Abstract
Group A streptococcal (GAS) serology is used for the diagnosis of post-streptococcal diseases, such as acute rheumatic fever, and occasionally for the diagnosis of streptococcal pharyngitis. Experts recommend that the upper limits of normal for streptococcal serology be determined for individual populations because of differences in the epidemiology of GAS between populations. Therefore, we performed a study to determine the values of the upper limit of normal for anti-streptolysin O (ASO) and anti-DNase B (ADB) titers in Fiji. Participants with a history of GAS disease, including pharyngitis or impetigo, were excluded. A total of 424 serum samples from people of all ages (with a sample enriched for school-aged children) were tested for their ASO and ADB titers. Reference values, including titers that were 80% of the upper limit of normal, were obtained by regression analysis by use of a curve-fitting method instead of the traditional nonparametric approach. Normal values for both the ASO titer and the ADB titer rose sharply during early childhood and then declined gradually with age. The estimated titers that were 80% of the upper limit or normal at age 10 years were 276 IU/ml for ASO and 499 IU/ml for ADB. Data from our study are similar to those found in countries with temperate climates, suggesting that a uniform upper limit of normal for streptococcal serology may be able to be applied globally.
Streptococcal antibody tests are used for the diagnosis of antecedent infections caused by the group A streptococcus (GAS) and are particularly useful for the diagnosis of acute rheumatic fever and acute post-streptococcal glomerulonephritis. Acute rheumatic fever is an autoimmune disease that follows infection with GAS; however, the isolation of GAS is uncommon (<15%), and so confirmation of the diagnosis often relies on streptococcal antibody tests (13). While a number of tests utilize different antigens of GAS, the most frequently performed tests are those that determine the anti-streptolysin O (ASO) titer and the anti-DNase B (ADB) titer (8, 18). Ideally, it is recommended that the titer be determined in the acute phase and then determined in the convalescent phase 14 to 28 days later, with a positive result defined as a rise in titer of twofold or more (26). However, it is not always practicable to obtain a second sample for titer determination, particularly in developing countries, where acute rheumatic fever is the most common. Therefore, it is generally accepted that if only a single specimen is available, a titer greater than the upper limit of normal at the initial testing can be considered presumptive evidence of a preceding streptococcal infection (10, 12, 26).
The upper limit of normal for streptococcal serology has been defined by separating the upper 20% from the lower 80% of the group distribution in a dichotomous fashion (4, 12, 26). The choice of the 80th centile cutoff rather than more traditional upper-limit-of-normal calculations (e.g., 2 standard deviations from the mean) is based upon studies that found that more than 80 to 90% of patients with acute rheumatic fever or post-streptococcal glomerulonephritis have streptococcal titers that are above the 80th centile for the healthy controls with no clinical evidence of recent streptococcal infection (4, 26). Therefore, it is assumed that in any population a proportion of apparently healthy individuals will have had a recent, subclinical GAS infection (4).
Streptococcal titers vary according to a number of factors, including age and population. In developed countries, where impetigo caused by GAS is uncommon, streptococcal titers in the population primarily reflect the incidence of pharyngeal infection with GAS; therefore, the titers in healthy people are low in early childhood, rise to a peak in children aged 5 to 15 years, decrease in late adolescence and early adulthood, and then flatten off after that (9, 12). In contrast, in populations with high rates of impetigo, background antistreptococcal titers are often very high, especially in children, probably because most children tested have had a recent streptococcal infection (15, 25).
Because of these differences in titers with age, it is recommended that age-stratified upper-limit-of-normal values be determined for populations of interest by testing people who have not had a recent streptococcal infection (12). Age-stratified upper-limit-of-normal reference values have been defined for the U.S. pediatric population, the Australian pediatric population, and the Indian pediatric population, among others (5, 7, 9, 11, 17). However, there has been no investigation of upper-limit-of-normal values for populations in the Pacific region, where some of the highest rates of acute rheumatic fever and acute post-streptococcal glomerulonephritis are known to occur and where impetigo is common in children (6, 21, 24).
