Abstract
Dendritic cells (DCs) internalize and process antigens as well as activate cellular immune responses. The aim of this study was to determine the capacity of DCs that contain antigen-coated magnetic beads to induce immunity against the nonstructural hepatitis C virus (HCV) antigen 5 (NS5). Splenocytes derived from Fms-like tyrosine kinase receptor 3 (Flt3) ligand-pretreated BALB/c mice were incubated with magnetic beads coated with HCV NS5, lipopolysaccharide (LPS), and/or anti-CD40; purified; and used for immunization. Cellular immunity was measured using cytotoxic T-lymphocyte (CTL) and T-cell proliferation assays, intracellular cytokine staining, and a syngeneic tumor challenge using NS5-expressing SP2/0 myeloma cells in vivo. Splenocytes isolated from animals vaccinated with DCs containing beads coated with NS5, LPS, and anti-CD40 secreted elevated levels of interleukin-2 (IL-2) and gamma interferon in the presence of NS5. The numbers of CD4+, IL-2-producing cells were increased >5-fold in the group immunized with DCs containing beads coated with NS5, LPS, and anti-CD40, paralleled by an enhanced splenocyte proliferative response. Immunization promoted antigen-specific CTL activity threefold compared to the level for control mice and significantly reduced the growth of NS5-expressing tumor cells in vivo. Thus, strategies that employ NS5-coated beads induce cellular immune responses in mice, which correlate well with the natural immune responses that occur in individuals who resolve HCV.
An estimated 170 million individuals (3% of the world's population) are infected with hepatitis C virus (HCV), leading to cirrhosis, end-stage liver disease, and hepatocellular carcinoma. The current standard-of-care therapy for chronically infected HCV patients is the combined administration of pegylated alpha interferon (IFN-α) and ribavirin (28). A sustained response is seen in approximately 50 to 60% of individuals (33). Treatment is long term (6 to 12 months), costly, and associated with substantial toxicity (42). Clearly, more-effective regimens are needed (10).
Clinical studies of the adaptive host immune response to acute HCV infection suggest a rationale for immunization approaches. Clearance of HCV is associated with early, multispecific, strong CD8+ T-cell immunity that is matched by vigorous and sustained CD4+ T-cell proliferation in response to multiple recombinant structural and nonstructural viral proteins (13, 18, 47). Activated T cells secrete proinflammatory cytokines (TH1 type), such as IFN-γ, coinciding with large reductions in viral load during acute infection (47). HCV infections that are successfully controlled result in durable memory populations (44). This observation is supported by a substantially lower rate of HCV persistence in reexposed humans with a history of acute resolving HCV (29). Rechallenge experiments with chimpanzees showed that antibody-mediated depletion of CD4+ T cells resulted in HCV persistence, which is in contrast to a marked reduction in duration and peak of viremia in nontreated animals (4, 20). The importance of CD4+ T cells is further emphasized by the loss of immune protection against reexposure to HCV and correlated with low CD4+ T-cell counts in intravenous drug users who had recovered from HCV but subsequently acquired human immunodeficiency virus infection (29). Whether recovery from acute HCV coincides ultimately with virus eradication is still a matter of debate (34). The disappearance of HCV-specific antibodies in some individuals 10 to 20 years after viral clearance indicates that a subgroup of patients achieves complete virus elimination (44).
Persistent HCV infection can be attributed to a number of viral evasion strategies. First, the rapid spread of HCV in a host outpaces the immune response by several weeks (39, 46). Second, HCV is a strong inducer of type I IFN but appears to render hepatocytes resistant to antiviral activity (45, 46). Third, NK cell function can be inhibited by cross-linking tetraspanin CD81 on the cell surface with the major envelope protein of HCV (HCV E2), thus blocking NK cell activation, proliferation, and cytokine production (12). Finally, persistent HCV infections correlate with the permanent loss of HCV-specific T-cell proliferation during acute HCV infection, the functional exhaustion of an initially vigorous response, and the inability of effector T cells to migrate into the infected liver (10, 13, 18, 36, 39, 46). Insufficient CD4+ T-cell activity appears to be a key event leading or contributing to chronic HCV. Failure to sustain the CD4+ helper response renders virus-specific CD8+ T cells inadequate (e.g., loss of cytotoxicity and IFN-γ production), thus contributing to persistent viremia (39).
Recapitulating successful immune responses and addressing HCV-specific defects in immunity are mandatory in efforts to improve current treatment options. The purported involvement of dendritic cells (DCs) in the impaired immune responses observed in patients with persistent HCV infection makes DCs a principal target for immunomodulatory approaches. Access to a sufficient quantity of mature DCs is a critical issue since only mature DCs are capable of targeting the antigen and inducing cellular immunity rather than tolerance. Previously, we outlined a novel method of generating large numbers of DCs in vivo (17). These, in turn, are enriched in vitro by phagocytosis of magnetic beads and separation in a magnetic field. Here we further demonstrate that, by immunizing mice with DCs that contain beads coated with the nonstructural HCV antigen NS5 and compounds known to induce DC maturation, significant levels of cellular immunity are elicited. The key elements of this approach reside in the combined enrichment, maturation, and antigen targeting of DCs in a single step and the generation of immune responses of the type known to promote viral clearance in humans.
MATERIALS AND METHODS
Mice.
Five- to 6-week-old female BALB/c (H-2d) mice were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN), and kept under specific-pathogen-free conditions in the animal facility of Rhode Island Hospital. All animal protocols have been reviewed and approved by the Life Span Animal Care and Use Committee.
Culture conditions.
DCs were cultured in serum-free, HEPES-buffered RPMI 1640 medium (Cambrex Bio Science, Wakersville, MD) supplemented with 2 mM l-glutamine, 1% essential and nonessential amino acids, 5 × 10−5 M 2-mercaptoethanol, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, and 5 U/ml gentamicin (all from Sigma-Aldrich, St. Louis, MO). Splenocytes obtained from immunized animals were cultured in the same medium additionally supplemented with heat-inactivated 10% fetal bovine serum.
