Abstract
Sera from patients involved in a Peruvian outbreak of dengue virus serotype 1 infection cross-neutralized the American genotype of dengue virus serotype 2 up to 100-fold more efficiently than they did the virulent Asian genotype of dengue virus serotype 2, as determined by a plaque reduction neutralization test (PRNT) with CV-1 fibroblasts modified to express human Fcγ receptor CD32. The concordant preferential immune enhancement of the Asian genotype of dengue virus serotype 2 in human monocytes suggests that such a modification might strengthen the correlation between the PRNT titer and protection.
Dengue fever is typically a self-limited, if incapacitating, illness, but a life-threatening hemorrhagic form (dengue hemorrhagic fever [DHF]) may complicate infection, especially in children. Dengue viruses (DENVs) are single positive-strand RNA viruses that exist as four antigenically distinct serotypes (DENV serotype 1 [DENV-1] to DENV-4) of considerable genotypic diversity that may contribute to pathogenicity (for a review, see reference 10). Durable protection is serotype specific, so that sequential infections with the four DENV serotypes are theoretically possible. The incidence of DHF has been linked to the introduction of new DENV serotypes into a region where dengue is endemic, with the sequence of DENV-1 infection followed by DENV-2 infection being particularly associated with more virulent Southeast Asian dengue epidemics (14). Asian genotype DENV-2 strains have been displacing the relatively more benign American genotype DENV-2 strains in the Americas, notably, accounting for the catastrophic 1981 DENV-2 epidemic in Cuba that followed an uneventful DENV-1 outbreak on the island 4 years earlier (3). The immune enhancement hypothesis offers an explanation for the paradox of increased disease severity in a population immune to DENV: it posits enhanced infection of Fcγ receptor (FcγR)-expressing monocytes (the primary DENV target cell type) by DENV immune complexes (ICs) comprised of antibodies from an earlier heterotypic DENV infection. It was therefore somewhat surprising that DHF was not observed during the 1995 DENV-2 outbreak in Iquitos, Peru, that followed a similarly large but benign primary DENV-1 epidemic (caused by strain IQT6152) 4 years previously (15). The new virus was an American genotype DENV-2 strain (strain IQT2913). Postoutbreak monotypic DENV-1-immune sera exhibited substantial neutralizing activity against the virus by the conventional 50% plaque reduction neutralization test (PRNT) performed with BHK-21 cells (5). Notably, these DENV-1-immune sera were found to exhibit less neutralizing activity against the prototypic virulent Asian genotype DENV-2 strain (strain 16681) (4).
Since the monocyte display of FcγR may be a pivotal determinant of whether DENV ICs are infectious or are neutralized in vivo, we reasoned that the introduction of FcγR expression into a conventional DENV PRNT that employs cells that lack Fc receptors would magnify the previously observed neutralization disparity between the American and Asian genotypes of DENV-2 by sera from the DENV-1 IQT6152-immune cohort. For this purpose, we selected CV-1 cells, which, like their simian virus 40-transformed COS cell derivative (12), we have found exhibit macrophage-like properties, such as FcγR signaling-dependent ingestion of antibody-coated particles (W. W. Rodrigo and J. J. Schlesinger, unpublished data) and DENV-stimulated alpha/beta interferon production (M. Quinn and X. Jin, unpublished data).
In the study described here, we reassayed sera from individuals involved in the Iquitos, Peru, DENV-1 outbreak for neutralizing activity against DENV-1 and American and Asian genotype DENV-2 strains IQT2913 and 16681, respectively, in FcγR-expressing CV-1 cells. These sera, collected in 1993 and 1994 (i.e., 2 to 3 years after the DENV-1 IQT6152 outbreak), were considered to reflect monotypic DENV infection since they were DENV-1 immunoglobulin G (IgG) positive and IgM negative and exhibited neutralizing activity only against DENV-1 (5). Because the amounts of sera remaining from the cohort were very small, equal volumes (∼50 to 100 μl) of 12 individual serum samples were pooled and assayed by using a microneutralization PRNT method and an automated enzyme-linked immunospot assay format that employs CV-1 cells stably transfected with human FcγRIIA (CD32) (11).
