Abstract
Dairy cattle in two commercial Holstein herds were randomly selected to be vaccinated twice with J5, at approximately 60 days and 28 days before the expected calving date, or to be untreated controls. Based on whether milk production changed following clinical mastitis or whether cows were culled or died within 30 days after onset, 51 mastitis cases were classified as severe or mild. J5-specific antibody responses were evaluated by enzyme-linked immunosorbent assay of all 32 severe and 19 mild cases. The amounts of J5-specific immunoglobulin M (IgM), IgG1, and IgG2 antibodies in sera from the 27 J5 vaccinates were compared with those of the 24 controls. At drying off (before J5 vaccination), all cows had similar amounts of J5-specific antibody. Immediately after calving (approximately 28 days after the second vaccination), J5 vaccinates had significantly higher production of J5-specific IgG1 and IgG2 than controls. When cows were tested following clinical mastitis, none of the three antibody classes differed significantly between the controls and the vaccinates. Vaccinates that contracted Escherichia coli mastitis had 75% less milk loss than controls. The cows that contracted clinical mastitis later in lactation, the unvaccinated controls, and those infected with E. coli had more milk loss following mastitis. The hazards of being culled for all reasons and of being culled for mastitis were significantly lower for J5 vaccinates. Vaccination with J5 was associated with protection against milk production loss and culling following clinical mastitis, and it was also significantly associated with changes in J5-specific IgM, IgG1, and IgG2 antibodies in sera of vaccinated cows.
Coliform mastitis is an important disease complex of dairy cows that is associated with intramammary infections (IMI) with any of several organisms, including Escherichia coli and Klebsiella, Enterobacter, and Citrobacter spp. (9, 11, 20, 22). Clinical mastitis (CM), including abnormal milk, necessity for treatment, milk loss, and death of cattle, can result from coliform mastitis (6, 10, 11, 20, 24). Vaccines against the E. coli rough mutant O111:B4 (J5) bacteria have been in use for 15 years (3, 7). The commercially available vaccines are often called J5 core antigen vaccines, but the antigen(s) and mechanism(s) of J5 immunization have not been clearly identified (2, 23). Associations between J5 vaccination of cattle, various measures of clinical severity of CM, and J5-specific antibodies have rarely been reported for naturally occurring cases of CM (2, 17, 19, 23). Use of survival (time-to-event) analysis to evaluate the relationships between J5 vaccination, antibody production, and time until culling or death has recently been reported for the first time (23). Time-to-event analysis as a measure of incidence of disease or any other event of interest precisely accounts for the time at risk for each animal for that event (5).
Naturally occurring cases of bovine CM were studied among J5 vaccinates and controls on two commercial United States dairy farms. The hypothesis was that vaccinates would have more J5-specific antibodies, longer survival times, and less milk production loss following coliform mastitis. J5-specific antibodies (immunoglobulin M [IgM], IgG1, IgG2, and the ratio of IgG1 to IgG2) were therefore compared among vaccinates and controls, as were the relationships of antibody to etiologic pathogen, milk production loss, clinical severity, and time until culling or death. These relationships were deemed important because of their practical relevance and were based upon results of previous intramammary E. coli challenge trials (15, 25).
MATERIALS AND METHODS
Study herds have been described previously (23). Briefly, the two herds used in the current study were housed on New York State commercial dairy farms milking Holstein cattle, with 330 and 630 lactating cows, respectively. Milk production for each herd was approximately 25,000 pounds (11,350 kg) per cow per lactation; both farms milked cows in fully automated milking parlors using good milking methods and computerized daily milk weight recording. Bulk tank milk somatic cell counts were consistently less than 250,000/ml, and contagious mastitis was well controlled. Historical milk culture data showed that the herds had predominantly environmental types of IMI before the study began.
Criteria for inclusion in study.
In order to be eligible for inclusion in the 20-month study, cows had to have completed at least one previous lactation, had to have a dry period (the time between cessation of milking and the end the previous lactation and the next calving) between 45 and 75 days, no detectable illness at the time of dryoff, and an individual cow somatic cell count of less than 1,000,000/ml for each of the three preceding months. Allocation to the J5 bacterin vaccinate group or control group was by randomization within blocks of 10 cows. Cows were blocked such that the mature equivalent 305-day (305ME) milk production value for each cow was within 10% of the mean 305ME value for the block. Dairy farm personnel were blind to the identity of the treatment groups. Despite randomly assigning cows to treatment groups at the time of dryoff, the investigators could not identify which treatment group cows were in during the experiment. The J5 vaccine (J Vac; Merial, Ltd., Duluth, GA) was administered by the investigators (2 ml) subcutaneously in the supramammary region just before cows were dried off to end the previous lactation and again at 21 to 28 days before the calving due date (during the mid-dry period), in accordance with label directions. Control cows did not receive any sham vaccination.
Reasons for exclusion of some initially enrolled animals.
There were 711 cows enrolled at the beginning of their dry period, 374 controls and 337 vaccinates. Detailed reasons have been described previously (23) for the loss or exclusion of 68 controls and 86 vaccinates from the study. Briefly, cows were excluded because of poor disease records, milk or blood samples required by protocol that had not been collected, vaccinates that had not been given their second vaccination, death, abortion, culling, or a dry period of <45 days or >75 days; the latter could only be determined after calving.
Case definition of clinical mastitis and measures of clinical severity.
Case definitions of CM were reviewed with farm personnel for uniformity, despite the fact that they were all familiar with the disease. Abnormal milk including clots, flakes, or watery appearance constituted CM. CM severity was scored as 1, abnormal milk, normal quarter; 2, abnormal milk, mild quarter swelling; 3, abnormal milk, severe quarter swelling; or 4, abnormal milk and cow showed signs of systemic illness (off feed, fever [rectal temperature of >39.7°C {103.5°F}], depression, and/or sunken eyes). All CM cases with onset during the first 200 days in milk (DIM, i.e., days after calving) or when the cow was confirmed pregnant were eligible for the larger study. Some cows had multiple CM episodes in the same quarter over time. Any such episode that occurred within 5 days of end of treatment (or end of milk withholding) or any episode from 6 to 14 days after recovery from the earlier episode with the same etiologic agent isolated from both episodes was considered a chronic case of mastitis. If a different mastitis pathogen was isolated or the episode occurred more than 14 days after recovery, it constituted a new CM case. Chronic CM cases were excluded from analysis, and only new cases meeting the above criteria were studied, because when results were compiled, there were only 9 chronic cases out of 230 cases of CM (3.5%).
Milk and blood sample collection.
Milk samples were aseptically collected for microbiological culture at calving (all four quarters were sampled individually) and at the onset of any episodes of CM (mastitic quarter[s] sampled) and frozen at −20°C. Samples were transported once each week to the microbiology laboratory (Quality Milk Production Services, Ithaca, NY) for culture according to protocols recommended by the National Mastitis Council (9). Results of cultures were defined as mixed pathogens when two or three types of bacteria other than Staphylococcus aureus were isolated, and contamination was defined as the isolation of more than three types of bacteria other than S. aureus.