Coating of magnetic beads with anti-CD40, LPS, and HCV NS5.
Immunomagnetic beads (Calbiochem; EMD Bioscience, San Diego, CA), 1.3 μm in diameter, were coated using methods previously described (17) and closely following the manufacturer's protocol. Briefly, beads were suspended in CDI buffer (Sigma-Aldrich, St. Louis, MO) and incubated for 10 min at 50°C, pelleted, and washed three times in borate buffer (Fluka; Sigma-Aldrich, St. Louis, MO). After the final wash, borate buffer was added with or without 100 μg of anti-CD40 (clone 3/23, 0.5 mg/ml) (BD Pharmingen, San Jose, CA), 10 μg of lipopolysaccharide (LPS) (Sigma-Aldrich, St. Louis, MO), and/or a total of 200 μg of NS5 protein (AG712 yeast-derived recombinant protein; Maine Biotechnology, Portland, ME) as indicated in the figure legends. After incubation for 10 min at 50°C, followed by an overnight incubation at 4°C under constant slow agitation, the beads were pelleted in a magnetic field and the supernatant was removed by pipetting; the beads were then washed and resuspended in 100 μl phosphate-buffered saline. Centrifugation was avoided at all steps to prevent bead aggregation.
According to the manufacturer, approximately 10% of the protein in solution is bound by the beads; therefore, the supernatant can be reused for additional coatings. We found that most of the beads were eliminated during the DC purification step (see below) and not taken up by phagocytic cells. Since a relatively small fraction of the purified DCs is injected into the animals (1 × 106 out of 1.5 × 107 purified cells), only minute amounts of antigen and/or anti-CD40 and LPS are finally injected into the animals.
Generation and purification of splenic DCs.
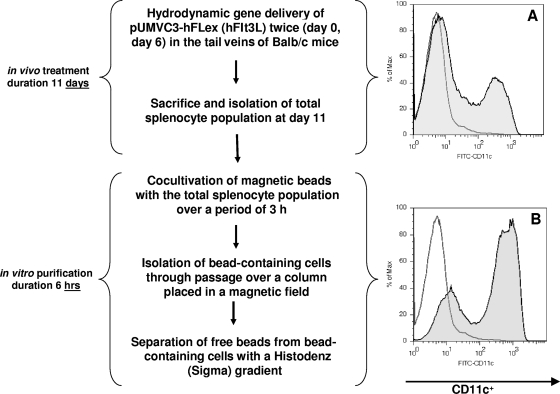
The DC population was expanded in vivo using methods previously described and outlined in Fig. 1 (17, 23, 27). Briefly, the plasmid pUMVC3-hFLex, expressing the secreted portion of the human Fms-like tyrosine kinase receptor 3 ligand (hFlt3L) (Vector Core Laboratory, University of Michigan), was injected twice (days 0 and 6) into the tail veins of mice (hydrodynamic gene delivery); the spleens were dissected on day 12. Single-cell splenocyte suspensions were mixed with beads and incubated for 3 h at 37°C. Cells were harvested, and those which had taken up beads were separated from the remaining population by use of magnetic force. Finally, free beads were removed from bead-containing cells by centrifugation on a 20% Histodenz gradient (Sigma-Aldrich, St. Louis, MO).
FIG. 1.
In vivo hFlt3L treatment and in vitro enrichment yield a CD11c+ cell population of 80%. (A) CD11c+ cells constitute ∼25% of the total splenocyte population isolated from hFlt3L-treated mice. (B) CD11c+ cells comprise 80% of the enriched, magnetic-bead-containing population. The unshaded area represents the isotype control, and the shaded area represents the anti-CD11c-stained population. Max, maximum; FITC, fluorescein isothiocyanate.
Immunization.
Groups of five mice were immunized with or without DCs as follows: (i) beads coated with LPS and anti-CD40; (ii) beads coated with NS5, LPS, and anti-CD40; (iii) DCs containing beads coated with LPS and anti-CD40; (iv) DCs containing beads coated with NS5; and (v) DCs containing beads coated with NS5, LPS, and anti-CD40. Unless noted otherwise, animals were inoculated subcutaneously three times at 2-week intervals with 50 μl of immunogen in each of two footpads (in the case of DCs, with 1 × 106 cells in 100 μl Hanks' balanced salt solution/immunization). Two weeks after the final immunization, mice were challenged or euthanized and splenocytes collected for subsequent analysis.
ICCS and fluorescence-activated cell sorter analyses.
Intracellular cytokine staining (ICCS) and fluorescence-activated cell sorter analyses were performed in accordance with methods described previously (17, 27). Briefly, 1 × 105 to 5 × 105 cells were incubated with excess anti-mouse CD16/32 (clone 93, rat isotype) to block the Fc receptor and then stained with 1 μg phycoerythrin-, fluorescein isothiocyanate-, or peridinin chlorophyll protein-labeled antibody specific for the mouse cell surface marker CD11c (clone N418), CD4 (clone L3T4), or CD8α (clone Lyt-2), respectively; the recommended isotype controls were included. ICCS was performed with anti-mouse interleukin-2 (IL-2) (clone JES6-5H4) and a cytofix/cytoperm kit (BD Pharmingen) according to the manufacturer's instructions. All antibodies were purchased from eBioscience (San Diego, CA) if not otherwise indicated.
Proliferation assay.
Cells (4 × 106) were labeled with 1 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes) in Hanks' balanced salt solution by incubation for 15 min at 37°C. Then, the cells were centrifuged, resuspended in prewarmed medium, and incubated for another 30 min to ensure complete modification of the probe. The labeled cells were incubated for 5 days in six-well plates containing 5 ml culture medium supplemented with 10% fetal bovine serum and 1 μg/ml HCV NS5. The cells were then washed, stained for CD4 or CD8, and evaluated for proliferation by flow cytometry.
Quantitation of cytokine production.
To quantify cytokine production, splenocytes derived from immunized and nonimmunized animals were cultured at 5 × 105 cells/200 μl/well in 96-well, flat-bottomed plates at 37°C. Cells were stimulated with 0.1 to 1 μg/ml recombinant HCV NS5 protein. After 24 h, the supernatants were collected and the levels of IFN-γ, IL-4, and IL-2 were quantified by using commercial enzyme-linked immunosorbent assay kits purchased from eBioscience according to the manufacturer's instructions.