For studies that determine streptococcal serology reference ranges, it is important that a representative group of individuals without a known recent streptococcal infection be sampled (12). The immune response to GAS infections should be considered in determining which subjects should be excluded from analysis (18). The ASO titer tends to rise a week following infection, peaks at 3 to 5 weeks, and begins to decline after 8 weeks; and it responds more vigorously to pharyngeal infection than skin infection. The ADB titer peaks at 6 to 8 weeks after infection and begins to decline at 12 weeks, and it responds vigorously to both pharyngeal and skin infections. Therefore, subjects with recent pharyngitis or skin infections should not be included in the sample. The exclusion of children with GAS throat carriage is not necessary, as all healthy pediatric populations include carriers of GAS (9).
A variety of statistical techniques for constructing age-specific reference values are available (27). Previous comparable studies investigating reference values for streptococcal serology have used nonparametric calculations by pooling data by age group and calculating an 80th centile cutoff value for each of the age groups (7, 9). However, more robust and efficient statistical methods that take advantage of parametric regression modeling techniques are available for the analysis of age-specific reference values (16).
In this study, we document for the first time the normal ranges of the ASO and ADB titers in all age groups in a Pacific island country and also present an alternative method for analyzing these data.
MATERIALS AND METHODS
Setting.
Fiji is a nation of approximately 330 islands located in the western Pacific. It has a population of 827,900 people comprising two major racial groups: indigenous Fijians (57.3%) and Indo-Fijians (37.6%) (3). Fiji is ranked 90 of 177 nations on the United Nations Development Programme's Human Development Index. It has a per capita gross domestic product of $6,066 and an infant mortality rate of 16.8 per 1,000 population (1, 2). Approximately 49% of the population lives in rural areas (3). The major hospital, the Colonial War Memorial Hospital, is located in the capital, Suva, on the main island of Viti Levu and predominantly serves the largest region of Fiji, the Central Division. GAS disease is common in Fiji, with high rates of invasive GAS disease, acute rheumatic fever, and rheumatic heart disease (14, 19, 22). In addition, there is a high prevalence (over 35%) of impetigo among schoolchildren (20), and the incidence of GAS culture-positive sore throat is similar to that in developed countries (approximately 15 cases per 100 child years) (23).
Sample.
Blood was collected from participant groups enrolled in two different studies. The first group comprised 280 volunteers of all ages identified through the Colonial War Memorial Hospital. The enrollment was designed so that there was an even distribution of participants from birth to age 65 years. All of these 280 participants were prospectively screened for current or recent GAS infection. We excluded any participant from enrollment by direct inquiry of the participant and by checking their medical records for the following: a history of acute rheumatic fever, rheumatic heart disease, or invasive GAS disease and a history in the previous 14 days of sore throat or skin sores. We also excluded participants who had a temperature of >38.0°C on the day of enrollment.
In order to enrich the sample for children in the age group that has been found in other studies to have the highest background antistreptococcal antibody titers, we included a second group of 423 schoolchildren aged 4 to 14 years enrolled as part of a separate prospective cohort study designed to study GAS pharyngitis and GAS impetigo. This study was conducted in three primary schools in the Central Division of Fiji. Two schools were located in a rural area, while a third school was located in Fiji's capital city, Suva. The participants in these schools were screened retrospectively for potential GAS disease. We excluded children with any of the following: rheumatic heart disease proven on echocardiogram (because these children are at a higher risk of acute rheumatic fever at any time); recent GAS pharyngitis; and any evidence of impetigo, which included dry, crusted, or pustular lesions.
Collection, transport, and testing of specimens.