Blood samples were collected from all cows at times of drying off, at 1 to 7 DIM following calving, and at 17 to 77 days following the end of treatment for all CM cases. Blood samples were transported cold to the Quality Milk Production Services Regional laboratory, centrifuged at 750 × g for 15 min at 5°C, and stored at −80°C in five 1-ml aliquots in microtubes.
Production and disease information.
Lactation number, DIM at the onset of each new case of CM, the number of survival days until dryoff, culling, or death and the primary reasons why cows died or were culled were collected for all cows and stored in a database. The quarter(s) with CM and clinical severity scores were recorded on paper forms at the farm. Mastitis pathogens were isolated, and results were recorded at the Quality Milk Production Services central laboratory in Ithaca, NY.
Measurement validation and standardization.
The accuracy of computerized daily milk weight recording was evaluated on both study farms by manually recording the identity and daily milk weight for each cow in the milking parlor during one milking and then comparing it to the computer-generated list of cows and their daily milk weights. On both farms, 97% of all cows' milk weights were recorded correctly in the computer data.
Selection of cases for antibody measurement.
Samples were selected for antibody measurement based upon changes in the mean daily milk production during the 14 days before the onset of CM in comparison to that during the 21 days after the end of treatment for CM. If there was no treatment, the latter measurement was the mean of milk production for the 21 days after CM onset. Cases of CM were defined as severe (the cow produced <85% of premastitic milk or was culled or died at <30 days after onset of CM and <150 DIM) or mild (the cow produced >100% of premastitic production). Antibody was evaluated by enzyme-linked immunosorbent assay (ELISA) of all severe and mild cases as defined above. These samples, having previously been frozen at −80°C, were transported on dry ice overnight to the laboratory at Michigan State University (J. L. Burton) and were stored frozen at −80°C until tested.
J5-specific antibody.
Antibody was measured by ELISA as described previously (2, 25). Briefly, flat-bottom 96-well ELISA plates (BD Falcon, Bedford, MA) were coated with 100 μl of J5 E. coli (1 × 109 CFU/ml in sterile saline, killed with 1% phenol), covered with an adhesive plate sealer, and left on a flat surface at room temperature for 12 h to enable adherence of the bacteria to the plate wells. There were four negative control wells with no E. coli added on each plate to assess nonspecific binding of antibodies to plastic. To perform the assay, plates were washed three times with a wash solution (0.1% Tween 20 in aqueous normal 0.9% saline), and various control and test samples were added to appropriate wells. Four wells of each plate received a 1:400 dilution of low-Ig fetal bovine serum (Life Technologies, Rockville, MD) as a negative control. An additional four wells received a 1:400 dilution of serum from a J5-hyperimmunized dairy cow as a positive control. Any plate for which the standard deviation of the mean of this positive control was more than 1.0 was repeated on a different assay day. Finally, additional control wells included two blanks (no E. coli or other test reagents) against which the plate reader was blanked, two wells with no E. coli or test serum but with all other test reagents added, two wells with E. coli but no other test reagents except serum, and two wells with E. coli plus all other test reagents except serum. Thus, there remained 80 wells of test samples (four dilutions of each of 20 samples) of sera per plate. In addition, each well received 125 μl of diluent (10% 10× phosphate-buffered saline [pH 7.3], 0.05% Tween 20 in sterile water).
Serum test samples for J5-specific IgG1 were plated in four serial dilutions, from 1:200 to 1:1,600. Serum test samples for J5-specific IgG2 and for J5-specific IgM were plated in four serial dilutions, from 1:50 to 1:400.
Statistical analysis.
Statistical analyses included chi-square (PROC FREQ; Statistical Analysis System, Cary, NC) and Fisher's exact test (PROC FREQ) to evaluate categorical outcomes and their associations with categorical cow factors (e.g., J5 vaccination and its association with the etiologic pathogen of CM). The J5-specific antibodies IgM, IgG1, and IgG2 and isotype ratios of IgG1 to IgG2 of vaccinates were evaluated at dryoff, postcalving, and following CM and compared with those of controls and were also evaluated as potential explanatory variables (covariates) with measures of outcome of mastitis. General linear models (PROC GLM) were used to evaluate factors (covariates) associated with continuous outcome variables such as milk production change following CM, including testing for the relationship to J5-specific antibodies. The authors have evaluated milk production following CM previously using the variable PRODDIFF, the change in mean daily milk production before and after CM as described previously, as a continuous outcome variable (1, 21, 23).
The relationship between possible explanatory variables, such as J5 vaccination, J5-specific antibody, and other variables, and the categorical outcomes of mild or severe CM or cow-side clinical measures of CM severity (via the four-point scale described above) were evaluated using logistic regression (PROC LOGIST) software.
The optical density (OD) value results for each of the four dilutions of each antibody class were tested using analysis of variance (ANOVA) to determine whether the sample storage box, the test run number, the 96-well plate tested, or the herd of origin was significantly associated with the OD value. Because there were no significant effects from any of these factors, the least diluted (most concentrated) dilution of each antibody class was used for the other analyses, i.e., 1:200 for IgG1 and 1:50 for IgG2 and IgM.
Evaluation of the time until events of CM onset, infection with particular agents, culling, or death used survival (time-to-event) analysis. Log-rank and Wilcoxon tests were used to evaluate J5 vaccination as the only explanatory variable possibly related to culling and dying, using the Kaplan-Meier method (PROC LIFETEST); and then, to test for other explanatory covariates as well, Cox's proportional hazards regression (PROC PHREG) was used. Only the subset of cases with antibody measurements was evaluated in this report. For example, the initial survival analysis (PROC LIFETEST) for factors associated with time until culling because of mastitis as the primary reason was h(t) = VACC, where h(t) is the probability of culling for mastitis within time t for cows surviving until the beginning of time t, and VACC is a J5 vaccinate or control.
The initial Cox proportional hazards model (PROC PHREG) tested was h(t) = h(0) EXP (VACC + LACT + DIM + DRYIGG1 + DRYIGG2 + DRYIGM + CALVIGG1… CALVIGM + MASTIGG1… MASTIGM + PRODDIFF + PREMEAN + SEVERITY + PATHOGEN + BOOSTTODUE + BOOSTTOCALVE), where h(t) is the probability of culling for mastitis within time t for cows surviving until the beginning of t; h(0) is a baseline hazard function; EXP (VACC + LACT + DIM + etc.) is a linear function of a set of fixed covariates, which are exponentiated; VACC is a J5 vaccinate or control; LACT is lactation number 2, 3, or 4 plus; DIM is DIM at onset of CM (censored at 200 DIM if the cow never got CM); DRYIGG1, is the dryoff sample OD value for IgG1; DRYIGG2, is the dryoff sample OD value for IgG2; CALVIGM is a postcalving sample OD value for IgM; MASTIGG1 is the postmastitic sample OD value for IgG1, etc.; PRODDIFF is the mean milk production during 14 days before CM onset (PREMEAN) subtracted from the mean milk production during 21 days following the end of treatment for CM (POSTMEAN) (e.g., if PREMEAN = 100 lb per day and POSTMEAN = 90 lb, the PRODDIFF = −10 lb); SEVERITY is the four levels of cow-side severity of CM described above; PATHOGEN is the etiologic pathogen isolated from CM, including negative or no sample; BOOSTTODUE is the number of days from the J5 booster to the expected due date of calving; and BOOSTTOCALVE is the number of days from the J5 booster to the actual date of calving.