In vitro cytotoxicity assay.
Cytotoxic T-lymphocyte (CTL) activity was assessed in accordance with methods described previously (17, 27). CTL activities expressed by splenocytes derived from immunized and control mice were determined following in vitro stimulation in which 1 × 108 cells in 30 ml of culture medium were incubated with recombinant murine IL-2 (5 U/ml; eBioscience, San Diego, CA) and recombinant HCV NS5 protein (0.3 μg/ml; Biodesign International, Saco, ME). After 3 days of culture, the cells were harvested and a standard 4-h 51Cr release assay was performed. The 51Cr-labeled syngeneic murine myeloma cells (SP2/0; ATCC, Manassas, VA) stably expressing HCV NS5 (SP-NS5 cells) were used as the targets. All assays were conducted in quadruplicate.
Tumor challenge model.
T-cell activity was assessed in vivo by tumor challenge as outlined previously (16, 27). SP-NS5 cells were harvested and washed three times in serum-free medium. The backs of mice were shaved, and each animal was inoculated subcutaneously in the right flank with 1 × 106 cells resuspended in 100 μl serum-free medium. The tumor size was measured daily with a caliper, starting on day 7. On day 15, the mice were euthanized; the tumors were dissected and weighed.
Statistical analysis.
Results were analyzed using the SigmaStat 3.0 statistics program (Jandel Scientific, San Rafael, CA). Individual means were compared using a nonpaired Student t test. When more than two groups were compared, a one-way analysis of variance was performed, followed by a Tukey test to determine which groups differed significantly (P < 0.05).
RESULTS
Generation, enrichment, and targeting of NS5 antigen to DCs.
In agreement with our previous findings, the hydrodynamic delivery of the hFlt3L-expressing plasmid pUMVC3-hFLex increased the total number of splenocytes 10-fold to an approximate 4 × 108 cells per spleen (17, 27). Twenty-five percent of the splenocytes were CD11c+, representing a 100-fold expansion of the murine DC population. After the spleens of Flt3L-treated mice were dissected, the total splenocyte population was incubated with coated magnetic beads for 3 h. The successful coating of the beads with recombinant NS5 was verified in advance by binding of anti-NS5 antibodies to the beads. An enriched DC population was obtained by passing the cells over a magnetic column and separating bead-containing cells from the remaining population. This procedure increased the CD11c+ cells from 25% (after Flt3L treatment) to greater than 80% of the total population (Fig. 1).
The majority of the remaining bead-containing, CD11c− cells have been characterized previously based on the expression of CD11b, CD4, CD8, and CD45R/B220 (17). The CD11c− cell population stained brightly positive for CD11b/Mac-1 and expressed low levels of CD4, CD8, and CD45R, which is characteristic of a macrophage population.
Cytokine secretion by the total splenocyte population.
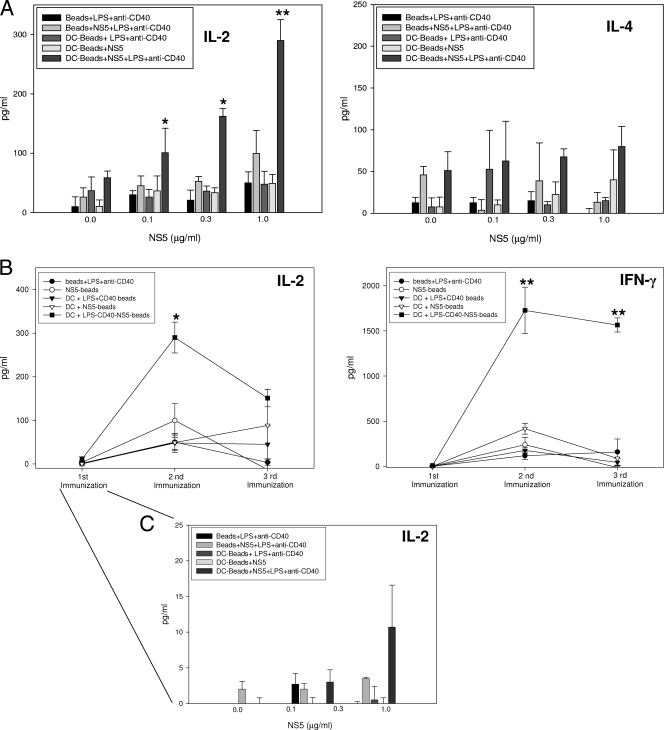
A series of experiments was undertaken to evaluate the potential of antigen-coated beads in stimulating antiviral responses in vitro and in vivo. Since a TH1 response is strongly associated with the resolution of HCV, we examined the ability of splenocytes derived from immunized animals to secrete IFN-γ and IL-2; IL-4 secretion was quantified as a measure of TH2-type responses. Relative to results for all other groups, splenocytes derived from mice immunized with DCs containing NS5-, LPS-, and anti-CD40-coated beads produced elevated concentrations of IL-2 when cultured in the presence of recombinant NS5 (Fig. 2A). IL-2 production was increased even in cultures that contained as little as 0.1 μg/ml NS5. In contrast, IL-4 production was relatively low and not significantly different between groups regardless of culture conditions. The production of IL-2 and IFN-γ was maximal in splenocyte cultures derived from mice immunized twice with DCs containing beads coated with NS5, LPS, and anti-CD40; additional immunizations did not promote greater cytokine production (Fig. 2B). A single immunization, however, elicited a splenocyte population capable of producing IL-2 in specific response to antigen (Fig. 2C).
FIG. 2.
