Abstract
The introduction of routine infant immunization with Haemophilus influenzae type b (Hib) conjugate vaccines in the United Kingdom in 1992 led to a significant reduction in invasive disease due to this organism. Subsequently, between 1999 and 2003 there was an increase in the number of immunized children with Hib infection. We investigated whether the rise in cases was related to changes in anti-polyribosylribitol phosphate (PRP) antibody concentration or avidity. Using stored sera, we analyzed temporal changes in antibody levels among 3- to 5-year-old children immunized between 1991 and 2000. Anti-PRP antibody concentrations were higher in 3- to 5-year-olds who received infant immunization in 1991 than those in subsequent years. This difference may be related to changes in either the mode of administration of Hib conjugate vaccines or the rates of Hib nasopharyngeal carriage. This study emphasizes the factors affecting anti-PRP antibody concentration following immunization with conjugate vaccines and the importance of these in long-term protection from invasive disease.
In 1992, the United Kingdom began routine immunization of infants with Haemophilus influenzae type b (Hib) conjugate vaccines given at 2, 3, and 4 months without a booster dose later in childhood (12). The annual incidence of invasive Hib disease fell from a preimmunization rate of 28.3 per 100,000 children under 5 years of age to 0.97 per 100,000 by 1998 (28). Subsequently, between 1999 and the end of 2003, there was a year-by-year increase in the number of fully immunized children who developed invasive Hib disease, with the overall incidence rising to 3.8/100,000 under 5 years of age (28).
Within the United Kingdom, the introduction of a diphtheria-tetanus-acellular pertussis (DTaP)-Hib vaccine was temporally associated with this increase in Hib vaccine failures (26), and a case-control study demonstrated a dose response relationship between the number of doses of DTaP-Hib vaccine and the probability of vaccine failure (30). Combination aP-Hib vaccines are known to be less immunogenic, in terms of anti-polyribosylribitol phosphate (PRP) antibody concentrations, than the Hib component given separately (16). However, prior to the widespread use of DTaP-Hib vaccine, there was already a progressive reduction in the age at which vaccine failures were occurring in successive United Kingdom birth cohorts (42). The possibility that there may have been additional factors responsible for the changes in direct individual protection due to changes in population levels of serum antibody against PRP (the Hib polysaccharide capsular) and immunological memory needs to be considered. Such additional factors include changes in rates of nasopharyngeal carriage of Hib, an alteration in the methods of administration of Hib vaccine, and the use of concomitant serogroup C meningococcal (MenC) vaccines.
Hib conjugate vaccines reduce Hib carriage rates (5). While a reduction in carriage will increase herd immunity, it may also decrease the “natural boosting” of anti-PRP antibody levels and affect the ability of individuals to maintain protective concentrations of antibody (33).
The efficacy of Hib conjugates was initially demonstrated when the vaccine was given at a separate site from other vaccines given at the same visit (22). After Hib vaccine introduction in the United Kingdom, immunization practice changed to include routine reconstitution of Hib with diphtheria-tetanus-whole-cell pertussis (DTwP) vaccines prior to administration at a single site and an increasing use of combination Hib-DTwP vaccines. The effect on vaccine effectiveness of these practices has not been studied.
The increase in vaccine failures from 1999 onwards was temporally associated with the introduction of universal infant immunization with MenC conjugate vaccine. The effect of concomitant MenC and Hib vaccine administrations on anti-PRP concentration was not assessed extensively prior to its introduction. A recent study, however, demonstrated that in some situations MenC conjugates may reduce the anti-PRP antibody responses to Hib conjugates (27).
Thus, several factors may have contributed to a decrease in the direct protection conferred by Hib conjugate vaccines for children immunized in infancy in the United Kingdom. Despite the potential importance of changes in population levels of direct protection, there is only one study that has considered changes in anti-PRP antibody levels in children since 1992 (41). In that study immunization details for the individuals whose serum samples were used were not given, and in addition, that study did not assess changes in any marker of immunological memory. In the current study we have investigated changes in the persistence of anti-PRP antibody concentration and the avidity index (as a marker of immunological memory) using the stored sera of children immunized between 1991 and 2000.
MATERIALS AND METHODS
Serum samples.
Samples were selected from sera stored at either −20°C or −80°C since their collection as part of clinical trials conducted by the Oxford Vaccine Group, University of Oxford. The sera were selected from studies in which children were 3 to 5 years of age and had received three doses of Hib polysaccharide-tetanus toxoid conjugate vaccine (PRP-T) and a wP-containing combination vaccine as part of primary immunization between 1991 and 2000. All children received their primary immunizations at 2, 3, and 4 months of age. To avoid selection bias, sera from all studies fitting the selection criteria were used. Sera fitting the selection criteria were available from five studies (Table 1). The Hib vaccine was given at a separate injection site from the DTwP vaccine (group A), was reconstituted by mixing together prior to administration at a single site (group C), or was given in the form of a combination vaccine (group D, ACT-Hib plus DPT; Pasteur-Merieux). For some children the method of administration of Hib vaccine could not be confirmed (groups B and E). Some of the children had received an infant schedule of MenC vaccine at the same time as the routine immunizations (groups C and E). Ethical approval for the use of these samples was obtained from the Oxford Clinical Research Ethics Committee (reference number C02.013).