RESULTS
Clinical mastitis cases classified as severe or mild.
Results of a larger study of naturally occurring CM, using some of the same cows, including mastitis cases not tested for antibodies because they were not defined as severe or mild, were reported previously (23). In the current study, there were 51 CM cases selected for antibody testing based on milk production change or survival. Antibody testing was performed for all 32 severe (17 controls, 15 vaccinates) and all 19 mild (7 controls, 12 vaccinates) cases; thus, the cases totaled 24 controls and 27 vaccinates. Of the 32 severe cases, 28 had milk production of <85% of premastitic production after CM, and the other 4 severe cases were culled at <30 days after onset, at <150 DIM.
Mastitis pathogens isolated from clinical mastitis cases.
Milk culture samples were collected from 49 of the 51 new cases of CM (24 controls, 25 J5 vaccinates). Two cases of CM were inadvertently not aseptically sampled for milk culture. Milk culture results for this group were similar to that of the larger data set as reported previously (23) (Table 1). As expected from characterization of the herds before the study, most isolates were environmental pathogens previously characterized as important agents of bovine mastitis (11, 20, 22). There were no contaminated or mixed pathogen results in this data set. The etiologic agent from the 49 CM cases cultured did not differ among vaccinates and controls or between the two herds of origin (for all, P > 0.05, chi-square or Fisher's exact test; PROC FREQ) (Table 1). The etiologic pathogen of CM was not significantly associated with severe or mild cases as defined by production loss (for all, P > 0.05, Fisher's exact test, PROC FREQ).
TABLE 1.
Etiologic agents of 49 CM casesa
| Pathogen | No. of isolates (% of total)
|
|
|---|---|---|
| J5 vaccinates (n = 25) | Controls (n = 24) | |
| No pathogen isolated | 4 (16.0) | 2 (8.3) |
| E. coli | 10 (40.0) | 3 (12.5) |
| Klebsiella sp. | 3 (12.0) | 5 (20.8) |
| Streptococcus sp. | 4 (16.0) | 6 (25.0) |
| S. aureus | 2 (8.0) | 5 (20.8) |
| Coagulase-negative staphylococci | 0 | 1 (4.2) |
| Arcanobacterium pyogenes | 0 | 1 (4.2) |
| Enterobacter sp. | 1 (4.0) | 0 |
| Yeast | 0 | 1 (4.2) |
| Fungi | 1 (4.0) | 0 |
Etiologic agents of 49 clinical mastitis cases selected for mild (>100% of premastitic daily milk production for 21 days following CM) or severe (<85% of premastitic daily milk production for 21 days following CM or culled or died at <30 days after onset of CM and <150 DIM) production loss among J5 vaccinates (n = 25/251) and controls (n = 24/306). No significant differences between vaccinates and controls (P = 0.17, Fisher's exact test) or between severe and mild CM cases (P = 0.16, chi-square test) in etiologic agent.
J5-specific IgM, IgG1, and IgG2 antibodies at dryoff and postcalving.
There were 51 blood samples tested for the J5-specific antibodies that were collected at dryoff and 48 blood samples collected 1 to 7 days postcalving. Three cows were inadvertently not blood sampled following calving. At dryoff (before J5 vaccinations were given), ODs for serum J5-specific IgM and IgG2 antibodies did not differ between cows in different herds or between those randomly selected to become vaccinates or controls (for all, P > 0.16, ANOVA; PROC ANOVA). However, the OD for the serum J5-specific IgG1 at dryoff was higher in cows that were randomly selected to become vaccinates by 0.14 (P = 0.05, ANOVA) (Table 2). The ODs for J5-specific IgM were not significantly different between J5 vaccinates and controls at dryoff or postcalving or following CM (for all, P > 0.17, ANOVA [Table 2]). Following calving (approximately 28 days after the second and last J5 immunization), ODs for J5-specific IgG1 (P < 0.01, ANOVA) and IgG2 (P < 0.05, ANOVA) antibodies were significantly higher in the sera of vaccinates than in controls (Table 2).
TABLE 2.
J5-specific antibody OD values for sera of J5 vaccinates and controlsa
| Antibodyb | J5 vaccinate serum OD (SD) | Control serum OD (SD) |
|---|---|---|
| Dryoff IgM | 0.53 (0.16) | 0.50 (0.17) |
| Dryoff IgG1 | 0.65 (0.24)* | 0.51 (0.23)* |
| Dryoff IgG2 | 0.28 (0.13) | 0.26 (0.09) |
| Dryoff IgG1:IgG2 ratio | 2.52 (1.09) | 2.11 (1.00) |
| Postcalving IgM | 0.63 (0.27) | 0.53 (0.25) |
| Postcalving IgG1 | 0.71 (0.25)*** | 0.47 (0.29)*** |
| Postcalving IgG2 | 0.43 (0.16)** | 0.34 (0.14)** |
| Postcalving IgG1:IgG2 ratio | 1.76 (0.60) | 1.41 (0.74) |
| Postmastitic IgM | 0.56 (0.15) | 0.52 (0.25) |
| Postmastitic IgG1 | 0.86 (0.27) | 0.75 (0.27) |
| Postmastitic IgG2 | 0.39 (0.12) | 0.36 (0.71) |
| Postmastitic IgG1:IgG2 ratio | 2.29 (0.79) | 2.20 (0.71) |
J5 vaccinates, n =27; controls, n = 24. *, significantly different at P = 0.05, ANOVA; **, significantly different at P < 0.05, ANOVA; ***, significantly different at P < 0.01, ANOVA. SD, standard deviation.
Dryoff, blood collected at the time of drying off, before the first J5 vaccination. Postcalving, blood collected 1 to 7 days postcalving, approximately 21 to 35 days after the second and last J5 immunization. Postmastitic, blood collected 17 to 77 days after the end of treatment for clinical mastitis.
General linear models were also used to evaluate factors associated with variations among cows of each class of postcalving J5-specific antibodies. The time from the mid-dry period booster with J5 until the actual date of calving was not significantly associated with the antibody after calving (for all, P ≥ 0. 52, type 3 SS F test, linear regression, PROC GLM). The only factor significantly related to antibody after calving was J5 vaccination, associated with increased J5-specific IgG1 (R2 = 0.18, P = 0.002) and IgG2 (R2 = 0.09, P = 0.04) among vaccinated cows (according to the GLM).