Mice immunized with DCs containing beads coated with NS5, LPS, and anti-CD40 exhibit a TH1-type cytokine response. (A and C) Splenocytes obtained from mice immunized two times were cultured with recombinant HCV NS5 at the concentrations listed. Culture supernatants were collected after 48 h; IFN-γ, IL-2, and IL-4 concentrations were quantified by an enzyme-linked immunosorbent assay. (B) Splenocytes derived from animals after each immunization were cultured with 1.0 μg/ml HCV NS5. Data represent the means ± standard deviations derived from four animals treated comparably in a single experiment. Experiments were repeated three times with similar results. *, P < 0.05; **, P < 0.005 (compared to the other groups incubated with the same concentrations of recombinant HCV NS5).
ICCS.
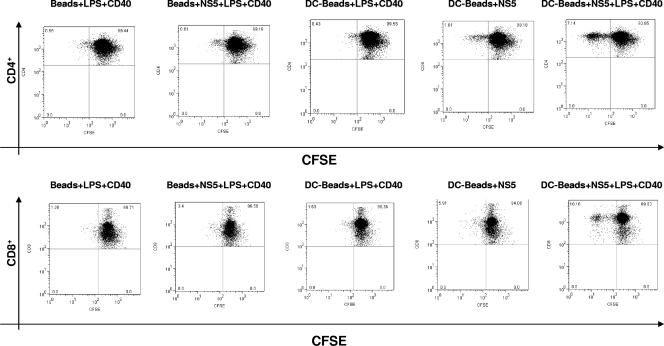
ICCS was performed to determine the cell source(s) of IL-2. Previously, we have shown that the CD4+ and CD8+ populations derived from animals immunized with DCs containing beads coated with NS5, LPS, and anti-CD40 displayed a significant (four- to fivefold) increase in the number of IFN-γ-producing cells in contrast to all of the control groups (17). Here we observed a somewhat different pattern when staining for IL-2. Only the CD4+ population obtained from animals immunized with DCs containing beads coated with NS5, LPS, and anti-CD40 displayed a marked increase in the number of IL-2-secreting cells (Fig. 3). None of the other groups had a substantial number of CD4+, IL-2-producing cells, nor did any of the groups have a sizable number of CD8+, IL-2-producing cells.
FIG. 3.
The CD4+ T-cell populations derived from animals immunized with DCs containing beads coated with NS5, LPS, and anti-CD40 secrete IL-2. The total splenocyte populations obtained from mice immunized as indicated were incubated in the presence of 1 μg/ml HCV NS5 for 24 h; GolgiPlug (BD Pharmingen) was added during the final 6 h of culture. ICCS of splenocytes was performed for IL-2. The phenotype of the cells was determined by staining for CD4+ and CD8+ cells. The plots are representative of at least two similar experiments with consistent results. The values within each grid represent the percentages of cells within the quadrants relative to the entire cell population analyzed per plot.
Cell proliferation in response to stimulation with recombinant NS5.
A CFSE proliferation assay with additional staining of CD4 and CD8 was performed to identify the cell types responsive to HCV NS5 immunization. A two- to threefold increase in the number of proliferating CD4+ cells was observed among splenocytes derived from mice immunized with DCs containing beads coated with NS5 only (Fig. 4). A much larger number of proliferating CD4+ cells was observed for the group immunized with DCs containing beads coated with NS5, LPS, and anti-CD40, i.e., a 6- to 10-fold increase in proliferating CD4+ cells compared to those for animals immunized with beads alone or with DCs containing beads coated with LPS and anti-CD40 but not NS5. Interestingly, animals immunized only with beads coated with NS5, LPS, and anti-CD40 (i.e., no DCs) displayed a weak, twofold increase in proliferating CD8+ cells. More proliferating CD8+ cells were detected in animals immunized with DCs containing beads coated with NS5 (fourfold). The strongest proliferative response, however, was seen with the group immunized with DCs containing beads coated with NS5, LPS, and anti-CD40; sixfold more CD8+ cells displayed a strong shift toward weak CFSE staining.
FIG. 4.
CD4+ and CD8+ T cells derived from mice immunized with DCs containing beads coated with NS5, LPS, and anti-CD40 display a vigorous proliferative response to HCV NS5. Proliferation was estimated from the decreased fluorescence intensity exhibited by splenocytes stained with CFSE and then cultured for 5 days in the presence of HCV NS5 (0.5 μg/ml). The phenotypes of the proliferating cells were determined by staining for CD4+ and CD8+ cells. The plots are representative of two or more comparable experiments. The values within each grid represent the percentages of cells within the quadrants relative to the entire cell population analyzed per plot.
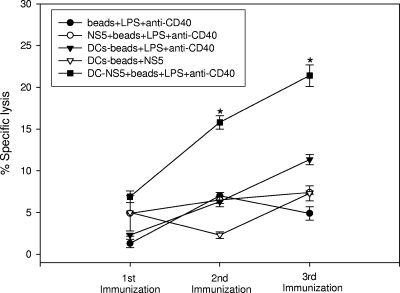
CTL response.
CTLs play a critical role in eliminating virus-infected host cells. Standard 51Cr release assays were performed to determine the capacity of bead-containing DCs to elicit CTL activity. After a single immunization, slightly greater CTL activity was expressed by splenocytes obtained from the group inoculated with DCs containing beads coated with NS5, LPS, and anti-CD40 (Fig. 5). Immunizing a second and third time significantly increased the CTL activity exhibited by splenocytes derived from this group relative to the activities of the other groups. Indeed, following the second immunization, splenocytes obtained from mice immunized with DCs containing beads coated with NS5, LPS, and anti-CD40 displayed ≥2-fold more CTL activity. Notably, splenocytes derived from mice immunized with DCs containing beads coated with NS5 alone did not exhibit more CTL activity than did splenocytes obtained from animals immunized with DCs containing non-NS5-coated beads (i.e., DCs containing beads coated with LPS and anti-CD40). Immunization with DCs induced a certain level of nonspecific cytolytic activity. Greater SP-NS5 tumor cell lysis was found in cocultures that contained splenocytes derived from mice immunized with DCs containing beads coated with LPS and anti-CD40 (no NS5) than in cocultures that contained splenocytes derived from mice immunized with beads coated with LPS and anti-CD40.
FIG. 5.