Blood specimens were taken and immediately stored in a cool box. The samples were transported to the laboratory on the same day, and on arrival at the laboratory, the samples were centrifuged and the serum was divided into three aliquots and stored at −70°C. One aliquot was thawed and tested for ADB in our Fiji laboratory. Another aliquot was transported to Queensland, Australia, where it was thawed and tested for ASO titer.
ASO titers were measured by a nephelometric technique (Beckman Coulter, Fullerton, CA), as described previously (2). ADB titers were measured by an enzyme inhibition assay (bioMerieux, Marcy l'Etoile, France), as described previously (2). Both methods provide an inexact figure for low titers (titers of <60 IU/ml for ASO and titers of <100 IU/ml for ADB); for these values, we estimated midtiter values (a titer of 30 IU/ml for ASO and a titer of 50 IU/ml for ADB).
Statistical methods.
We followed a previously described technique for constructing age-specific reference ranges (16). The raw data for both ASO and ADB titers were logarithmically transformed from a positively skewed distribution to a normal distribution. A regression analysis of the log values was performed by using fractional polynomials to handle nonlinearity. With this method, we created smoothed fitted curves against age. From the fitted curves, we obtained median and 80% upper-limit-of-normal values for five age groups from age 1 year to 65 years and also by year of age for children aged 5 to 14 years. Data were analyzed by using the Stata (version 10.0) program (Stata-Corp, College Station, TX).
Ethical approval.
Ethical approval was obtained from the Fiji National Research Ethics Review Committee, the Fiji National Health Research Committee, the University of Melbourne Human Research Ethics Committee, and the Queensland Institute of Medical Research Human Research Ethics Committee. We approached all participants for their consent, and prior to enrollment information sheets in Fijian and English were provided. We required that all participants provide written informed consent before information was collected. Children were enrolled only if written consent was obtained from a parent or guardian, and we also insisted on written assent by children aged 10 years or older.
RESULTS
Sample.
All samples from the 280 participants in the first study group were included in the analysis. Of this group, there were 26 participants who were inpatients for non-GAS disease, 87 blood donors, and 167 outpatients. Among the 423 participants in the second group, 3 participants with rheumatic heart disease were excluded from the analysis, there were no children with a history of GAS pharyngitis in the preceding 14 days, and 273 children with evidence of impetigo were excluded from the analysis. When the data were log transformed, it was noted that there were three extreme outliers (one participant with an ASO titer of >1,999 and two participants with ADB titers of >2,999), and these values were excluded from the analysis. Therefore, a total of 424 blood samples were included in the study. Table 1 summarizes the demographic features of the study participants.
TABLE 1.
Demographic details of study participantsa
| Characteristic | No. (%) of subjects |
|---|---|
| Age (yr) | |
| <5 | 44 (10.4) |
| 5-14 | 186 (43.8) |
| >14 | 194 (45.8) |
| Gender | |
| Female | 202 (47.6) |
| Male | 222 (52.4) |
| Ethnicity | |
| Indigenous Fijian | 230 (54.3) |
| Indo-Fijian | 164 (38.7) |
| Other | 30 (7.0) |
The data are for 424 participants.
ASO and ADB titer analysis.
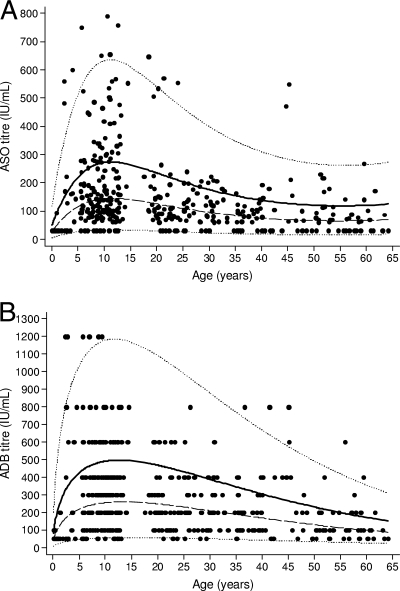
There was a peak in the mean titers of both ASO and ADB in the 5- to 14-year-old age group, with a gradual decrease occurring following this peak (Fig. 1). The estimated median and 80% upper-limit-of-normal values for five age groups are presented in Table 2. There are no values presented in Table 2 for children aged less than 1 year because of the small number of values and the steep incline of the curve. Table 3 presents these data in more detail for children aged 5 to 14 years.