TABLE 1.
Characteristics of the groups of 3- to 5-year-old children whose stored sera were used in the study of anti-PRP antibody concentration and avidity
| Group | Yr of primary immunization | Yr of serum collection | No. of serum samples | Median age in yr (range) | PRP-T (Hib) vaccination modea | MenC vaccine in primary schedule |
|---|---|---|---|---|---|---|
| A | 1991 | 1994 | 87 | 3.6 (3.5-3.7) | Separate | No |
| B | 1995 to 1996 | 2000 | 79 | 4.5 (4.0-5.2) | NA | No |
| C | 1995 to 1996 | 2000 | 64 | 4.5 (4.1-4.9) | Reconstituted with DTwP | Mixed |
| D | 1997 to 1998 | 2002 | 72 | 4.0 (3.6-5.0) | Combination vaccine† | No |
| E | 1999 to 2000 | 2003 | 42 | 3.2 (3.0-3.4) | NA | Yes |
PRP-T (Hib) vaccination mode refers to whether the Hib vaccine was given at a separate site to other vaccines, reconstituted with DTwP vaccine prior to giving it at the same site, or in the form of a combination vaccine. NA, no confirmation available regarding the mode of administration of the PRP-T vaccine. †, two children given separate PRP-T vaccines and no confirmation of the mode of administration of the PRP-T vaccine for eight children.
Hib anti-PRP ELISA.
Anti-PRP antibody concentrations were measured for all serum samples by using a standard Hib enzyme-linked immunosorbent assay (ELISA) (9). Ninety-six-well polystyrene plates (Nunc Polysorb) were coated with PRP conjugated to human albumin (obtained from the National Institute for Biological Standards and Controls) at a concentration of 2 μg/ml in phosphate-buffered saline buffer and incubated at 37°C for 90 min before storing at 4°C until use. Sera and control samples were diluted and added to the PRP-coated plates and incubated for 2 h. Immunoglobulin G (IgG) antibodies were then detected with goat anti-human IgG alkaline phosphatase-conjugated sera (Sigma A9544) left on the plates for 1 h. IgG binding was quantified by adding p-nitrophenyl phosphate (Sigma 104) diluted in diethanolamine substrate buffer. The reaction was monitored by taking absorbance readings at 450 nm (MRX-TC microplate reader; Revelation). The reaction was stopped by the addition of 3 M NaOH at a set absorbance reading for wells containing the anti-PRP reference serum (lot 1983; FDA). The antibody concentrations were determined by reference to a standard curve, generated using four-parameter sigmoid curve fitting, from known dilutions of an anti-PRP reference serum (lot 1983; FDA). The minimum sensitivity of the assay was estimated to be an anti-PRP concentration of 0.1 μg/ml.
Hib anti-PRP avidity assay.
Anti-PRP antibody avidity was determined using a thiocyanate elution assay described elsewhere (19). Serum samples were first assayed for anti-PRP concentration using the anti-PRP ELISA. Due to the sensitivity of the ELISA, only sera with a titer of at least 0.2 μg/ml were analyzed for anti-PRP antibody avidity. A dilution was then calculated that would yield an optical density of either 0.5 or 1.0 at 450 nm in the ELISA. Serum samples at the predetermined dilution were added to the 96-well plates described above and incubated for 2 h. Ammonium thiocyanate of various concentrations (0.8 M, 0.4 M, 0.2 M, 0.1 M, 0.05 M, and 0.25 M) was then added to duplicate wells of each serum sample tested, with one set of duplicate wells having no ammonium thiocyanate added. After 15 min of incubation, the plate was washed and the ELISA method was continued to the end, from the addition of anti-human IgG conjugate to the addition of alkaline phosphatase. The avidity index of anti-PRP antibody in a serum sample was expressed as the molar concentration of thiocyanate required to reduce the optical density of the final ELISA reading by 50%.
Statistical analysis.
For each group of sera, the Hib anti-PRP antibody concentration and avidity index were log transformed and geometric means were calculated, together with 95% confidence intervals. For each group, the proportion of individuals with concentrations above those considered necessary for both long- and short-term protection (1.0 μg/ml and 0.15 μg/ml, respectively) was calculated. These groups were compared using the chi-square test for trend. The effects of year of serum collection, concomitant MenC conjugate vaccine, and age on the logarithmically transformed anti-PRP antibody concentration and avidity index were investigated independently by simple linear regression analysis.
RESULTS
Anti-PRP antibody concentrations at 3 to 5 years of age.