J5-specific IgM, IgG1, and IgG2 antibodies following clinical mastitis.
There were 33 blood samples collected at 17 to 77 days after the end of treatment for CM and tested for the three classes of J5-specific antibodies. Antibodies were not significantly different between vaccinates and controls following CM (for all, P > 0.27, ANOVA) (Table 2).
General linear models evaluated variation among cows in postmastitic IgM, IgG1, and IgG2 antibodies specific for J5. The time from the end of CM treatment until the collection of the blood sample did not affect postmastitic antibody (P = 0.38, type 3 SS F test, linear regression, PROC GLM). Higher production of postmastitic IgM was associated with higher IgM production after calving (P < 0.0001, type 3 SS F test, linear regression, PROC GLM) and with CM caused by E. coli, Klebsiella, or negative culture results in comparison to those caused by other agents (for all, P < 0.04) (R2 = 0.70, P < 0.0001 for overall model, PROC GLM).
Postmastitic IgG1 response was increased in association with higher IgG1 response after calving (P = 0.03, PROC GLM) and with CM caused by E. coli, Klebsiella, or negative culture results (P = 0.005) (R2 = 0.39, P < 0.001 for overall model, PROC GLM).
Postmastitic J5-specific IgG2 was increased in association with higher IgG2 production after calving (P < 0.003, PROC GLM) and was higher in cases caused by E. coli, Klebsiella, or negative culture results than in those caused by other agents (for all, P < 0.02) (R2 = 0.64, P < 0.001 for overall model, PROC GLM).
Thus, there was an overall pattern indicating that higher J5-specific antibody production in all three classes following CM was associated with a higher postcalving response with the same antibody (higher postcalving IgG1 and IgG2 production was more likely among vaccinates) and with CM caused by coliforms or with negative milk culture results.
Ratios of J5-specific IgG1 to IgG2.
Linear regression was used to evaluate the ratios of J5-specific IgG1 to IgG2 at different time points. The IgG1/IgG2 ratios were not significantly different at dryoff, postcalving, and following CM among J5 vaccinates and controls (for all, P ≥ 0.08, type 3 SS F test, linear regression) (Table 2). Nevertheless, there was a trend approaching significance indicating that the IgG1/IgG2 ratio was lower at postcalving, after the second vaccination but before the onset of CM.
J5 vaccination, J5-specific antibodies, and mild or severe outcomes of clinical mastitis.
A logistic regression model was developed to evaluate factors associated with whether cases of mastitis were mild or severe, using only information available up until the onset of CM. The final model was explanatory (85.7% concordant pairs) and included J5 vaccination, DIM, and the interaction of those two factors (Table 3). The model showed that J5 vaccination and CM onset earlier in lactation were associated with milder cases of CM; as DIM at onset increased, severity was more common, and the protective effect of vaccination waned (for all, P < 0.03, type III Wald chi-square, logistic regression, PROC LOGIST) (Table 3). No J5-specific antibody variables at any time point were significantly associated with a mild or severe outcome of CM, even with J5 vaccination removed from the model (for all, P > 0.12, PROC LOGIST).
TABLE 3.
Significant factors associated with CM outcomea
| Parameterb | Estimate (SE)c | Wald chi-square test | Pr > t valued |
|---|---|---|---|
| Intercept | 2.79 (1.27) | 4.78 | 0.0287 |
| J5 Vacc | 3.00 (1.27) | 5.54 | 0.0186 |
| DIM | −0.05 (0.02) | 7.41 | 0.0065 |
| DIM*J5 Vacc | −0.04 (0.02) | 4.77 | 0.0289 |
Data are a logistic regression model of significant factors associated with whether CM had a mild (>100% of premastitic daily milk production for 21 days following CM) or severe (<85% of premastitic daily milk production for 21 days following CM or culled or died at <30 days after onset of CM and <150 DIM) outcome. Quantitative effects of significant variables on mild CM outcome.
J5 Vacc, effect of being vaccinated with J5; DIM, effect of each 1-day increase in days in milk at onset of CM; DIM*J5 Vacc, likelihood of mild outcome for J5 vaccinates for each 1-day increase in DIM at onset of CM. Overall logistic regression model of 85.7% concordant pairs, with P < 0.0001, likelihood ratio.
Positive estimates mean a mild outcome is more likely as this factor increases; negative estimates mean a severe outcome is more likely as this factor increases.
Chi-square test P value.
Cow-side clinical severity of mastitis.
Clinical severity of CM was evaluated using the four-point scale for clinical signs described above. Neither vaccination status nor etiologic pathogen (when all pathogens were evaluated together) was significantly associated with CM clinical severity (P = 0.21 and 0.34, respectively; Fisher's exact test). Nevertheless, of the CM cases caused by Klebsiella, 5/7 (71.4%) had clinical severity scores of 3 (four cases) or 4 (one case), and for all other etiologic agents, 5/39 (12.8%) had severity scores of 3 (none had severity score 4). This increased clinical severity of CM among Klebsiella cases was significant (P = 0.002, Fisher's exact test).
Vaccination with J5 did not reduce the clinical severity of Klebsiella cases (P = 0.43, Fisher's exact test). Postcalving serum IgM, IgG1, and IgG2 antibodies specific for J5, as well as the IgG1/IgG2 ratio after calving, were also not associated with cow-side severity (for all, P > 0.14, type III Wald chi-square, logistic regression, PROC LOGIST).
Milk production change following mastitis.
Of the 51 cases of CM, 4 cases did not have any daily milk production recorded either before the onset of CM or after the end of treatment. Therefore, 47 cases were evaluated for PRODDIFF following CM. Most cows lost milk production following CM; the mean PRODDIFF was −15.5 lb (−7.0 kg). The case definitions of severe and mild CM dictated that the mild cases had positive PRODDIFF values and the severe cases had negative PRODDIFF values. For descriptive purposes, the PREMEANs, POSTMEANs, and PRODDIFFs are reported as follows: for mild, 80.1 lb (36.4 kg), 91.5 lb (41.6 kg), and 11.4 lb (5.2 kg) (n = 19); and for severe, 91.1 lb (41.4 kg), 57.3 lb (26.0 kg), and −33.8 lb (15.4 kg) (n = 28). The raw numerical difference in PRODDIFF values between those of vaccinates and those of controls was small. For 21 controls, the PRODDIFF was −15.8 lb (7.2 kg), and for 26 J5 vaccinates, the PRODDIFF was −15.3 lb (7.0 kg), which were not significantly different values (P = 0.96, ANOVA).