FIG. 1.
ASO titer (A) and ADB titer (B) versus age. The solid line represents the 80th centile (upper limit of normal), the dashed line represents the geometric mean (equivalent to the median) titer, and the dotted lines represent the 2.5th and the 97.5th centiles.
TABLE 2.
Median and 80% upper-limit-of-normal reference values for ASO titer and ADB titer, by age groupa
| Age (yr) | No. of subjects | ASO titer (IU/ml)
|
ADB titer (IU/ml)
|
||
|---|---|---|---|---|---|
| Median | 80% upper limit of normal | Median | 80% upper limit of normal | ||
| 1-4 | 18 | 89 | 170 | 185 | 366 |
| 5-14 | 186 | 145 | 276 | 257 | 499 |
| 15-24 | 50 | 126 | 238 | 249 | 473 |
| 25-34 | 51 | 95 | 177 | 211 | 390 |
| ≥35 | 93 | 69 | 127 | 148 | 265 |
The data were derived from the fractional polynomial regression model for log titer.
TABLE 3.
Median and 80% upper-limit-of-normal reference values for ASO and ADB titers, by yearly age group for children aged 5 to 14 years
| Age (yr) | No. of subjects | ASO titer (IU/ml)
|
ADB titer (IU/ml)
|
||
|---|---|---|---|---|---|
| Median | 80% upper limit of normal | Median | 80% upper limit of normal | ||
| 5 | 16 | 116 | 222 | 220 | 433 |
| 6 | 13 | 126 | 241 | 232 | 455 |
| 7 | 17 | 134 | 255 | 241 | 472 |
| 8 | 24 | 139 | 265 | 248 | 484 |
| 9 | 27 | 143 | 272 | 253 | 493 |
| 10a | 27 | 145 | 276 | 257 | 499 |
| 11 | 22 | 146 | 278 | 259 | 503 |
| 12 | 32 | 146 | 277 | 261 | 504 |
| 13 | 6 | 145 | 275 | 261 | 504 |
| 14 | 2 | 144 | 272 | 261 | 503 |
DISCUSSION
We found the normal values for streptococcal serology to be similar to those that have been reported from other regions. In comparison to data from the United States and from Australia, we found that the overall values for the ASO titers were only slightly lower in our study and that the values for the ADB titers were only slightly higher (8, 9). The slightly higher ADB titers are probably due to the fact that impetigo is endemic in Fiji, particularly in children (20). Although we excluded children with a recent history of impetigo, the ADB titers remain elevated for many months; hence, we are likely to have included some children whose ADB titers were in the process of returning to their baseline level after a case of impetigo.
Although some have claimed that normal ranges for streptococcal antibody titers are higher in populations with endemic streptococcal infections, this is incorrect. The studies on which these statements were based did not meticulously exclude children with recent streptococcal infections. For example, a study with an aboriginal community in Australia, in which impetigo was very common among the children of the community, found median titers of 256 IU/ml and 3,172 IU/ml for ASO and ADB, respectively, but did not exclude children with current or recent impetigo (15). The very close similarities of the titers between our study in a tropical country and studies in temperate zones provide evidence and impetus for the notion that single upper-limit-of-normal values for ASO and ADB titers may be able to be applied globally. Similar data from India support this notion (11,12).