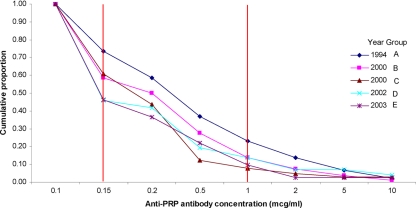
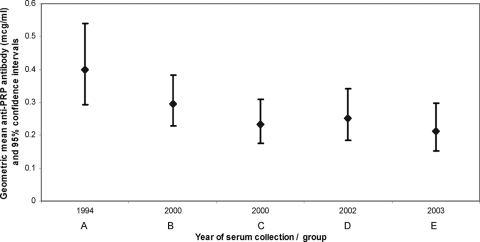
The reverse cumulative distribution of anti-PRP antibody concentrations (Fig. 1) demonstrates that there was a reduction in the proportion of individuals with anti-PRP concentrations greater than 0.15 μg/ml during the period of 1994 to 2003 (chi-square test for trend, P < 0.02). However, there was no significant trend for the proportion with concentrations greater than 1.0 μg/ml across the same period (chi-square test for trend, P > 0.2). Geometric mean anti-PRP concentrations were highest in the earliest periods of serum collection (Fig. 2). Linear regression analysis of anti-PRP antibody concentration against the variables of age, sex, avidity index, concomitant MenC immunization, and year of serum collection (Table 2) demonstrated a significant effect for year of serum collection (P = 0.03) and concomitant MenC vaccine (P = 0.04). There was a negative linear trend across the time period (P = 0.003). The direction of the effect for concomitant MenC vaccine was for lower anti-PRP antibody concentrations.
FIG. 1.
Reverse cumulative distribution of anti-PRP antibody concentrations (μg/ml) for groups of children whose sera were collected between 1994 and 2003. The two vertical lines indicate the cutoff for the titers associated with short-term (>0.15 μg/ml) and long-term (>1.0 μg/ml) protection from invasive Hib disease.
FIG. 2.
Geometric mean anti-PRP antibody concentrations (μg/ml) with 95% confidence intervals (error bars) for children aged 3 to 5 years with a primary immunization schedule of 2, 3, and 4 months in studies conducted between 1995 and 2003. Studies are identified by year of serum collection and group letter (see Table 1).
TABLE 2.
Linear regression analysis of factors likely to influence anti-PRP antibody concentrations and avidity indexes at 3 to 5 years of agea
| Factor | Log anti-PRP antibody | Log anti-PRP avidity index |
|---|---|---|
| Log anti-PRP avidity index | 0.052 | NA |
| Log anti-PRP antibody | NA | 0.052 |
| Yr of serum collection | 0.03* | 0.06 |
| Linear trend | 0.003* | 0.66 |
| Age | 0.97 | 0.004* |
| Concomitant MenC | 0.04* | 0.09 |
Values are P values, and asterisks indicate those that are significant (P values of <0.05). NA, not applicable.
Anti-PRP avidity index at 3 to 5 years of age.
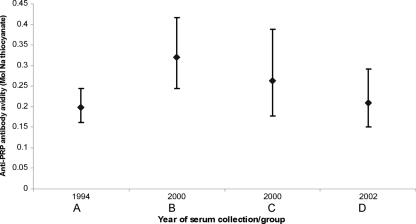
The geometric mean avidity index by year of serum collection is shown in Fig. 3. Linear regression analysis (Table 2) demonstrated a significant effect of age on anti-PRP avidity (P = 0.004), but there was no significant linear trend (P = 0.66) across the time period of the analysis.
FIG. 3.
Geometric mean anti-PRP antibody avidity indexes (M ammonium thiocyanate) together with 95% confidence intervals (error bars) by year of serum collection, for children aged 3 to 5 years and immunized on a primary schedule at 2, 3, and 4 months, from sera collected in studies conducted between 1994 and 2002. Studies are identified by year of serum collection and group letter (see Table 1).
The effect of mode of administration of PRP-T vaccine (whether at a separate injection site, reconstituted with DTwP, or in a combination vaccine) could not be analyzed by simple linear regression analysis, as there was information from too few groups to allow this to be done independently of other factors (e.g., year of serum collection and concomitant MenC administration).
DISCUSSION
In this study there is a trend for the proportion of immunized 3- to 5-year-old children with anti-PRP antibody concentrations of >0.15 μg/ml to be greater the earlier they were immunized in relation to the introduction of routine infant immunization. There was no consistent trend in the anti-PRP antibody avidity index during the same study period.
In all groups of sera there was a relatively low proportion of children with anti-PRP levels above the threshold for long-term protection (1.0 μg/ml). This has been well described previously for the United Kingdom population. The lack of large numbers of vaccine failures, despite low population levels of anti-PRP antibody, had been ascribed to the protection conferred by immunological memory (23). However, subsequent analyses of effectiveness data have suggested the vulnerability conferred by low anti-PRP concentrations and, together with studies of vaccine failures, questioned the protection conferred by immunological memory (32).
There is only one previous study of anti-PRP sero-epidemiology in the United Kingdom covering the time period before and after the introduction of routine Hib conjugate infant immunization (41). In that study cross-sectional age-specific sera were used for each of five time periods between 1991 and 2003. Except for the effect of the “catch-up” campaign, there were no changes in the anti-PRP concentration for any age group. However, details of the immunizations were not available for these children, and in addition, that study did not assess any markers of immunological memory. Other authors have noted an apparent decline in the immunogenicity of Hib conjugate vaccines coincident with the widespread use of infant Hib immunization, and some have speculated on the role of declining Hib carriage rates (20, 37).