Linear regression (PROC GLM) was used to evaluate factors, each adjusted for the influence of the others, which might be associated with variations in PRODDIFF among cows. J5 vaccination was only significant as part of the interaction with E. coli CM, not as the main effect (Table 4). DIM at onset of CM was evaluated in ranges of DIM as categorical variables, but the linear variable DIM was most significant, with a nearly linear relationship indicating that as CM onset occurred later in lactation, more milk production was lost (0.3 lb [0.1 kg] of PRODDIFF value lost for each day of increased DIM at the onset of CM) (P < 0.0001, type 3 SS F test, linear regression, PROC GLM). Mastitis caused by E. coli was associated with a large daily milk production loss, 48.0 lb (21.8 kg), but the interaction showed that J5 vaccinates lost 43.8 lb (19.9 kg) less per day than controls did (P = 0. 002); most of the loss in daily milk production following E. coli CM occurred in control cows.
TABLE 4.
Significant factors associated with change in daily milk production from 14 days before the onset to 21 days following the end of treatment for CMa
| Parameterb | Estimated PRODDIFF (lb/kg) (SE)c | t value | P value |
|---|---|---|---|
| Intercept | 4.2/1.9 (9.37) | 0.45 | 0.6560 |
| J5 Vacc | −7.7/−3.5 (7.10) | −1.08 | 0.2859 |
| DIM | −0.3/−0.1 (0.06) | −5.04 | <0.0001 |
| E. coli | −48.0/−21.8 (14.85) | −3.23 | 0.0025 |
| J5 Vacc*E. coli | 43.8/19.9 (17.80) | 2.46 | 0.0185 |
| CALV IGG1 | 13.4/6.1 (6.93) | 1.93 | 0.0607 |
| MAST IGG1 | 21.3/9.7 (7.99) | 2.67 | 0.0112 |
Data from a linear regression model of significant factors associated with the change in daily milk production from 14 days before the onset of CM to 21 days following the end of treatment (PRODDIFF).
J5 Vacc, effect on PRODDIFF following CM of being vaccinated with J5; DIM, effect of each 1-day increase in DIM at onset of CM; E. coli, effect on PRODDIFF of being infected with E. coli; J5 Vacc*E. coli, effect on PRODDIFF of E. coli infection in J5 vaccinates compared with E. coli in controls; CALV IGG1, effect of J5-specific IgG1 antibody postcalving with OD values from 0.3 to 1.0 compared with higher or lower OD values for IgG1; MAST IGG1, effect of J5-specific IgG1 antibody after CM with OD values of ≤1.0 compared with higher OD values for IgG1. Overall linear regression model R2, 0.61, P < 0.0001.
Estimated effects on PRODDIFF following CM, shown in lb and kg.
Cows that calved with J5-specific IgG1 with OD values from 0.3 to 1.0 (P = 0.06) and cows with postmastitic IgG1 with OD values of ≤1.0 (P = 0.01) had significantly lower daily milk losses following CM (Table 4). Other studies have suggested that OD values of <0.3 or >1.0 for J5-specific IgG1 may represent extremely high and low values, respectively (2, 16). There were 17/48 (35.4%) cases with an OD for IgG1 of >1.0 following CM, and nearly all had at least 20% loss of milk production, with a mean of 23% loss, from approximately 90 lb (41 kg) to 69 lb (31 kg) per day before and after CM, respectively. While not statistically significant, there were trends evident when ODs were evaluated as continuous variables; a higher OD for postcalving IgG1 (J5 specific) was associated with less milk loss following CM, but a higher OD for postmastitic IgG1 was associated with more production loss (data not shown).
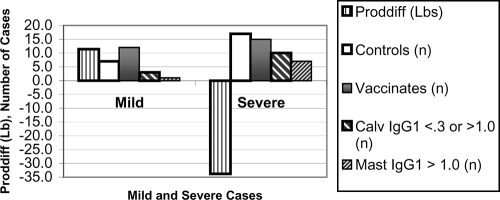
There was benefit shown in this model from J5 vaccination for cows that eventually contracted E. coli CM. Vaccination was associated with 83% of vaccinates having postcalving serum IgG1 OD values in the beneficial range of 0.3 to 1.0, while 63% of controls had values in this range, a nearly statistically significant difference (P = 0.06, Fisher's exact test, PROC FREQ). However, as noted earlier, following CM, the serum J5-specific IgG1 was elevated and production of these antibodies was similar among J5 vaccinates and controls; differences associated with vaccination were obscured by the disease (Table 2). The proportions of cows with antibodies in the beneficial range of postmastitic IgG1 OD values of ≤1.0 were 50% among controls and 48% among vaccinates, values which were not significantly different (P = 0.86, Fisher's exact test). The trends for unvaccinated controls, cows calving with J5-specific IgG1 OD values of <0.3 or >1.0, and cows with postmastitic IgG1 OD values of >1.0 indicating a higher likelihood of having severe CM are shown in Fig. 1.
FIG. 1.
Mild and severe CM defined by production change. Greater milk production loss following CM (severe by definition) is likely among controls, cows calving with IgG1 OD values of <0.3 or >1.0, and cows with postmastitic IgG1 OD values of >1.0. The significant factors are among those in the final linear regression model for milk production change following CM (Table 4).
The lactation number, pathogen causing CM, clinical severity, time from booster immunization to either the due date or the actual calving date, IgG1/IgG2 ratios and IgG2 and IgM, considered either as continuous or as categorical range variables, were not significantly associated with PRODDIFF following CM (for all, P > 0.20, type 3 SS F test, linear regression, PROC GLM). The IgG2 and IgM variables had some relationship to PRODDIFF but were displaced as significant factors from the final model by IgG1.
Culling and death loss as a categorical variable.
When all cows in the two study herds were considered, overall culling (26.7% and 26.4%, respectively) and death loss rates (1.9% and 6.0%, respectively) were not significantly different according to the chi-square test. The 51 new CM cases in the data set affected 45 cows: 17 cows with the 19 mild cases (2 cows each had two mild cases), and 28 cows with the 32 severe cases (4 cows each had two severe cases). Therefore, there were 45 cows each with the possibility of surviving until the end of lactation, until being culled, or until dying.
The four severe-CM cows that were selected based upon being culled or dying soon after CM were culled or died as follows: three cows were culled at 1, 7, and 25 days after onset of CM, between 26 and 118 DIM; one cow died 9 days after onset of CM, at 133 DIM, with the reason for death noted as mastitis. None of these four cases returned from the treated cow group whose milk was discarded, and therefore they had no records of milk postmastitic production. Of the remaining 24 severe CM cows, 16 more were culled (at >30 days after onset of CM and >150 DIM). Therefore, of the cows that contracted at least one severe CM case, 19/28 (67.9%) were culled for all reasons combined. Eventually 5/17 (29.4%) cows that contracted at least one mild case were culled, but all 5 survived for between 83 and 382 days following CM. For all cows, the proportion culled was 24/45 (53.3%). As a categorical variable (culled or not culled), the culling for all reasons among the cows with severe CM was higher than that for the cows with mild CM (P = 0.01, chi-square). The 45 cows included 22 controls and 23 J5 vaccinates. When culling for all reasons was compared only as a categorical variable among controls (14/22; 63.6%) and vaccinates (10/23, 43.5%), the proportion of culls was not significantly different (P = 0.18, chi-square) (Table 5).