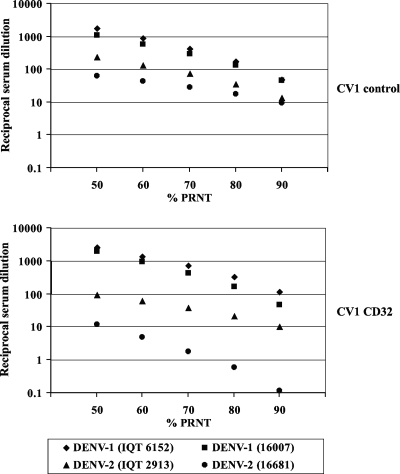
Figure 1 shows the 50 to 90% PRNT endpoint titers of pooled DENV-1 antibodies measured against DENV-1 and DENV-2 strains of the American and the Asian genotypes. The results obtained with control CV-1 cells were similar to those obtained earlier with BHK-21 cells (5): the neutralizing activity over the range of PRNT endpoint titers was greatest against DENV-1 of the American genotype (strain IQT6152) and the Asian genotype (strain 16007), the cross-neutralization of DENV-2 IQT2913 was comparable (P = 0.10, Mann-Whitney U-test), and the cross-neutralization of Asian genotype DENV-2 16681 was significantly reduced (P = 0.02). Notably, this hierarchical virus neutralization pattern was magnified in CD32-expressing CV-1 cells. The neutralization profile for DENV-1 in these cells was essentially unchanged from that in control CV-1 cells, but the amounts of antibody required to neutralize DENV-2 16681 were strikingly increased, ∼10- to 100-fold (P = 0.01), over the range of PRNT determinations. The concentrations of antiserum required for neutralization were also somewhat increased (∼10-fold) for DENV-2 IQT2913 (P = 0.01). The results of PRNT with another contemporaneously circulating Peruvian DENV-2 strain, strain IQT2124, were identical to those obtained with strain IQT2913 (data not shown).
FIG. 1.
Differential neutralization of the American and Asian genotypes of DENV-1 and DENV-2 by monotypic DENV-1 antibodies from the 1995 Iquitos, Peru, DENV-1 outbreak (5). Pooled DENV-1-immune serum samples (n = 12) were titrated against DENV-1 strain IQT6152 or 16007 and American genotype DENV-2 strain IQT2913 or Asian genotype DENV-2 strain 16681 in a plaque reduction microneutralization test (11) performed with control and CD32-expressing CV-1 cell monolayers. Endpoint serum dilutions corresponding to 50% to 90% PRNT titers were calculated by probit analysis of triplicate samples with GraphPad Prism5 software. The results of PRNTs were compared by the Mann-Whitney U test by the use of (i) control cells and American genotype DENV-1 6152 versus DENV-2 IQT2913 (P = 0.10) and American genotype DENV-1 6152 versus DENV-2 16681 (P = 0.02) and (ii) CD32-expressing CV-1 cells and DENV-1 6152 versus DENV-2 IQT2913 (P = 0.01) and DENV-1 6152 versus DENV-2 16681 (P = 0.01). The results are representative of those from two independent experiments.