Changes in population levels of anti-PRP antibody after the introduction of routine Hib immunization may be due to the following: (i) reduction in “natural boosting” due to Hib carriage or organisms with cross-reactive antigens, (ii) changes in the way in which the Hib component of the immunization was given (separately, reconstituted with DTwP vaccine, or given as a combination vaccine), or (iii) the use of concomitant MenC conjugate vaccine.
The effect of Hib carriage.
In the prevaccine era, the anti-PRP antibody concentration fell in the first few months of life as maternally acquired antibody was lost (2). The nadir of anti-PRP antibody coincided with the peak of invasive Hib disease in early childhood. The subsequent rise in anti-PRP antibody concentration was attributed to the immune response to nasopharyngeal carriage of Hib or exposure to cross-reactive organisms such as Escherichia coli K-100 (6, 36). This phenomenon of carriage inducing antibody has been termed natural boosting. A prominent feature of all glyco-conjugate vaccines against the common encapsulated bacterial pathogens of childhood is that they significantly reduce nasopharyngeal carriage in an immunized population (7). The advantage of this reduction in carriage is the resulting herd immunity, whereby there is a decrease in disease, even in unvaccinated individuals, and enhanced protection of those who have been vaccinated (21, 25, 35, 38). However, in addition to generating herd immunity, a decrease in carriage will also reduce the opportunities for natural boosting and may result in lower levels of persistent antibody than would have otherwise been the case. In Oxfordshire, United Kingdom, carriage rates in young children declined from 3.98% in an unvaccinated population, prior to 1992, to 0% by 1997 and 2000 (31). The serum samples in this study span this period, and the observed reduction in the proportion of individuals with protective anti-PRP antibody concentrations is consistent with a reduction in natural boosting. There are already some data from the United Kingdom demonstrating that anti-PRP antibody concentrations in unvaccinated adults decline with decreasing rates of carriage and that this is associated with an increase in the rates of invasive disease (33).
Method of administration of Hib vaccine.
The reconstitution of PRP-T with DTP prior to administration was given official support by the Department of Health in early 1999 (15) but was also used prior to this. Reconstituting Hib glyco-conjugate vaccines with DTwP can result in lower anti-PRP concentrations immediately following infant immunization compared to administering vaccines concurrently at different sites (3, 17, 18, 24). However, other studies have found no significant difference between groups receiving mixed or separate vaccines (4, 8, 34, 39). Almost all of the comparative studies relate to anti-PRP concentrations at 1 month after primary immunization, and there is little information on whether differences in immunogenicity are reflected in subsequent antibody persistence.
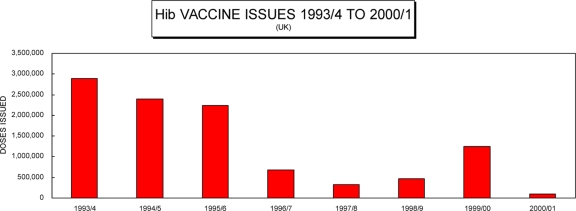
Hib-DTwP combination vaccines were introduced in the United Kingdom in 1996, and their use was associated with a decline in the use of separate Hib vaccine (Fig. 4). Information is limited regarding the relative immunogenicity of Hib combination vaccines compared to that of other modes of administration. A prelicensure study had shown comparable levels of immunogenicity for a DTwP-PRP-T combination vaccine and a PRP-T vaccine reconstituted with DTwP immediately prior to immunization (1). However, the data comparing combination vaccines to vaccines given separately relate to HibOC (a CRM197-conjugated oligosaccharide Hib conjugate vaccine). Two studies demonstrated a lower anti-PRP response for the HibOC vaccines given separately than for the combination form (9a, 37). Once again it is not clear whether differences in initial immunogenicity levels are reflected in subsequent long-term antibody persistence. There is little data allowing direct comparison of levels of antibody persistence for Hib conjugate vaccines given as part of combination vaccines and for those given separately.
FIG. 4.
Doses of the single Hib vaccine issued by the Department of Health over the period from 1993 and 1994 to 2000 and 2001. (Data are from the United Kingdom Department of Health, Vaccine Supply and Finance Team.)
In the current study there were too few groups with complete information on the method of administration of Hib vaccine to allow an analysis to be undertaken independently of other factors (such as study period and concomitant MenC administration). This was due to the fact that the only group who had Hib vaccine confirmed as given separately also had no concomitant MenC vaccine and was the earliest group in relation to the introduction of routine Hib immunization in the United Kingdom.
Concomitant MenC glyco-conjugate vaccine.