TABLE 5.
Comparison of J5 vaccinates and controls leaving the herd because of CMa
| Reason for leaving the herd | No. of J5 vaccinates (%) | No. of controls (%) |
|---|---|---|
| Culled for mastitis | 1 (4)* | 5 (23)* |
| Culled for all reasons | 10 (44) | 14 (64) |
| Died from clinical mastitis | 1 (4) | 0 |
| Died from all causes | 2 (9) | 0 |
Data compare J5 vaccinate cows (n = 23/251) and control cows (n = 22/306), of the 45 cows selected for antibody testing that left the herd because of severe or mild CM. *, P = 0.096.
There were no deaths among the 17 mild-CM cows. Among severe-CM cows, one more died at 198 DIM, with no reason stated for death, for a total of 2/28 severe-CM cows (7.1%) that died. Death as a categorical variable was not significantly different among the severe- or mild-CM cows (P = 0.52, Fisher's exact test), or among controls (0/22) and J5 vaccinates (2/23, 8.7%) (P = 0.49) (Table 5).
Culling for mastitis as the primary reason was recorded for six cows, four severe (14.3%) and two mild (11.8%) cases, which was not significantly different among the two severity groups (P = 0.81, Fisher's exact test). Culling for mastitis as the categorical variable was also evaluated by J5 vaccination status: five controls (22.7%) and one vaccinate (4.3%), a difference approaching significance (P = 0.096, Fisher's exact test) (Table 5). Only one cow, the severe-CM case described above and also a vaccinate, died with mastitis stated as the reason. Death from mastitis was statistically nonsignificant among severe- and mild-CM cows or J5 vaccinates and controls (for both, P > 0.90, Fisher's exact test).
Survival analysis of culling and death loss.
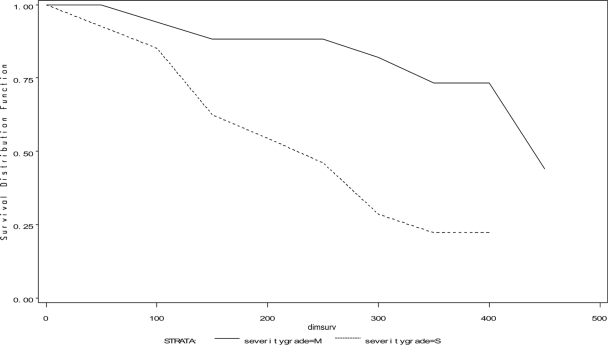
Survival (time-to-event) analysis (PROC LIFETEST) was used to evaluate the hazard of cows being culled or dying over time, rather than simply evaluating whether they were culled or died. Severe-CM cows were more likely to be culled for all reasons and culled sooner during lactation (P < 0.002, time-to-event analysis and log-rank and Wilcoxon tests) than mild-CM cows (Fig. 2). Severe- and mild-CM cows showed no differences in the hazard of death (P > 0.16), the hazard of death from mastitis (P = 0.35), or being culled because of mastitis (P > 0.18).
FIG. 2.
Time to culling for all reasons among mild- and severe-CM case cows. dimsurv, DIM of survival during lactation. Mild-CM case cows (grade M, solid line), severe-CM case cows (grade S, dashed line). A significantly greater hazard of culling for all reasons was shown among cows with one or two severe cases of CM, using time-to-event analysis and log-rank and Wilcoxon tests, P < 0.002.
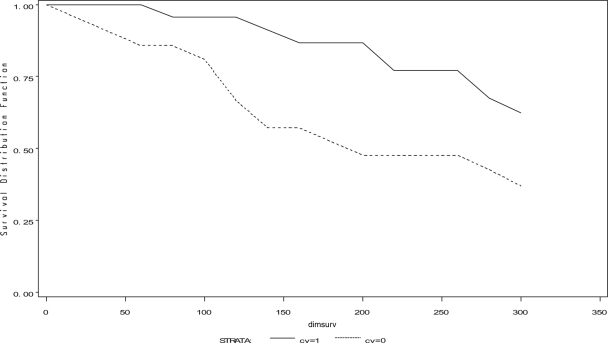
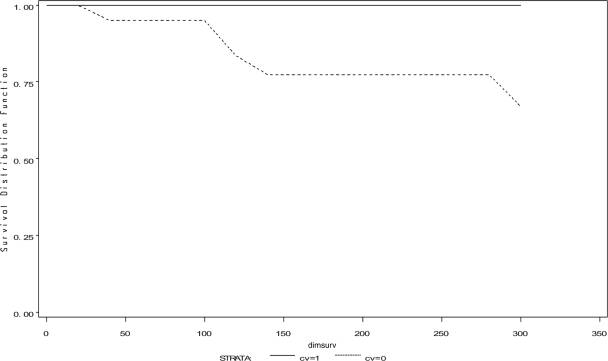
The hazard of being culled for all reasons was significantly less for J5 vaccinates than for controls, and vaccinates were especially less likely to be culled during early lactation (P ≤ 0.05, time-to-event analysis, log-rank and Wilcoxon tests) (Fig. 3). The hazard of culling for mastitis as the reason was also significantly less for vaccinates (P < 0.03) (Fig. 4). Hazards of dying, whether from all reasons or from mastitis, were not different among the J5 vaccinates and the controls (for both, P > 0.27).
FIG. 3.
Time to culling for all reasons among J5 vaccinates and controls. dimsurv, DIM of survival during lactation. J5 vaccinates (solid line); controls (dashed line). A significantly greater hazard of culling for all reasons was shown among controls, using log-rank and Wilcoxon tests, P ≤ 0.05.
FIG. 4.
Time to culling for mastitis among J5 vaccinates and controls. dimsurv, DIM of survival during lactation. J5 vaccinates (solid line); controls (dashed line). A significantly greater hazard of culling for mastitis was shown among controls, using log-rank and Wilcoxon tests, P < 0.03.
Cox's proportional hazards regression model (PROC PHREG) was used to test for multiple factors associated with the likelihood of cows being culled for all reasons. The final model had only one significant factor, severe CM (likelihood ratio chi-square value of 11.2, 1 degree of freedom, P < 0.001; hazard ratio, 5.80), associated with culling. Vaccination with J5 and the J5-specific antibody response were not significantly associated with the likelihood of cows being culled for all reasons (for all, P > 0.05, likelihood ratio chi-square). Cox's proportional hazards regression analysis found no factors related to whether cows were culled for mastitis.