Splenocytes derived from mice vaccinated with DCs containing beads coated with NS5, LPS, and anti-CD40 exhibit elevated antigen-specific CTL activity. Groups of four mice were inoculated subcutaneously three times at 2-week intervals with beads or DCs containing beads coated with the factors indicated. Splenocytes obtained from mice 1 week after each inoculation were cultured for 3 days in the presence of 0.5 μg/ml recombinant NS5. Subsequently, the cells were cocultured with 51Cr-labeled, SP-NS5 myeloma target cells. Values are the means ± standard deviations of specific cytotoxicity calculated from quadruplicate wells in a single experiment representative of two similar experiments. CTL activity was assessed after each immunization at an effector-to-target cell ratio of 30:1. *, P < 0.001 compared to the other groups.
Tumor challenge.
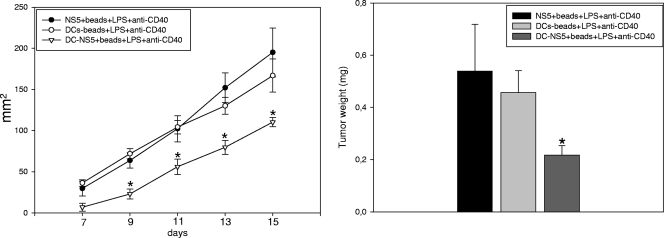
To determine the efficacy of DC-based immunization in vivo, mice were challenged with the same stable, SP-NS5-expressing tumor cell line used to assess CTL activity in vitro. Levels of tumor growth were compared in animals vaccinated with DCs containing beads coated with LPS and anti-CD40 with or without NS5 and in animals immunized with only beads coated with NS5, LPS, and anti-CD40, i.e., no DCs (Fig. 6). After 15 days of observation, the animals were euthanized; the tumors were dissected and weighed. Mice immunized with beads alone or with DCs containing beads coated with LPS and anti-CD40 displayed fast tumor growth. Tumor weight paralleled the rate of tumor growth observed, with an average weight of 0.5 g/animal in the two control groups (beads coated with NS5, LPS, and anti-CD40 and DCs containing beads coated with LPS and anti-CD40). By comparison, the tumors dissected from animals immunized with DCs containing beads coated with NS5, LPS, and anti-CD40 were less than half the size (≤0.2 g/mouse).
FIG. 6.
Vaccination with DCs containing beads coated with NS5, LPS, and anti-CD40 induces NS5-specific immunity in vivo. Groups of animals were inoculated three times at 2-week intervals with beads coated with NS5, LPS, and anti-CD40; DCs that contained beads coated with LPS and anti-CD40; or DCs that contained beads coated with NS5, LPS, and anti-CD40. Vaccinated animals were subcutaneously administered 1 × 106 myeloma cells stably expressing HCV NS5 2 weeks after the last inoculation. Mice vaccinated with DCs that contained beads coated with NS5, LPS, and anti-CD40 showed significantly less NS5-specific tumor cell growth than the other two groups (*, P < 0.01) (left). On day 15 of growth, the tumors were dissected, weighed, and compared. Data are the means ± standard errors of the means of weights of tumors obtained from eight mice in each group (*, P < 0.01 compared to the other two groups) (right).
DISCUSSION
Robust memory CD4+ and CD8+ T-lymphocyte activities characterize host immune responses leading to the resolution of HCV infection (44). Strategies to enhance immunity should recapitulate these naturally occurring immune responses. In this regard, we and others have investigated the ability of DNA-based immunization to evoke sustained cellular immune responses against HCV antigens (6, 15, 16, 32). Even though significant CTL activity was observed in vitro and in vivo, success was limited by a lack of memory and an overall weak CD4+ T-cell response (16, 27). Notably, sustained CD4+ T-cell responses appear to be a key factor in the control of HCV infection; HCV persistence, on the other hand, is related to impaired CD4+ T-cell activity (39). The latter might be explained by a reduction in DC activity. Indeed, DCs obtained from individuals chronically infected with HCV display a decreased stimulatory capacity, possibly due to a reduction in their abilities to produce IL-12 and IFN-γ (3, 25). In addition, DCs from persistently HCV-positive individuals failed to mature in response to conventional stimuli, evidenced by diminished costimulatory molecule expression and antigen uptake (2). The underlying cause(s) for these changes in DC function can be attributed to the ability of HCV to infect DCs (3, 19) and/or the effects of HCV proteins on DC function (14, 37, 38), though the latter concept is controversial. Moreover, the microenvironment regulates DC maturation and function; T cells are required (40). The CD4+ T cells associated with chronic HCV infection produce anti-inflammatory cytokines, such as IL-4 and IL-10 (48, 49). A significant number of HCV-specific CD8+ cells are negative for the CD38 and CD69 activation markers (22).
The impaired function of DCs during chronic HCV infections contrasts with the ability of naïve DCs to induce a strong T-cell response. This observation and the failure of current vaccine strategies to induce a vigorous HCV antigen-specific CD4+ T-cell response have brought recent attention to the role of DCs in this regard. Targeting DCs is an approach capable of interrupting the vicious cycle of impaired DC function that causes insufficient T-cell priming and, in turn, the generation of anergic T cells not capable of stimulating DC maturation.
Insufficient DC maturation is addressed herein by the ingestion of beads coated with LPS (a Toll-like receptor 4 ligand) and antibodies specific for CD40, a member of the tumor necrosis factor receptor family. Thus, two receptor families involved in DC maturation are engaged. Toll-like receptor 4 and CD40 are integral membrane proteins (5, 7), which likely interact with LPS and anti-CD40 bound to beads prior to ingestion. Accordingly, beads coated with both LPS and anti-CD40 induced phenotypic maturation of the CD11c+ population, as we have documented recently, by elevated expression of maturation markers and increased secretion of IFN-γ, IL-2, tumor necrosis factor alpha, and IL-12 (17). Mature, IL-12-secreting DCs polarize CD4+ T cells toward a TH1-type response (9).
