
Although the MNM-OMV vaccine contained only fHbp antigens in the v.1 and v.2 groups, the immunized mice developed serum bactericidal activity against strains with fHbp from all three antigenic groups. This result was expected. There is more amino acid sequence identity and cross-reactivity between fHbps in the v.2 and v.3 groups than between fHbps in the v.1 and v.2 groups or between fHbps in the v.1 and v.3 groups (1, 3, 22, 26), which is one reason that the Zlotnick antigenic classification system combined v.2 and v.3 fHbps into a single subfamily, designated “A” (11). Our data indicated that a native OMV vaccine with a heterologous fHbp in the v.2 group may be sufficient for eliciting protective antibodies against strains with either v.2 or v.3 fHbp. However, the results were based on testing a small number of strains in each of the antigenic groups, and additional studies are required.
In summary, the LpxL1 KO mutation in the MNM-OMV vaccine provided a means to attenuate endotoxin activity, which may circumvent the need for detergent treatment of the vaccine. The vaccine elicited broad serum bactericidal antibodies in mice against genetically diverse N. meningitidis strains. However, one needs to be cautious in extrapolating immunogenicity results from mice to humans since there can be important species differences in responses to adjuvants and T-lymphocyte receptor signaling (34). Thus, the contribution of naturally occurring adjuvants, such as PorB (5, 27, 33), lipid A (34, 47), or the lipid portion of fHbp, to the ability of the MNM-OMV vaccine to elicit broad anti-fHbp bactericidal responses in humans is not known. Additional studies with animal models, including nonhuman primates, or a clinical trial is required to assess the potential safety and effectiveness of this vaccine approach in humans.
Acknowledgments
This work was supported, in part, by Public Health Service grant R01 AI46464 from the National Institute of Allergy and Infectious Diseases, NIH.
Footnotes
Published ahead of print on 24 December 2008.
REFERENCES
- 1.Beernink, P. T., and D. M. Granoff. 2008. Bactericidal antibody responses induced by meningococcal recombinant chimeric factor H-binding protein vaccines. Infect. Immun. 762568-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beernink, P. T., J. A. Welsch, M. Bar-Lev, O. Koeberling, M. Comanducci, and D. M. Granoff. 2008. Fine antigenic specificity and cooperative bactericidal activity of monoclonal antibodies directed at the meningococcal vaccine candidate factor H-binding protein. Infect. Immun. 764232-4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beernink, P. T., J. A. Welsch, L. H. Harrison, A. Leipus, S. L. Kaplan, and D. M. Granoff. 2007. Prevalence of factor H-binding protein variants and NadA among meningococcal group B isolates from the United States: implications for the development of a multicomponent group B vaccine. J. Infect. Dis. 1951472-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brehony, C., K. A. Jolley, and M. C. Maiden. 2007. Multilocus sequence typing for global surveillance of meningococcal disease. FEMS Microbiol. Rev. 3115-26. [DOI] [PubMed] [Google Scholar]
- 5.Burke, J. M., L. M. Ganley-Leal, A. Khatri, and L. M. Wetzler. 2007. Neisseria meningitidis PorB, a TLR2 ligand, induces an antigen-specific eosinophil recall response: potential adjuvant for helminth vaccines? J. Immunol. 1793222-3230. [DOI] [PubMed] [Google Scholar]
- 6.Cartwright, K., R. Morris, H. Rumke, A. Fox, R. Borrow, N. Begg, P. Richmond, and J. Poolman. 1999. Immunogenicity and reactogenicity in UK infants of a novel meningococcal vesicle vaccine containing multiple class 1 (PorA) outer membrane proteins. Vaccine 172612-2619. [DOI] [PubMed] [Google Scholar]