Interactions may occur between concomitantly administered protein-polysaccharide vaccines. The administration of a CRM197-conjugated 9-valent pneumococcal polysaccharide-MenC combination vaccine (PnC9-MenC) resulted in reduced MenC bactericidal titers compared to the MenC component given alone (11). This has been attributed to the phenomenon of carrier-induced epitope suppression, whereby a limited amount of T-cell help is divided between increasing numbers of antigens and may thereby reduce the magnitude of response to an individual antigen. A similar effect has also been seen for vaccines with similar carrier proteins given at different sites. A study of a tetravalent pneumococcal PRP-T conjugate given at a separate site to the PRP-T vaccine showed reduced anti-PRP concentrations compared to the vaccine given alone (13). In addition, noncarrier-specific suppression was seen in the PnC9-MenC study reduction of anti-PRP responses to a PRP-T Hib conjugate vaccine when given with the PnC9-MenC conjugate, despite not sharing carrier proteins (11). These effects are not easily predictable. In a study involving DTwP-PRP-T given at a separate site to a MenC vaccine conjugated to either CRM197 or tetanus toxoid, a better anti-PRP response was seen with the tetanus toxoid conjugate (27). A recent review of the effect of concomitant MenC conjugates on the responses to Hib conjugate vaccines, including previously unpublished trial data, indicated that there was a trend to lower anti-PRP antibodies in children given concomitant CRM197-conjugated MenC vaccines (14). However, the conclusions of the review were drawn from comparisons between studies rather than direct comparisons within a trial. Most of the information on the interference of conjugate vaccines relates to immediate immunogenicity, and there is little information on the persistence of antibody concentration.
In groups C and E, infant immunization included a CRM197-conjugated MenC polysaccharide vaccine. At 3 to 5 years of age, there was an association of borderline significance between concomitant administration of MenC conjugate vaccine and lower anti-PRP antibody concentrations. However, within group C, individuals had been randomized to receive MenC or hepatitis B vaccine as part of the primary immunization schedule, and there was no between-group difference in anti-PRP antibody concentrations at 3 years of age (data not shown). Hence, in the current study the evidence is weak that concomitant MenC administration was an important contributor to lower anti-PRP antibody concentrations.
Anti-PRP antibody avidity.
Antibody produced following administration of Hib conjugate vaccine undergoes a gradual increase in avidity in the weeks following primary immunization in infancy (19). The increase in antibody avidity occurs due to the selection of B cells with higher affinity for antigen in germinal centers, and avidity has been proposed as a marker of successful induction of immunological memory (19). The avidity of anti-PRP antibody varies with the particular Hib conjugate vaccine used. Such variations have been linked to the function of antibody, higher levels of avidity corresponding to more functional antibody (40). Hib vaccine failures in the United Kingdom occurred despite evidence of immunological memory among individuals with vaccine failure (32). However, this leaves open the question of whether there were differences in some aspect of immunological memory between vaccine failures and children who remained protected. Immunological studies of a small number of Hib vaccine failures from Holland measured a lower avidity in vaccine failures than in controls who did not have invasive Hib disease (10). This is consistent with work on United Kingdom vaccine failures (29). Unlike with anti-PRP antibody concentration, there are no recognized correlates of protection for the anti-PRP antibody avidity index, but it has been presumed that a higher avidity is associated with more effective priming (19).
In the current study there was not a significant effect of year of serum collection on the anti-PRP avidity index and there was no consistent trend across the time period. There is thus no evidence of a systematic change in avidity as an additional marker of antibody function and immunological memory, which could explain increasing numbers of vaccine failures.
Limitations.
In this study a relatively restricted number of groups of serum samples were available in relation to the number of variables that might have affected the anti-PRP concentration and avidity index (e.g., Hib carriage, age, mode of administration of Hib vaccine, and concomitant MenC vaccine). This limited the power to determine which of these elements were independently responsible for the decline in antibody concentration observed. Following a schedule of infant immunization with Hib conjugate vaccine at 2, 3, and 4 months, and in the absence of further boosting, an initial peak anti-PRP antibody level declines quickly over the following months (23). United Kingdom data indicate that population values for anti-PRP concentrations reach a constant level between 2 and 5 years, before gradually increasing again (41). The sera that are the subject of the current study are from individuals between 3 and 5 years of age, when, in the absence of other factors, one would expect relatively constant anti-PRP antibody concentrations, and thus, age is unlikely to have confounded the analysis of anti-PRP concentrations. A further consideration for this study is that serum samples were obtained from individuals recruited for previous studies rather than randomly selected from the general population and thus may not be truly representative of trends within the population. However, all the children were recruited from the general population, and they were thus exposed to the same opportunities for natural boosting through Hib carriage as that population. Furthermore, the trend to decreasing use of immunization with separate Hib vaccine seen within the groups studied is reflected in the changes in practice within the wider population.
Conclusions.
A reduction in the persistence of anti-PRP antibody concentrations after the introduction of routine Hib immunization in the United Kingdom may have been a significant factor in the epidemiology of Hib vaccine failures prior to 1999 (42). Despite a decrease in anti-PRP concentrations between successive cohorts of children studied, there was no change in a marker of immunological memory, the anti-PRP avidity index. Factors that are likely to have influenced anti-PRP antibody concentrations in the children in the current study, such as a reduction in the rate of nasopharyngeal carriage of Hib, may also have exaggerated any decrease in antibody persistence that arose from the subsequent use of aP-Hib combination vaccines and ultimately precipitated an increase in vaccine failures from 1999 onwards.