The ingestion of coated microbeads not only stimulated DC maturation but served as a mechanism for antigen delivery. In the present study, beads were coated with the entire recombinant HCV NS5 protein, rather than known immunogenic peptides, to avoid the possible exclusion of important epitopes (52). Cells that ingested magnetic microbeads were readily separated from the remaining population; ≥80% of the bead-positive cells derived from hFlt3L-treated animals were CD11c+ DCs (Fig. 1).
The adoptive transfer of DCs containing beads coated with LPS, anti-CD40, and recombinant HCV NS5 induced an antigen-specific cellular immune response that was evident in vitro and in vivo. Splenocytes derived from animals vaccinated with DCs containing beads coated with NS5, LPS, and anti-CD40 secreted elevated levels of IL-2 and IFN-γ in the presence of NS5 (Fig. 2). The maturation of DCs in the presence of LPS and anti-CD40 appears to be critical; cytokine production by splenocytes derived from animals inoculated with DCs containing beads coated with NS5 only was sharply diminished (Fig. 2). Interestingly, the CD4+ T-cell population was identified as a main source of cytokine production and proliferative response when animals were immunized with DCs containing beads coated with NS5, LPS, and anti-CD40 (Fig. 3 and 4). These findings suggest that additional maturation signals are required to elicit a DC population capable of inducing a vigorous TH1-type CD4+ T-cell response. In the absence of these factors, the cellular immune response was weak. The importance of CD4+ T cells for an effective anti-HCV response is evidenced by patient studies (18) and underscored by a recent chimpanzee study (30) that demonstrated a temporal relationship between the recurrence of HCV viremia after apparent viral clearance and the loss of a detectable CD4+ T-cell response. Importantly, a vigorous CD8+ T-cell response is linked to the presence of HCV-specific CD4+ T-cell activity. A recovery of CD8+ T-cell effector function and a significant decrease in HCV viremia occurred at the time when CD4+ T cells became detectable (47). Other animal models support the critical role of CD4+ T cells in the immune response to viral infections. CD8+ T-cell-dependent control of lymphocytic choriomeningitis virus and herpesvirus infections, for example, is progressively lost in the absence of CD4+ T cells (8, 24). Notably, a recent patient study by Kaplan et al. (26) showed that sustained HCV clearance depended upon a CD4+ T-cell response that included both antigen-specific proliferation and IFN-γ production. Viremia was controlled only transiently by either HCV-specific CD4+ T-cell proliferation or IFN-γ production alone. In contrast, the resolution of HCV infection did not correlate with the HCV-specific response of CD8+ T cells or the production of virus-specific antibody.
The adoptive transfer of DCs containing beads coated with NS5, LPS, and anti-CD40 induced CTL activity in vitro (Fig. 5) and antitumor immunity in vivo (Fig. 6), activities required for immunity against HCV. The observation that DCs internalizing recombinant NS5 protein are capable of inducing CD8+ T-cell responses underscores the ability of DCs to cross-present antigens, previously described by several investigators (1, 21, 35). Albeit statistically significant, the CTL response and antitumor immunity demonstrated here show the need for improvement. Recent studies employing microparticles for DC-based immunization suggest a number of possible strategies. These include using biodegradable beads (31); encapsulating or coating the beads with DC maturation compounds, such as CpG motifs (41), granulocyte-macrophage colony-stimulating factor (50), and monophospholipid A (11); and targeting DCs in vivo by coating the beads with DC-specific receptor antibodies (e.g., anti-DEC 205) (43).
In conclusion, an orchestrated cell-mediated immune response to viral antigens that includes proliferation and TH1 cytokine secretion by CD4+ T cells is required to resolve acute HCV infections and to prevent viral persistence. The documented link between DC impairment and chronic HCV infection emphasizes targeting antigen to DCs specifically as a natural cell-based adjuvant against HCV. Although these studies are performed with an experimental animal model system, the applicability of this immunization technique to a human setting may be achievable through the employment of biodegradable, superparamagnetic beads and the use of compounds that optimize maturation of human DCs, e.g., poly(I:C), CpG motifs, and R-848 (51). The correlation between the type of cellular immune responses induced by DCs containing coated beads and the natural immune responses of individuals who resolve HCV illustrates the potential of this approach.
Acknowledgments
We thank Donna Pratt for editorial assistance in producing the manuscript.
The work was supported in part by NIH grants CA-35711, AA-08169, and AA-02666. Stephan Gehring was supported by Deutsche Forschungsgemeinschaft (DFG) grant GE1193/1-1, and Philip Wintermeyer was supported by DFG grant WI12683/1-1.
Footnotes
Published ahead of print on 17 December 2008.