- 7.Cox, A. D., W. Zou, M. A. Gidney, S. Lacelle, J. S. Plested, K. Makepeace, J. C. Wright, P. A. Coull, E. R. Moxon, and J. C. Richards. 2005. Candidacy of LPS-based glycoconjugates to prevent invasive meningococcal disease: developmental chemistry and investigation of immunological responses following immunization of mice and rabbits. Vaccine 235045-5054. [DOI] [PubMed] [Google Scholar]
- 8.de Kleijn, E. D., R. de Groot, J. Labadie, A. B. Lafeber, G. van den Dobbelsteen, L. van Alphen, H. van Dijken, B. Kuipers, G. W. van Omme, M. Wala, R. Juttmann, and H. C. Rumke. 2000. Immunogenicity and safety of a hexavalent meningococcal outer-membrane-vesicle vaccine in children of 2-3 and 7-8 years of age. Vaccine 181456-1466. [DOI] [PubMed] [Google Scholar]
- 9.de Kleijn, E. D., R. de Groot, A. B. Lafeber, J. Labadie, C. J. van Limpt, J. Visser, G. A. Berbers, L. van Alphen, and H. C. Rumke. 2001. Prevention of meningococcal serogroup B infections in children: a protein-based vaccine induces immunologic memory. J. Infect. Dis. 18498-102. [DOI] [PubMed] [Google Scholar]
- 10.Finne, J., M. Leinonen, and P. H. Makela. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii355-357. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher, L. D., L. Bernfield, V. Barniak, J. E. Farley, A. Howell, M. Knauf, P. Ooi, R. P. Smith, P. Weise, M. Wetherell, X. Xie, R. Zagursky, Y. Zhang, and G. W. Zlotnick. 2004. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect. Immun. 722088-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frasch, C., L. Van Alphen, J. Holst, J. Poolman, and E. Rosenqvist. 2001. Outer membrane protein vesicle vaccines for meningococcal disease, p. 81-107. In A. J. Pollard and M. C. Maiden (ed.), Meningococcal vaccines: methods and protocols. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 13.Fredriksen, J. H., E. Rosenqvist, E. Wedege, K. Bryn, G. Bjune, L. O. Froholm, A. K. Lindbak, B. Mogster, E. Namork, U. Rye, et al. 1991. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 1467-80. [PubMed] [Google Scholar]
- 14.Giuliani, M. M., J. Adu-Bobie, M. Comanducci, B. Arico, S. Savino, L. Santini, B. Brunelli, S. Bambini, A. Biolchi, B. Capecchi, E. Cartocci, L. Ciucchi, F. Di Marcello, F. Ferlicca, B. Galli, E. Luzzi, V. Masignani, D. Serruto, D. Veggi, M. Contorni, M. Morandi, A. Bartalesi, V. Cinotti, D. Mannucci, F. Titta, E. Ovidi, J. A. Welsch, D. Granoff, R. Rappuoli, and M. Pizza. 2006. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. USA 10310834-10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Granoff, D. M., J. A. Welsch, and S. Ram. 2009. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect. Immun. 77764-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayrinen, J., H. Jennings, H. V. Raff, G. Rougon, N. Hanai, R. Gerardy-Schahn, and J. Finne. 1995. Antibodies to polysialic acid and its N-propyl derivative: binding properties and interaction with human embryonal brain glycopeptides. J. Infect. Dis. 1711481-1490. [DOI] [PubMed] [Google Scholar]
- 16.Holst, J. 2007. Strategies for development of universal vaccines against meningococcal serogroup B disease: the most promising options and the challenges evaluating them. Hum. Vaccines 3290-294. [DOI] [PubMed] [Google Scholar]
- 17.Holst, J., B. Feiring, L. M. Naess, G. Norheim, P. Kristiansen, E. A. Hoiby, K. Bryn, P. Oster, P. Costantino, M. K. Taha, J. M. Alonso, D. A. Caugant, E. Wedege, I. S. Aaberge, R. Rappuoli, and E. Rosenqvist. 2005. The concept of “tailor-made,” protein-based, outer membrane vesicle vaccines against meningococcal disease. Vaccine 232202-2205. [DOI] [PubMed] [Google Scholar]