DISCUSSION
Vaccination of dairy cows with J5 was associated with less milk production loss and a lower rate of culling, especially culling during early lactation, following naturally occurring cases of CM, compared with nonvaccinated control cows. This was an important distinction from the case when culling was evaluated only as a categorical variable; J5 protection was shown only when the length of time the cows survived was evaluated. Immunization with J5 appears to confer protection at least partly by increasing antibody against J5. J5 vaccinates had more postcalving J5-specific antibody, with class switching from IgM to IgG1 and IgG2 as an indicator of immunological memory. After calving (approximately 28 days following the second and last J5 immunization), production of J5-specific IgG1 and IgG2 antibody was significantly higher in the sera of vaccinates than in controls, and statistically, J5 vaccination was the main factor associated with increased postcalving IgG1 and IgG2. IgM was not significantly associated with J5 vaccination. Cows with the highest IgG1 antibody production (high responders) at J5 postcalving had the lowest milk loss. These results are similar to those of a previous study indicating that high-antibody-responder cows had less CM (19). This is the first report to show that J5 vaccination was associated with increased survival following naturally occurring cases of CM and with class switching to J5-specific IgG1 and IgG2.
Protection conferred by J5 vaccination decreased as lactation progressed and the time since the last vaccination increased. According to the estimates in the milk production loss linear model, the advantage for J5 vaccinates disappeared at 75 DIM. Waning protection during lactation may be from a lack of long-term memory type immunity associated with this vaccine, at least after two doses, a common practice in the dairy industry. This has not been reported previously.
There was also evidence that there were optimal ranges of anti-J5 antibody production by the cows, above and below which the outcome of mastitis was poor. Both postcalving and postmastitic IgG1 production was associated with less daily milk loss following CM if the antibody response was in the optimal range; if both cases were true, the associated milk production benefits were additive. A threshold of J5-specific IgG1 above which cows are protected against coliform mastitis has been reported (17), but an optimal range of J5-specific IgG1 or IgG2 antibody production above or below which protection was poor has not been described previously for bovine J5. The present results support previous evidence that OD values of <0.3 and >1.0 for IgG1 represent extreme values (2, 16), but those studies did not measure milk production change following disease. These findings are similar to those of a recent intramammary E. coli challenge trial (25), in which cows with the highest production of milk IgM or IgG2 antibodies against J5 following challenge had significantly greater milk production loss. Increased total IgG and IgM specific for the pathogen causing chronic mastitis has been reported for experimentally induced Salmonella enterica serovar Dublin mastitis of 175 days duration in dairy cattle (14). Possibly the highest level of antibodies against mastitis pathogens is found in cows with the most severe or chronic disease or in cows whose immune response is unable to clear the infection from the mammary gland.
The etiologic agent of CM affected antibody production following mastitis. Clinical mastitis caused by coliforms (or negative milk culture results) resulted in significantly higher J5-specific antibodies in serum. This agrees with previous reports that J5-specific antibody was more reactive with coliform bacteria and less reactive with other pathogens in vitro (16, 18). J5 vaccinates lost 75% less milk per day than controls did when infected with E. coli. Particularly strong protection by J5 vaccination against milk production loss following E. coli mastitis, even compared with that of Klebsiella cases, was evident in this study. This contrasted with previous studies that have indicated that J5 worked similarly against E. coli and Klebsiella in terms of reducing the occurrence of CM signs (8) and cross-reactivity of serum from a J5-immunized calf (18). It was also observed in the present study that more J5 vaccinates contracted E. coli CM than controls, which contrasts with some earlier reports that coliform IMI are less likely to develop clinical signs among vaccinates than among controls (7, 8). The protective effects of vaccination in this study were reduced milk loss and delayed culling, not prevention of coliform CM.
Vaccinates and controls had similar J5-specific serum ODs for IgM, IgG1, and IgG2 at the time of dryoff, before vaccination. Vaccinates had higher ODs for IgG1 and IgG2, especially IgG1, after calving (and following vaccination) than controls. After CM, however, the production of J5-specific IgG1 and IgG2 was higher and similar among both vaccinates and controls. Vaccination with J5 appears to result in class switching from IgM to IgG1 and IgG2 antibodies and increased antibody production, similar to that of natural infection resulting in CM, but vaccinates achieve this before the infection and, thus, should be more protected against milk production loss from the disease. It has been speculated that natural infection with coliform mastitis cannot produce the same amount of antibody that J5 vaccination can, partly because prior exposure was not found associated with increased titers against J5 (4, 12, 13). The present results suggest that at least from 17 to 77 days after CM, both natural infection and J5 vaccination produce similar antibody responses specific for J5.
Cows characterized as severe cases (more milk loss) following CM were significantly more likely to be culled for all reasons combined. It could be quite practical if the ability to categorize cows by 21 days following the end of treatment for CM was predictive of long-term survivability in other herds. Any farm with daily milk weight recording could calculate the difference in mean daily milk production from the 14 days before onset and the 21 days following CM. This could be a useful area for further study. The question of whether an even shorter period for calculating mean postmastitic production to determine the mean production loss could be a useful prognostic tool is of interest. It might allow earlier identification of cows that may possibly be culled, so that fewer resources are expended upon them before they are sold.
An important question arising from these results is that of the optimum vaccination schedule for J5 bacterin. If J5 vaccination stimulates protection against mastitis in association with class switching to IgG1 and IgG2 antibodies, resulting in immunological memory, it is quite possible that vaccinating cows more than two or three times per lactation, as is now the convention in the dairy industry, may be cost effective for improving protection against clinical mastitis, as well as for the welfare of dairy cows. Significantly increased J5-specific IgM, IgG1, and IgG2 antibodies were observed following the third of 12 doses of J5 bacterin administered over 170 days in five Holstein steers. Compared to the response after three doses, antibody increased significantly again following the 12th immunization for IgM, the sixth immunization for IgG1, and the seventh immunization for IgG2 (2). In the current study, protection with J5 vaccine appeared to decline as lactation (and the time since vaccination) progressed, disappearing by approximately 75 DIM. It may be true that if most cows can seroconvert to long-lasting high IgG1 levels and/or IgG2 against J5, they might be better protected against mastitis throughout lactation. This requires further investigation.
Acknowledgments
This work was funded by grants from USDA NRICGP, Merial Limited, and the NYS Centers for Advanced Technology Program.
We thank the owners and personnel of the participating dairy farms for their cooperation and the staff members of the Canton Northern Regional Laboratory and the Ithaca Central Regional Laboratory of the Quality Milk Production Services. We also thank Phil Sears and the staff in his laboratory, as well as the laboratory staff of the late Jeanne Burton, both at Michigan State University, for their helpfulness.
Footnotes
Published ahead of print on 3 December 2008.