REFERENCES
- 1.Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 39286-89. [DOI] [PubMed] [Google Scholar]
- 2.Auffermann-Gretzinger, S., E. B. Keeffe, and S. Levy. 2001. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood 973171-3176. [DOI] [PubMed] [Google Scholar]
- 3.Bain, C., A. Fatmi, F. Zoulim, J. P. Zarski, C. Trepo, and G. Inchauspe. 2001. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology 120512-524. [DOI] [PubMed] [Google Scholar]
- 4.Bassett, S. E., B. Guerra, K. Brasky, E. Miskovsky, M. Houghton, G. R. Klimpel, and R. E. Lanford. 2001. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology 331479-1487. [DOI] [PubMed] [Google Scholar]
- 5.Bodmer, J. L., P. Schneider, and J. Tschopp. 2002. The molecular architecture of the TNF superfamily. Trends Biochem. Sci. 2719-26. [DOI] [PubMed] [Google Scholar]
- 6.Brinster, C., S. Muguet, Y. C. Lone, D. Boucreux, N. Renard, A. Fournillier, F. Lemonnier, and G. Inchauspe. 2001. Different hepatitis C virus nonstructural protein 3 (Ns3)-DNA-expressing vaccines induce in HLA-A2.1 transgenic mice stable cytotoxic T lymphocytes that target one major epitope. Hepatology 341206-1217. [DOI] [PubMed] [Google Scholar]
- 7.Brodsky, I., and R. Medzhitov. 2007. Two modes of ligand recognition by TLRs. Cell 130979-981. [DOI] [PubMed] [Google Scholar]
- 8.Cardin, R. D., J. W. Brooks, S. R. Sarawar, and P. C. Doherty. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cella, M., D. Scheidegger, K. Palmer-Lehmann, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chisari, F. V. 2005. Unscrambling hepatitis C virus-host interactions. Nature 436930-932. [DOI] [PubMed] [Google Scholar]
- 11.Chong, C. S., M. Cao, W. W. Wong, K. P. Fischer, W. R. Addison, G. S. Kwon, D. L. Tyrrell, and J. Samuel. 2005. Enhancement of T helper type 1 immune responses against hepatitis B virus core antigen by PLGA nanoparticle vaccine delivery. J. Control. Release 10285-99. [DOI] [PubMed] [Google Scholar]
- 12.Crotta, S., A. Stilla, A. Wack, A. D'Andrea, S. Nuti, U. D'Oro, M. Mosca, F. Filliponi, R. M. Brunetto, F. Bonino, S. Abrignani, and N. M. Valiante. 2002. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J. Exp. Med. 19535-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diepolder, H. M., R. Zachoval, R. M. Hoffmann, E. A. Wierenga, T. Santantonio, M. C. Jung, D. Eichenlaub, and G. R. Pape. 1995. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet 3461006-1007. [DOI] [PubMed] [Google Scholar]
- 14.Dolganiuc, A., K. Kodys, A. Kopasz, C. Marshall, T. Do, L. Romics, Jr., P. Mandrekar, M. Zapp, and G. Szabo. 2003. Hepatitis C virus core and nonstructural protein 3 proteins induce pro- and anti-inflammatory cytokines and inhibit dendritic cell differentiation. J. Immunol. 1705615-5624. [DOI] [PubMed] [Google Scholar]
- 15.Encke, J., J. zu Putlitz, M. Geissler, and J. R. Wands. 1998. Genetic immunization generates cellular and humoral immune responses against the nonstructural proteins of the hepatitis C virus in a murine model. J. Immunol. 1614917-4923. [PubMed] [Google Scholar]
- 16.Gehring, S., S. H. Gregory, N. Kuzushita, and J. R. Wands. 2005. Type 1 interferon augments DNA-based vaccination against hepatitis C virus core protein. J. Med. Virol. 75249-257. [DOI] [PubMed] [Google Scholar]
- 17.Gehring, S., S. H. Gregory, P. Wintermeyer, M. San Martin, C. Aloman, and J. R. Wands. 2008. Generation and characterization of an immunogenic dendritic cell population. J. Immunol. Methods 33218-30. [DOI] [PubMed] [Google Scholar]
- 18.Gerlach, J. T., H. M. Diepolder, M. C. Jung, N. H. Gruener, W. W. Schraut, R. Zachoval, R. Hoffmann, C. A. Schirren, T. Santantonio, and G. R. Pape. 1999. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology 117933-941. [DOI] [PubMed] [Google Scholar]
- 19.Goutagny, N., A. Fatmi, V. De Ledinghen, F. Penin, P. Couzigou, G. Inchauspe, and C. Bain. 2003. Evidence of viral replication in circulating dendritic cells during hepatitis C virus infection. J. Infect. Dis. 1871951-1958. [DOI] [PubMed] [Google Scholar]
- 20.Grakoui, A., N. H. Shoukry, D. J. Woollard, J. H. Han, H. L. Hanson, J. Ghrayeb, K. K. Murthy, C. M. Rice, and C. M. Walker. 2003. HCV persistence and immune evasion in the absence of memory T cell help. Science 302659-662. [DOI] [PubMed] [Google Scholar]
- 21.Guermonprez, P., J. Valladeau, L. Zitvogel, C. Thery, and S. Amigorena. 2002. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 20621-667. [DOI] [PubMed] [Google Scholar]
- 22.He, X. S., B. Rehermann, F. X. Lopez-Labrador, J. Boisvert, R. Cheung, J. Mumm, H. Wedemeyer, M. Berenguer, T. L. Wright, M. M. Davis, and H. B. Greenberg. 1999. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc. Natl. Acad. Sci. USA 965692-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He, Y., A. A. Pimenov, J. V. Nayak, J. Plowey, L. D. Falo, Jr., and L. Huang. 2000. Intravenous injection of naked DNA encoding secreted flt3 ligand dramatically increases the number of dendritic cells and natural killer cells in vivo. Hum. Gene Ther. 11547-554. [DOI] [PubMed] [Google Scholar]
- 24.Kalams, S. A., and B. D. Walker. 1998. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 1882199-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanto, T., N. Hayashi, T. Takehara, T. Tatsumi, N. Kuzushita, A. Ito, Y. Sasaki, A. Kasahara, and M. Hori. 1999. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J. Immunol. 1625584-5591. [PubMed] [Google Scholar]
- 26.Kaplan, D. E., K. Sugimoto, K. Newton, M. E. Valiga, F. Ikeda, A. Aytaman, F. A. Nunes, M. R. Lucey, B. A. Vance, R. H. Vonderheide, K. R. Reddy, J. A. McKeating, and K. M. Chang. 2007. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology 132654-666. [DOI] [PubMed] [Google Scholar]