- 18.Hou, V. C., O. Koeberling, J. A. Welsch, and D. M. Granoff. 2005. Protective antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed genome-derived neisserial antigen 1870. J. Infect. Dis. 192580-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ieva, R., C. Alaimo, I. Delany, G. Spohn, R. Rappuoli, and V. Scarlato. 2005. CrgA is an inducible LysR-type regulator of Neisseria meningitidis, acting both as a repressor and as an activator of gene transcription. J. Bacteriol. 1873421-3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jodar, L., I. Feavers, D. Salisbury, and D. M. Granoff. 2002. Development of vaccines against meningococcal disease. Lancet 3591499-1508. [DOI] [PubMed] [Google Scholar]
- 21.Kelly, C., R. Arnold, Y. Galloway, and J. O'Hallahan. 2007. A prospective study of the effectiveness of the New Zealand meningococcal B vaccine. Am. J. Epidemiol. 166817-823. [DOI] [PubMed] [Google Scholar]
- 22.Koeberling, O., A. Seubert, and D. M. Granoff. 2008. Bactericidal antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed factor H-binding protein and genetically attenuated endotoxin. J. Infect. Dis. 198262-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koeberling, O., J. A. Welsch, and D. M. Granoff. 2007. Improved immunogenicity of a H44/76 group B outer membrane vesicle vaccine with over-expressed genome-derived Neisserial antigen 1870. Vaccine 251912-1920. [DOI] [PubMed] [Google Scholar]
- 24.Litt, D. J., S. Savino, A. Beddek, M. Comanducci, C. Sandiford, J. Stevens, M. Levin, C. Ison, M. Pizza, R. Rappuoli, and J. S. Kroll. 2004. Putative vaccine antigens from Neisseria meningitidis recognized by serum antibodies of young children convalescing after meningococcal disease. J. Infect. Dis. 1901488-1497. [DOI] [PubMed] [Google Scholar]
- 25.Madico, G., J. A. Welsch, L. A. Lewis, A. McNaughton, D. H. Perlman, C. E. Costello, J. Ngampasutadol, U. Vogel, D. M. Granoff, and S. Ram. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 177501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masignani, V., M. Comanducci, M. M. Giuliani, S. Bambini, J. Adu-Bobie, B. Arico, B. Brunelli, A. Pieri, L. Santini, S. Savino, D. Serruto, D. Litt, S. Kroll, J. A. Welsch, D. M. Granoff, R. Rappuoli, and M. Pizza. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197789-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massari, P., A. Visintin, J. Gunawardana, K. A. Halmen, C. A. King, D. T. Golenbock, and L. M. Wetzler. 2006. Meningococcal porin PorB binds to TLR2 and requires TLR1 for signaling. J. Immunol. 1762373-2380. [DOI] [PubMed] [Google Scholar]
- 28.Moe, G. R., P. Zuno-Mitchell, S. N. Hammond, and D. M. Granoff. 2002. Sequential immunization with vesicles prepared from heterologous Neisseria meningitidis strains elicits broadly protective serum antibodies to group B strains. Infect. Immun. 706021-6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pagotto, F. J., H. Salimnia, P. A. Totten, and J. R. Dillon. 2000. Stable shuttle vectors for Neisseria gonorrhoeae, Haemophilus spp. and other bacteria based on a single origin of replication. Gene 24413-19. [DOI] [PubMed] [Google Scholar]
- 30.Plested, J. S., B. L. Ferry, P. A. Coull, K. Makepeace, A. K. Lehmann, F. G. MacKinnon, H. G. Griffiths, M. A. Herbert, J. C. Richards, and E. R. Moxon. 2001. Functional opsonic activity of human serum antibodies to inner core lipopolysaccharide (galE) of serogroup B meningococci measured by flow cytometry. Infect. Immun. 693203-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sacchi, C. T., A. M. Whitney, T. Popovic, D. S. Beall, M. W. Reeves, B. D. Plikaytis, N. E. Rosenstein, B. A. Perkins, M. L. Tondella, and L. W. Mayer. 2000. Diversity and prevalence of PorA types in Neisseria meningitidis serogroup B in the United States, 1992-1998. J. Infect. Dis. 1821169-1176. [DOI] [PubMed] [Google Scholar]
- 32.Schneider, M. C., R. M. Exley, H. Chan, I. Feavers, Y. H. Kang, R. B. Sim, and C. M. Tang. 2006. Functional significance of factor H binding to Neisseria meningitidis. J. Immunol. 1767566-7575. [DOI] [PubMed] [Google Scholar]