The accretion of a number of individually small differences in immunogenicity may be relatively unnoticed when licensing is undertaken with comparisons of immunogenicity levels with currently licensed vaccines rather than with efficacy studies. Efficacy studies are impractical in vaccinated populations, and it is therefore important to maintain good quality surveillance over a period of many years following the introduction of a vaccine in order to detect any changes in effectiveness.
These findings are of significance for all protein-polysaccharide conjugate vaccines for which carriage may be an important mechanism for maintaining protective immunity and where there is the potential for interactions with other vaccines.
Acknowledgments
We express our gratitude to the children and families who participated in the various studies and from whom the sera are derived and to the clinical research team at the Oxford Vaccine Group, University of Oxford. A.J.P. and E.R.M. are Jenner Institute Investigators. This study was funded by a Wellcome Trust entry-level fellowship to D.F.K.
Conflict of interest statement: A.J.P. has conducted clinical trials on behalf of Oxford University, sponsored vaccine manufacturers, and received assistance from vaccine manufacturers to attend scientific meetings. Industry-sourced honoraria for lecturing or writing are paid directly to an independent charity or an educational/administrative fund held by the Department of Paediatrics, University of Oxford. E.R.M. has received assistance from vaccine manufacturers to attend scientific meetings and serves on the Scientific Advisory Board of Novartis Vaccines and Diagnostics. D.F.K. and L.M.Y. have no conflicts of interest.
Footnotes
Published ahead of print on 10 September 2008.
REFERENCES
- 1.Amir, J., R. Melamed, J. Bader, C. Ethevenaux, B. Fritzell, J. R. Cartier, F. Arminjon, and R. Dagan. 1997. Immunogenicity and safety of a liquid combination of DTP-PRP-T vs lyophilized PRP-T reconstituted with DTP. Vaccine 15149-154. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, P., R. B. Johnston, Jr., and D. H. Smith. 1972. Human serum activities against Hemophilus influenzae, type b. J. Clin. Investig. 5131-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo, O. O., E. Forleo-Neto, G. N. Vespa, R. F. Puccini, L. W. Weckx, E. S. Carvalho, and C. K. Farhat. 2000. Associated or combined vaccination of Brazilian infants with a conjugate Haemophilus influenzae type b (Hib) vaccine, a diphtheria-tetanus-whole-cell pertussis vaccine and IPV or OPV elicits protective levels of antibodies against Hib. Vaccine 19367-375. [DOI] [PubMed] [Google Scholar]
- 4.Avendano, A., C. Ferreccio, R. Lagos, I. Horwitz, M. Cayazzo, B. Fritzell, C. Meschievitz, and M. Levine. 1993. Haemophilus influenzae type b polysaccharide-tetanus protein conjugate vaccine does not depress serologic responses to diphtheria, tetanus or pertussis antigens when coadministered in the same syringe with diphtheria-tetanus-pertussis vaccine at two, four and six months of age. Pediatr. Infect. Dis. J. 12638-643. [DOI] [PubMed] [Google Scholar]
- 5.Barbour, M. L. 1996. Conjugate vaccines and the carriage of Haemophilus influenzae type b. Emerg. Infect. Dis. 2176-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbour, M. L., R. Booy, D. W. Crook, H. Griffiths, H. M. Chapel, E. R. Moxon, and D. Mayon-White. 1993. Haemophilus influenzae type b carriage and immunity four years after receiving the Haemophilus influenzae oligosaccharide-CRM197 (HbOC) conjugate vaccine. Pediatr. Infect. Dis. J. 12478-484. [DOI] [PubMed] [Google Scholar]
- 7.Barbour, M. L., R. T. Mayon-White, C. Coles, D. W. Crook, and E. R. Moxon. 1995. The impact of conjugate vaccine on carriage of Haemophilus influenzae type b. J. Infect. Dis. 17193-98. [DOI] [PubMed] [Google Scholar]
- 8.Begg, N. T., E. Miller, C. K. Fairley, H. M. Chapel, H. Griffiths, P. A. Waight, and L. A. Ashworth. 1995. Antibody responses and symptoms after DTP and either tetanus or diphtheria Haemophilus influenzae type B conjugate vaccines given for primary immunisation by separate or mixed injection. Vaccine 131547-1550. [DOI] [PubMed] [Google Scholar]