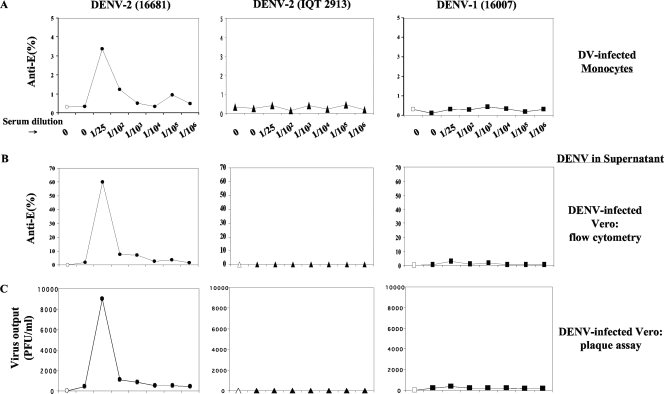
In parallel experiments, we measured the capacities of these DENV-1 antibodies to enhance Asian genotype DENV-2 16681 and American genotype DENV-2 IQT2913 infection and replication in human monocytes by flow cytometry and by an amplification plaque assay with Vero cells using previously reported methods (6). Figure 2 shows the DENV (multiplicity of infection [MOI], 2.0) and DENV IC infectivity results obtained with serially diluted monotypic DENV-1 immune serum and DENV-2 16681 or DENV-2 IQT2913 and DENV-1 (16007). DENV-1 antibodies substantially increased (∼10-fold) the percentage of DENV-2 16681-infected CD14-positive monocytes (Fig. 2A), but DENV-1 16007- and DENV-2 IQT2913-infected monocytes were not detected. In parallel with the increased number of Asian genotype DENV-2 16681-infected monocytes, surrogate amplification assays with Vero cells revealed the enhanced replication of Asian genotype DENV-2 16681 (Fig. 2B and C) at low serum dilutions (1/25, 1/100, 1/1,000) similar to those that only weakly neutralized Asian genotype DENV-2 16681 in the CD32-expressing CV-1 cells. A trivial degree of enhancement was also observed with DENV-1 16007, but little or no measurable American genotype DENV-2 IQT2913 was present in the monocyte supernatants, as determined by surrogate amplification assays with Vero cells (Fig. 2B and C). Relatively low-level DENV-2 IQT2913 replication in human monocytes compared to that of DENV-2 16681 has been observed by use of the much more sensitive quantitative reverse transcription-PCR assay (2).
FIG. 2.
Antibody-dependent enhancement of the American and Asian genotypes of DENV-1 or DENV-2 in human monocytes. (A) DENV ICs were formed with DENV-2 IQT2913, DENV-2 16681, or DENV-1 16007 and serially diluted human monotypic DENV-1 immune serum. The percentages of CD14-positive monocytes infected with DENV-1 (MOI, 2.0) or DENV-2 ICs were determined by a previously described flow cytometry method (6). Open symbols indicate mock infection. DV, dengue virus. (B) The amount of virus released from monocytes after 48 h was measured with directly inoculated Vero cells by flow cytometry, as described previously (6). (C) The amount of virus released from monocytes after 48 h was measured by titrating supernatants in duplicate in a Vero cell plaque assay (11).
Conventional DENV neutralization assays employ mammalian cell types that do not express Fc receptors (e.g., Vero, LLC-MK2, and BHK cells) and often use DENV genotypes of uncertain virulence. It is generally acknowledged that the correlation between protection and the neutralizing antibody titer is imperfect (13). This may not be surprising, since in vivo, DENV target monocytes display FcγRs that mediate the infectivity of DENV ICs. Furthermore, DENV neutralization is very likely governed by mechanics that differ between cells that display FcγRs and those that do not. For example, neutralization is ordinarily a measure of antibody blockade of virus attachment or fusion (or both) (9), but the FcγR-mediated entry of DENV and other viruses can involve FcγR-specific signaling pathways (12) and may trigger regulatory mechanisms of innate immunity that influence the fate of the internalized virus IC (1, 7). Notable in this regard is the capacity of DENV-2 16681 NS2A, NS4A, and NS4B to inhibit alpha/beta interferon signaling, a property that might contribute to its virulence by suppressing a key innate immune mechanism (8). The fact that the DENV-2 16681 ICs formed with DENV-1 antibodies were disproportionately much less susceptible to neutralization in CD32-expressing CV-1 cells than DENV-2 IQT2913 ICs invites speculation that such mechanisms might also be contributing to the magnified divergence in neutralization observed between the CD32-expressing cells and the control CV-1 cells used in this assay. We are currently investigating whether the Asian and American genotypes of DENV-2, free and antibody coated, differ in their capacities to modulate alpha/beta interferon and other elements of innate immune pathways in FcγR-expressing CV-1 cells. Ultimately, whether the introduction of the human FcγR display to conventional neutralization assays will improve the correlation between PRNT titers and protection (or immune enhancement) will require verification in studies with well-characterized field sera and DENV isolates.
Acknowledgments
This work was funded by the Pediatric Dengue Vaccine Initiative of the International Vaccine Institute, awards TR 03/04 (to J.J.S.) and TR16 (to X.J.).
T.J.K. and K.R.P. are military service members or employees of the U.S. government. This work was prepared as part of their official duties.
We declare no conflicts of interest.
Footnotes
Published ahead of print on 26 November 2008.