REFERENCES
- 1.Bartlett, P. C., J. van Wijk, D. J. Wilson, C. D. Green, G. Y. Miller, G. A. Majewski, and L. E. Heider. 1991. Temporal patterns of lost milk production following clinical mastitis in a large Michigan Holstein herd. J. Dairy Sci. 741561-1572. [DOI] [PubMed] [Google Scholar]
- 2.Chaiyotwittayakun, A., J. L. Burton, P. S. Weber, K. Kizilkaya, F. F. Cardoso, and R. J. Erskine. 2004. Hyperimmunization of steers with J5 Escherichia coli bacterin: effects on isotype-specific serum antibody responses and cross reactivity with heterogeneous gram-negative bacteria. J. Dairy Sci. 873375-3385. [DOI] [PubMed] [Google Scholar]
- 3.Cullor, J. S. 1991. The Escherichia coli J5 vaccine: investigating a new tool to combat mastitis. Vet. Med. 86836-844. [Google Scholar]
- 4.Dosogne, H., F. Vangroenweghe, and C. Burvenich. 2002. Potential mechanism of action of J5 vaccine in protection against severe bovine coliform mastitis. Vet. Res. 331-12. [DOI] [PubMed] [Google Scholar]
- 5.Fleming, T. R., and D. Y. Lin. 2000. Survival analysis in clinical trials: past developments and future directions. Biometrics 56971-983. [DOI] [PubMed] [Google Scholar]
- 6.Fuquay, J., A. Zook, and W. Poe. 1975. Metabolic and physiologic response of dairy cattle to coliform mastitis. J. Dairy Sci. 58751-752. [Google Scholar]
- 7.González, R., J. Cullor, D. Jasper, T. Farver, R. Bushnell, and M. Oliver. 1989. Prevention of clinical coliform mastitis in dairy cows by a mutant Escherichia coli vaccine. Can. J. Vet. Res. 53301-305. [PMC free article] [PubMed] [Google Scholar]
- 8.González, R. N., D. J. Wilson, H. N. Mohammed, P. M. Sears, A. L. Rivas, and S. G. Campbell. 1996. A placebo-controlled trial of an Escherichia coli J5 bacterin and the ribotyping-based assessment of coliform bacteria diversity on a dairy farm, p. 277-280. In Proceedings of the 19th World Buiatrics Congress, Edinburgh, Scotland.
- 9.Hogan, J. S., R. N. González, R. J. Harmon, S. C. Nickerson, S. P. Oliver, J. W. Pankey, and K. L. Smith. 1999. Laboratory handbook on bovine mastitis, revised ed., p. 85-111. NMC Inc., Madison, WI.
- 10.Jackson, E., and J. Bramley. 1983. Coliform mastitis. In Pract. 5135-146. [DOI] [PubMed] [Google Scholar]
- 11.Jones, G., and G. Ward. 1989. Cause, occurrence, and clinical signs of mastitis and anorexia in cows in a Wisconsin study. J. Am. Vet. Med. Assoc. 1951108-1113. [PubMed] [Google Scholar]
- 12.Mallard, B. A., L. C. Wagter, M. J. Ireland, and J. C. Dekkers. 1997. Effects of growth hormone, insulin-like growth factor-I, and cortisol on periparturient antibody response profiles of dairy cattle. Vet. Immunol. Immunopathol. 6061-76. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Carlo, V., R. A. Wilson, J. S. McDonald, J. S., and R. A. Packer. 1984. Biochemical and serologic properties of Escherichia coli isolated from cows with acute mastitis. Am. J. Vet. Res. 451771-1774. [PubMed] [Google Scholar]
- 14.Spier, S. J., B. P. Smith, J. S. Cullor, H. J. Olander, L. D. Roden, and G. W. Dilling. 1991. Persistent experimental Salmonella dublin intramammary infection in dairy cows. J. Vet. Intern. 5341-350. [DOI] [PubMed] [Google Scholar]
- 15.Tomita, G. M., C. H. Ray, S. C. Nickerson, W. E. Owens, E., and G. F. Gallo. 2000. A comparison of two commercially available Escherichia coli J5 vaccines against E. coli intramammary challenge. J. Dairy Sci. 832276-2281. [DOI] [PubMed] [Google Scholar]
- 16.Tomita, G. M., D. A. Todhunter, J. S. Hogan, and K. L. Smith. 1995. Antigenic crossreactivity and lipopolysaccharide neutralization properties of bovine immunoglobulin G. J. Dairy Sci. 782745-2752. [DOI] [PubMed] [Google Scholar]
- 17.Tyler, J. W., J. S. Cullor, B. I. Osburn, R. B. Bushnell, and B. W. Fenwick. 1988. Relationship between serological recognition of Escherichia coli O111:B4 (J5) and clinical coliform mastitis in cattle. Am. J. Vet. Res. 491950-1954. [PubMed] [Google Scholar]
- 18.Tyler, J., H. Spears, J. Cullor, W. Smith, R. Nelson, and J. Martin. 1991. Antigenic homology among gram-negative organisms isolated from cattle with clinical mastitis. J. Dairy Sci. 741235-1242. [DOI] [PubMed] [Google Scholar]
- 19.Wagter, L. C., B. A. Mallard, B. N. Wilkie, K. E. Leslie, P. J. Boettcher, and J. C. Dekkers. 2000. A quantitative approach to classifying Holstein cows based on antibody responsiveness and its relationship to peripartum mastitis occurrence. J. Dairy Sci. 83488-498. [DOI] [PubMed] [Google Scholar]
- 20.Wenz, J. R., G. M. Barrington, F. B. Garry, K. D. McSweeney, R. P. Dinsmore, G. Goodell, and R. J. Callan. 2001. Bacteremia associated with naturally occurring acute coliform mastitis in dairy cows. J. Am. Vet. Med. Assoc. 219976-981. [DOI] [PubMed] [Google Scholar]
- 21.Wilson, D. J., P. C. Bartlett, J. H. Kirk, R. W. Mellenberger, and E. C. Mather. 1991. N-Acetyl-beta-d-glucosaminidase as a predictor of milk loss and recovery after clinical mastitis. Am. J. Vet. Res. 521110-1116. [PubMed] [Google Scholar]
- 22.Wilson, D. J., R. N. González, and H. H. Das. 1997. Bovine mastitis pathogens in New York and Pennsylvania: prevalence and effects on somatic cell count and milk production. J. Dairy Sci. 802592-2598. [DOI] [PubMed] [Google Scholar]
- 23.Wilson, D. J., Y. T. Grohn, G. J. Bennett, R. N. González, Y. H. Schukken, and J. Spatz. 2007. Comparison of J5 vaccinates and controls for incidence, etiologic agent, clinical severity, and survival in the herd following naturally occurring cases of clinical mastitis. J. Dairy Sci. 904282-4288. [DOI] [PubMed] [Google Scholar]
- 24.Wilson, D. J., P. S. Herer, and P. M. Sears. 1991. N-Acetyl-beta-d-glucosaminidase, etiologic agent, and duration of clinical signs for sequential episodes of chronic clinical mastitis in dairy cows. J. Dairy Sci. 741539-1543. [DOI] [PubMed] [Google Scholar]
- 25.Wilson, D. J., B. A. Mallard, J. L. Burton, Y. H. Schukken, and Y. T. Grohn. 2007. Milk and serum J5-specific antibody responses, milk production change, and clinical effects following intramammary Escherichia coli challenge for J5 vaccinate and control cows. Clin. Vaccine Immunol. 14693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]