- 33.Singleton, T. E., P. Massari, and L. M. Wetzler. 2005. Neisserial porin-induced dendritic cell activation is MyD88 and TLR2 dependent. J. Immunol. 1743545-3550. [DOI] [PubMed] [Google Scholar]
- 34.Steeghs, L., A. M. Keestra, A. van Mourik, H. Uronen-Hansson, P. van der Ley, R. Callard, N. Klein, and J. P. van Putten. 2008. Differential activation of human and mouse Toll-like receptor 4 by the adjuvant candidate LpxL1 of Neisseria meningitidis. Infect. Immun. 763801-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steeghs, L., J. Tommassen, J. H. Leusen, J. G. van de Winkel, and P. van der Ley. 2004. Teasing apart structural determinants of ‘toxicity’ and ‘adjuvanticity’: implications for meningococcal vaccine development. J. Endotoxin Res. 10113-119. [DOI] [PubMed] [Google Scholar]
- 36.Taha, M. K., M. L. Zarantonelli, J. M. Alonso, L. M. Naess, J. Holst, B. Feiring, and E. Rosenqvist. 2007. Use of available outer membrane vesicle vaccines to control serogroup B meningococcal outbreaks. Vaccine 252537-2538. [DOI] [PubMed] [Google Scholar]
- 37.Tappero, J. W., R. Lagos, A. M. Ballesteros, B. Plikaytis, D. Williams, J. Dykes, L. L. Gheesling, G. M. Carlone, E. A. Hoiby, J. Holst, H. Nokleby, E. Rosenqvist, G. Sierra, C. Campa, F. Sotolongo, J. Vega, J. Garcia, P. Herrera, J. T. Poolman, and B. A. Perkins. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 2811520-1527. [DOI] [PubMed] [Google Scholar]
- 38.van der Ley, P., L. Steeghs, H. J. Hamstra, J. ten Hove, B. Zomer, and L. van Alphen. 2001. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect. Immun. 695981-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welsch, J. A., G. R. Moe, R. Rossi, J. Adu-Bobie, R. Rappuoli, and D. M. Granoff. 2003. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J. Infect. Dis. 1881730-1740. [DOI] [PubMed] [Google Scholar]
- 40.Welsch, J. A., S. Ram, O. Koeberling, and D. M. Granoff. 2008. Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J. Infect. Dis. 1971053-1061. [DOI] [PubMed] [Google Scholar]
- 41.Welsch, J. A., R. Rossi, M. Comanducci, and D. M. Granoff. 2004. Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine. J. Immunol. 1725606-5615. [DOI] [PubMed] [Google Scholar]
- 42.Westphal, O., K. Jann, and K. Himmelspach. 1983. Chemistry and immunochemistry of bacterial lipopolysaccharides as cell wall antigens and endotoxins. Prog. Allergy 339-39. [PubMed] [Google Scholar]
- 43.Wetzler, L. M. 1994. Immunopotentiating ability of neisserial major outer membrane proteins. Use as an adjuvant for poorly immunogenic substances and potential use in vaccines. Ann. N. Y. Acad. Sci. 730367-370. [DOI] [PubMed] [Google Scholar]
- 44.Weynants, V. E., C. M. Feron, K. K. Goraj, M. P. Bos, P. A. Denoel, V. G. Verlant, J. Tommassen, I. R. Peak, R. C. Judd, M. P. Jennings, and J. T. Poolman. 2007. Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of Neisseria meningitidis. Infect. Immun. 755434-5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu, D., Y. Zhang, V. Barniak, L. Bernfield, A. Howell, and G. Zlotnick. 2005. Evaluation of recombinant lipidated P2086 protein as a vaccine candidate for group B Neisseria meningitidis in a murine nasal challenge model. Infect. Immun. 736838-6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zollinger, W. D., E. E. Moran, S. J. Devi, and C. E. Frasch. 1997. Bactericidal antibody responses of juvenile rhesus monkeys immunized with group B Neisseria meningitidis capsular polysaccharide-protein conjugate vaccines. Infect. Immun. 651053-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zughaier, S., L. Steeghs, P. van der Ley, and D. S. Stephens. 2007. TLR4-dependent adjuvant activity of Neisseria meningitidis lipid A. Vaccine 254401-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]