REFERENCES
- 1.Chareonsirisuthigul, T., S. Kalayanarooj, and S. Ubol. 2007. Dengue virus (DENV) antibody-dependent enhancement of infection upregulates the production of anti-inflammatory cytokines, but suppresses anti-DENV free radical and pro-inflammatory cytokine production, in THP-1 cells. J. Gen. Virol. 88365-375. [DOI] [PubMed] [Google Scholar]
- 2.Cologna, R., and R. Rico-Hesse. 2003. American genotype structures decrease dengue virus output from human monocytes and dendritic cells. J. Virol. 773929-3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzman, M. G., G. P. Kouri, J. Bravo, M. Soler, S. Vazquez, and L. Morier. 1990. Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am. J. Trop. Med. Hyg. 42179-184. [DOI] [PubMed] [Google Scholar]
- 4.Halstead, S. B., and P. Simasthien. 1970. Observations related to the pathogenesis of dengue hemorrhagic fever. II. Antigenic and biologic properties of dengue viruses and their association with disease response in the host. Yale J. Biol. Med. 42276-292. [PMC free article] [PubMed] [Google Scholar]
- 5.Kochel, T. J., D. M. Watts, S. B. Halstead, C. G. Hayes, A. Espinoza, V. Felices, R. Caceda, C. T. Bautista, Y. Montoya, S. Douglas, and K. L. Russell. 2002. Effect of dengue-1 antibodies on American dengue-2 viral infection and dengue haemorrhagic fever. Lancet 360310-312. [DOI] [PubMed] [Google Scholar]
- 6.Kou, Z., M. Quinn, H. Chen, W. W. Rodrigo, R. C. Rose, J. J. Schlesinger, and X. Jin. 2008. Monocytes, but not T or B cells, are the principal target cells for dengue virus (DV) infection among human peripheral blood mononuclear cells. J. Med. Virol. 80134-146. [DOI] [PubMed] [Google Scholar]
- 7.Mahalingam, S., and B. A. Lidbury. 2002. Suppression of lipopolysaccharide-induced antiviral transcription factor (STAT-1 and NF-kappa B) complexes by antibody-dependent enhancement of macrophage infection by Ross River virus. Proc. Natl. Acad. Sci. USA 9913819-13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munoz-Jordan, J. L., G. G. Sanchez-Burgos, M. Laurent-Rolle, and A. Garcia-Sastre. 2003. Inhibition of interferon signaling by dengue virus. Proc. Natl. Acad. Sci. USA 10014333-14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parren, P. W., and D. R. Burton. 2001. The antiviral activity of antibodies in vitro and in vivo. Adv. Immunol. 77195-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rico-Hesse, R. 2007. Dengue virus evolution and virulence models. Clin. Infect. Dis. 441462-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigo, W. W., D. C. Alcena, R. C. Rose, X. Jin, and J. J. Schlesinger. An automated dengue virus microneutralization plaque assay performed in human Fcγ receptor-expressing CV-1 cells. Am. J. Trop. Med. Hyg., in press. [PubMed]
- 12.Rodrigo, W. W., X. Jin, S. D. Blackley, R. C. Rose, and J. J. Schlesinger. 2006. Differential enhancement of dengue virus immune complex infectivity mediated by signaling-competent and signaling-incompetent human FcγRIA (CD64) or FcγRIIA (CD32). J. Virol. 8010128-10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roehrig, J. T. 2007. Guidelines for plaque reduction neutralization testing of human antibodies to dengue viruses, p. 1-23. World Health Organization, Geneva, Switzerland. [DOI] [PubMed]
- 14.Vaughn, D. W., S. Green, S. Kalayanarooj, B. L. Innis, S. Nimmannitya, S. Suntayakorn, T. P. Endy, B. Raengsakulrach, A. L. Rothman, F. A. Ennis, and A. Nisalak. 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 1812-9. [DOI] [PubMed] [Google Scholar]
- 15.Watts, D. M., K. R. Porter, P. Putvatana, B. Vasquez, C. Calampa, C. G. Hayes, and S. B. Halstead. 1999. Failure of secondary infection with American genotype dengue 2 to cause dengue haemorrhagic fever. Lancet 3541431-1434. [DOI] [PubMed] [Google Scholar]