- 27.Kuzushita, N., S. H. Gregory, N. A. Monti, R. Carlson, S. Gehring, and J. R. Wands. 2006. Vaccination with protein-transduced dendritic cells elicits a sustained response to hepatitis C viral antigens. Gastroenterology 130453-464. [DOI] [PubMed] [Google Scholar]
- 28.McHutchison, J. G., and M. W. Fried. 2003. Current therapy for hepatitis C: pegylated interferon and ribavirin. Clin. Liver Dis. 7149-161. [DOI] [PubMed] [Google Scholar]
- 29.Mehta, S. H., A. Cox, D. R. Hoover, X. H. Wang, Q. Mao, S. Ray, S. A. Strathdee, D. Vlahov, and D. L. Thomas. 2002. Protection against persistence of hepatitis C. Lancet 3591478-1483. [DOI] [PubMed] [Google Scholar]
- 30.Nascimbeni, M., E. Mizukoshi, M. Bosmann, M. E. Major, K. Mihalik, C. M. Rice, S. M. Feinstone, and B. Rehermann. 2003. Kinetics of CD4+ and CD8+ memory T-cell responses during hepatitis C virus rechallenge of previously recovered chimpanzees. J. Virol. 774781-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Hagan, D. T., M. Singh, and J. B. Ulmer. 2006. Microparticle-based technologies for vaccines. Methods 4010-19. [DOI] [PubMed] [Google Scholar]
- 32.Ou-Yang, P., L. H. Hwang, M. H. Tao, B. L. Chiang, and D. S. Chen. 2002. Co-delivery of GM-CSF gene enhances the immune responses of hepatitis C viral core protein-expressing DNA vaccine: role of dendritic cells. J. Med. Virol. 66320-328. [DOI] [PubMed] [Google Scholar]
- 33.Pearlman, B. L. 2004. Hepatitis C treatment update. Am. J. Med. 117344-352. [DOI] [PubMed] [Google Scholar]
- 34.Pham, T. N., S. A. MacParland, P. M. Mulrooney, H. Cooksley, N. V. Naoumov, and T. I. Michalak. 2004. Hepatitis C virus persistence after spontaneous or treatment-induced resolution of hepatitis C. J. Virol. 785867-5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pooley, J. L., W. R. Heath, and K. Shortman. 2001. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8− dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J. Immunol. 1665327-5330. [DOI] [PubMed] [Google Scholar]
- 36.Rehermann, B., and M. Nascimbeni. 2005. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 5215-229. [DOI] [PubMed] [Google Scholar]
- 37.Sarobe, P., J. J. Lasarte, N. Casares, A. Lopez-Diaz de Cerio, E. Baixeras, P. Labarga, N. Garcia, F. Borras-Cuesta, and J. Prieto. 2002. Abnormal priming of CD4+ T cells by dendritic cells expressing hepatitis C virus core and E1 proteins. J. Virol. 765062-5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarobe, P., J. J. Lasarte, A. Zabaleta, L. Arribillaga, A. Arina, I. Melero, F. Borras-Cuesta, and J. Prieto. 2003. Hepatitis C virus structural proteins impair dendritic cell maturation and inhibit in vivo induction of cellular immune responses. J. Virol. 7710862-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shoukry, N. H., A. G. Cawthon, and C. M. Walker. 2004. Cell-mediated immunity and the outcome of hepatitis C virus infection. Annu. Rev. Microbiol. 58391-424. [DOI] [PubMed] [Google Scholar]
- 40.Shreedhar, V., A. M. Moodycliffe, S. E. Ullrich, C. Bucana, M. L. Kripke, and L. Flores-Romo. 1999. Dendritic cells require T cells for functional maturation in vivo. Immunity 11625-636. [DOI] [PubMed] [Google Scholar]
- 41.Standley, S. M., I. Mende, S. L. Goh, Y. J. Kwon, T. T. Beaudette, E. G. Engleman, and J. M. Frechet. 2007. Incorporation of CpG oligonucleotide ligand into protein-loaded particle vaccines promotes antigen-specific CD8 T-cell immunity. Bioconjug. Chem. 1877-83. [DOI] [PubMed] [Google Scholar]
- 42.Strader, D. B., T. Wright, D. L. Thomas, and L. B. Seeff. 2004. Diagnosis, management, and treatment of hepatitis C. Hepatology 391147-1171. [DOI] [PubMed] [Google Scholar]
- 43.Tacken, P. J., I. J. de Vries, R. Torensma, and C. G. Figdor. 2007. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat. Rev. Immunol. 7790-802. [DOI] [PubMed] [Google Scholar]
- 44.Takaki, A., M. Wiese, G. Maertens, E. Depla, U. Seifert, A. Liebetrau, J. L. Miller, M. P. Manns, and B. Rehermann. 2000. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat. Med. 6578-582. [DOI] [PubMed] [Google Scholar]
- 45.Tan, S. L., and M. G. Katze. 2001. How hepatitis C virus counteracts the interferon response: the jury is still out on NS5A. Virology 2841-12. [DOI] [PubMed] [Google Scholar]
- 46.Thimme, R., J. Bukh, H. C. Spangenberg, S. Wieland, J. Pemberton, C. Steiger, S. Govindarajan, R. H. Purcell, and F. V. Chisari. 2002. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc. Natl. Acad. Sci. USA 9915661-15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 1941395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai, S. L., Y. F. Liaw, M. H. Chen, C. Y. Huang, and G. C. Kuo. 1997. Detection of type 2-like T-helper cells in hepatitis C virus infection: implications for hepatitis C virus chronicity. Hepatology 25449-458. [DOI] [PubMed] [Google Scholar]
- 49.Ulsenheimer, A., J. T. Gerlach, N. H. Gruener, M. C. Jung, C. A. Schirren, W. Schraut, R. Zachoval, G. R. Pape, and H. M. Diepolder. 2003. Detection of functionally altered hepatitis C virus-specific CD4 T cells in acute and chronic hepatitis C. Hepatology 371189-1198. [DOI] [PubMed] [Google Scholar]
- 50.van Broekhoven, C. L., C. R. Parish, C. Demangel, W. J. Britton, and J. G. Altin. 2004. Targeting dendritic cells with antigen-containing liposomes: a highly effective procedure for induction of antitumor immunity and for tumor immunotherapy. Cancer Res. 644357-4365. [DOI] [PubMed] [Google Scholar]
- 51.Warger, T., P. Osterloh, G. Rechtsteiner, M. Fassbender, V. Heib, B. Schmid, E. Schmitt, H. Schild, and M. P. Radsak. 2006. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood 108544-550. [DOI] [PubMed] [Google Scholar]
- 52.Wertheimer, A. M., C. Miner, D. M. Lewinsohn, A. W. Sasaki, E. Kaufman, and H. R. Rosen. 2003. Novel CD4+ and CD8+ T-cell determinants within the NS3 protein in subjects with spontaneously resolved HCV infection. Hepatology 37577-589. [DOI] [PubMed] [Google Scholar]