- 9.Booy, R., S. A. Taylor, S. R. Dobson, D. Isaacs, G. Sleight, S. Aitken, H. Griffiths, H. Chapel, R. T. Mayon-White, J. A. Macfarlane, et al. 1992. Immunogenicity and safety of PRP-T conjugate vaccine given according to the British accelerated immunisation schedule. Arch. Dis. Child. 67475-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Botet Asensi, F. I., A. Veronese, M. Del Carmen Otero, M. Desamparados Tamarit Pérez, J. L. Hontangas Lopez, and S. Viviani. 2003. Immunogenicity and safety in infants of a DTwPHib full liquid vaccine. Acta Paediatr. 92:541-545. [DOI] [PubMed] [Google Scholar]
- 10.Breukels, M. A., E. Jol-van der Zijde, M. J. van Tol, and G. T. Rijkers. 2002. Concentration and avidity of anti-Haemophilus influenzae type b (Hib) antibodies in serum samples obtained from patients for whom Hib vaccination failed. Clin. Infect. Dis. 34191-197. [DOI] [PubMed] [Google Scholar]
- 11.Buttery, J. P., A. Riddell, J. McVernon, T. Chantler, L. Lane, J. Bowen-Morris, L. Diggle, R. Morris, A. Harnden, S. Lockhart, A. J. Pollard, K. Cartwright, and E. R. Moxon. 2005. Immunogenicity and safety of a combination pneumococcal-meningococcal vaccine in infants: a randomized controlled trial. JAMA 2931751-1758. [DOI] [PubMed] [Google Scholar]
- 12.Cartwright, K. A. 1992. Vaccination against Haemophilus influenzae b disease. BMJ 305485-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dagan, R., J. Eskola, C. Leclerc, and O. Leroy. 1998. Reduced response to multiple vaccines sharing common protein epitopes that are administered simultaneously to infants. Infect. Immun. 662093-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dagan, R., J. T. Poolman, and F. Zepp. 2008. Combination vaccines containing DTPa-Hib: impact of IPV and coadministration of CRM197 conjugates. Expert Rev. Vaccines 797-115. [DOI] [PubMed] [Google Scholar]
- 15.Department of Health. 1999. Current vaccine issues: action update. PL/CPHO/99/4. Department of Health, London, United Kingdom.
- 16.Eskola, J., J. Ward, R. Dagan, D. Goldblatt, F. Zepp, and C. A. Siegrist. 1999. Combined vaccination of Haemophilus influenzae type b conjugate and diphtheria-tetanus-pertussis containing acellular pertussis. Lancet 3542063-2068. [DOI] [PubMed] [Google Scholar]
- 17.Ferreccio, C., J. Clemens, A. Avendano, I. Horwitz, C. Flores, L. Avila, M. Cayazzo, B. Fritzell, M. Cadoz, and M. Levine. 1991. The clinical and immunologic response of Chilean infants to Haemophilus influenzae type b polysaccharide-tetanus protein conjugate vaccine coadministered in the same syringe with diphtheria-tetanus toxoids-pertussis vaccine at two, four and six months of age. Pediatr. Infect. Dis. J. 10764-771. [DOI] [PubMed] [Google Scholar]
- 18.Gold, R., D. Scheifele, L. Barreto, S. Wiltsey, G. Bjornson, W. Meekison, R. Guasparini, and L. Medd. 1994. Safety and immunogenicity of Haemophilus influenzae vaccine (tetanus toxoid conjugate) administered concurrently or combined with diphtheria and tetanus toxoids, pertussis vaccine and inactivated poliomyelitis vaccine to healthy infants at two, four and six months of age. Pediatr. Infect. Dis. J. 13348-355. [DOI] [PubMed] [Google Scholar]
- 19.Goldblatt, D., A. R. Vaz, and E. Miller. 1998. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J. Infect. Dis. 1771112-1115. [DOI] [PubMed] [Google Scholar]
- 20.Granoff, D. M. 2001. Assessing efficacy of Haemophilus influenzae type b combination vaccines. Clin. Infect. Dis. 33(Suppl. 4)S278-S287. [DOI] [PubMed] [Google Scholar]
- 21.Gray, S. J., C. L. Trotter, M. E. Ramsay, M. Guiver, A. J. Fox, R. Borrow, R. H. Mallard, and E. B. Kaczmarski. 2006. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J. Med. Microbiol. 55887-896. [DOI] [PubMed] [Google Scholar]
- 22.Heath, P. T. 1998. Haemophilus influenzae type b conjugate vaccines: a review of efficacy data. Pediatr. Infect. Dis. J. 17S117-S122. [DOI] [PubMed] [Google Scholar]
- 23.Heath, P. T., R. Booy, H. J. Azzopardi, M. P. Slack, J. Bowen-Morris, H. Griffiths, M. E. Ramsay, J. J. Deeks, and E. R. Moxon. 2000. Antibody concentration and clinical protection after Hib conjugate vaccination in the United Kingdom. JAMA 2842334-2340. [DOI] [PubMed] [Google Scholar]
- 24.Hoppenbrouwers, K., R. Lagos, B. Swennen, C. Ethevenaux, J. Knops, M. M. Levine, and J. Desmyter. 1998. Safety and immunogenicity of an Haemophilus influenzae type b-tetanus toxoid conjugate (PRP-T) and diphtheria-tetanus-pertussis (DTP) combination vaccine administered in a dual-chamber syringe to infants in Belgium and Chile. Vaccine 16921-927. [DOI] [PubMed] [Google Scholar]
- 25.Hviid, A., and M. Melbye. 2004. Impact of routine vaccination with a conjugate Haemophilus influenzae type b vaccine. Vaccine 22378-382. [DOI] [PubMed] [Google Scholar]
- 26.Kelly, D. F., E. R. Moxon, and A. J. Pollard. 2004. Haemophilus influenzae type b conjugate vaccines. Immunology 113163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitchin, N. R., J. Southern, R. Morris, F. Hemme, S. Thomas, M. W. Watson, K. Cartwright, and E. Miller. 2007. Evaluation of a diphtheria-tetanus-acellular pertussis-inactivated poliovirus-Haemophilus influenzae type b vaccine given concurrently with meningococcal group C conjugate vaccine at 2, 3 and 4 months of age. Arch. Dis. Child. 9211-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ladhani, S., M. P. Slack, P. T. Heath, and M. E. Ramsay. 2006. Changes in ascertainment of Hib and its influence on the estimation of disease incidence in the United Kingdom. Epidemiol. Infect. [Epub ahead of print.] doi: 10.1017/S0950268806007382. [DOI] [PMC free article] [PubMed]
- 29.Lee, Y. C., D. Kelly, L. M. Yu, M. P. E. Slack, R. Booy, P. T. Heath, C.-A. Siegrist, R. E. Moxon, and A. J. Pollard. 2008. Haemophilus influenzae type b vaccine failure in children is associated with inadequate production of high-quality antibody. Clin. Infect. Dis. 46186-192. [DOI] [PubMed] [Google Scholar]
- 30.McVernon, J., N. Andrews, M. P. Slack, and M. E. Ramsay. 2003. Risk of vaccine failure after Haemophilus influenzae type b (Hib) combination vaccines with acellular pertussis. Lancet 3611521-1523. [DOI] [PubMed] [Google Scholar]
- 31.McVernon, J., A. J. Howard, M. P. Slack, and M. E. Ramsay. 2004. Long-term impact of vaccination on Haemophilus influenzae type b (Hib) carriage in the United Kingdom. Epidemiol. Infect. 132765-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McVernon, J., P. D. Johnson, A. J. Pollard, M. P. Slack, and E. R. Moxon. 2003. Immunologic memory in Haemophilus influenzae type b conjugate vaccine failure. Arch. Dis. Child. 88379-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McVernon, J., C. L. Trotter, M. P. Slack, and M. E. Ramsay. 2004. Trends in Haemophilus influenzae type b infections in adults in England and Wales: surveillance study. BMJ 329655-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, M. A., C. K. Meschievitz, G. A. Ballanco, and R. S. Daum. 1995. Safety and immunogenicity of PRP-T combined with DTP: excretion of capsular polysaccharide and antibody response in the immediate post-vaccination period. Pediatrics 95522-527. [PubMed] [Google Scholar]
- 35.Musher, D. M. 2006. Pneumococcal vaccine-direct and indirect (“herd”) effects. N. Engl. J. Med. 3541522-1524. [DOI] [PubMed] [Google Scholar]
- 36.Ostrow, P. T., E. R. Moxon, N. Vernon, and R. Kapko. 1979. Pathogenesis of bacterial meningitis. Studies on the route of meningeal invasion following Hemophilus influenzae inoculation of infant rats. Lab. Investig. 40678-685. [PubMed] [Google Scholar]
- 37.Paradiso, P. R., D. A. Hogerman, D. V. Madore, H. Keyserling, J. King, K. S. Reisinger, M. M. Blatter, E. Rothstein, H. H. Bernstein, and J. Hackell. 1993. Safety and immunogenicity of a combined diphtheria, tetanus, pertussis and Haemophilus influenzae type b vaccine in young infants. Pediatrics 92827-832. [PubMed] [Google Scholar]
- 38.Rushdy, A., M. Ramsay, P. T. Heath, H. J. Azzopardi, and M. P. Slack. 1999. Infant Hib vaccination and herd immunity. J. Pediatr. 134253-254. [DOI] [PubMed] [Google Scholar]
- 39.Scheifele, D., L. Barreto, W. Meekison, R. Guasparini, and B. Friesen. 1993. Can Haemophilus influenzae type b-tetanus toxoid conjugate vaccine be combined with diphtheria toxoid-pertussis vaccine-tetanus toxoid? CMAJ 1491105-1112. [PMC free article] [PubMed] [Google Scholar]
- 40.Schlesinger, Y., D. M. Granoff, et al. 1992. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. JAMA 2671489-1494. [PubMed] [Google Scholar]
- 41.Trotter, C. L., J. McVernon, N. J. Andrews, M. Burrage, and M. E. Ramsay. 2003. Antibody to Haemophilus influenzae type b after routine and catch-up vaccination. Lancet 3611523-1524. [DOI] [PubMed] [Google Scholar]
- 42.Trotter, C. L., M. E. Ramsay, and M. P. Slack. 2003. Rising incidence of Haemophilus influenzae type b disease in England and Wales indicates a need for a second catch-up vaccination campaign. Commun. Dis. Public Health 655-58. [PubMed] [Google Scholar]