By applying the simple nonparametric technique that has been used in previous studies to analyze our data, we found cutoff values similar to those obtained by the parametric method. However, the parametric method of data analysis that we used in this study has some advantages over the nonparametric method. The nonparametric method often produces implausible irregular patterns in the centiles with age, unless a large sample is used and wide age intervals are specified. The results may be artificially affected by the choice of age groups, especially when titers have a complex pattern of change with age. In comparison, the curve-fitting method produces smooth centile curves (that is, the reference value varies smoothly with age), and it allows both the level and the spread of the reference distribution to change with time.
We will recommend that Fijian clinicians use a single upper-limit-of-normal cutoff value for children aged 5 to 14 years (that is, the estimated 80% upper-limit values at 10 years, which were 276 IU/ml for the ASO titer and 499 IU/ml for the ADB titer; Table 2) rather than the cutoff values for subgroups, such as 4 to 5 years, 6 to 9 years, and 10 to 14 years, as recommended in other studies. This is because only minimal variability in the year-by-year values was found for children aged 5 to 14 years (Table 3). The use of a single cutoff value for this age group also makes it far simpler for laboratory staff to report results and for clinicians to interpret them (12).
This study provides upper-limit-of-normal values for streptococcal serology for people of all ages in Fiji determined by using a robust and readily repeatable parametric statistical technique. These upper-limit-of-normal values will guide clinicians in Fiji when they consider the diagnosis of post-streptococcal diseases in their patients and will provide useful baseline data for future studies of interventions against GAS disease in Fiji. Our data could also be applied to surrounding Pacific island countries.
Acknowledgments
This study was funded by a grant from the National Institute of Allergy and Infectious Diseases (grant U01AI60579).
There is no conflict of interest for any author.
We acknowledge the following people: all people who agreed to participate in the study; the Fiji Group A Streptococcal Project team, including Laisiana Matatolu, Frances Matanatabu, Maureen Ah-Kee, and Loraine Kelpie; the laboratory and clinical staff at the Colonial War Memorial Hospital, particularly Eka Buadromo, Joe Bolaqace, and Tagica Taratai; Robert Gibb at the Department of Microbiology, Pathology Queensland; and the staff of the Streptococcal Laboratory of the Queensland Institute of Medical Research, in particular, the director, Michael Good.
Footnotes
Published ahead of print on 3 December 2008.
REFERENCES
- 1.Anonymous. 2007. Fiji facts and figures as at July 2007. Fiji Islands Bureau of Statistics, Suva, Fiji Islands.
- 2.Anonymous. 2006. Human development report 2006. United Nations Development Programme, New York, NY.
- 3.Anonymous. 2007. Statistical news press release no. 52. Fiji Islands Bureau of Statistics, Suva, Fiji Islands.
- 4.Ayoub, E. M., and L. W. Wannamaker. 1962. Evaluation of the streptococcal deoxyribonuclease B and diphosphopyridine nucleotide antibody tests in acute rheumatic fever and acute glomerulonephritis. Pediatrics 29527-538. [Google Scholar]
- 5.Blyth, C. C., and P. W. Robertson. 2006. Anti-streptococcal antibodies in the diagnosis of acute and post-streptococcal disease: streptokinase versus streptolysin O and deoxyribonuclease B. Pathology 38152-156. [DOI] [PubMed] [Google Scholar]
- 6.Carapetis, J. R., A. C. Steer, E. K. Mulholland, and M. Weber. 2005. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5685-694. [DOI] [PubMed] [Google Scholar]
- 7.Danchin, M. H., J. B. Carlin, W. Devenish, T. M. Nolan, and J. R. Carapetis. 2004. New normal ranges of antistreptolysin O and anti-deoxyribonuclease B titres for Australian children. J. Paediatr. Child Health 41583-586. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan, E. L., P. Ferrieri, and L. W. Wannamaker. 1974. Comparison of the antibody response to streptococcal cellular and extracellular antigens in acute pharyngitis. J. Pediatr. 8421-28. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan, E. L., C. D. Rothermel, and D. R. Johnson. 1998. Antistreptolysin O and anti-deoxyribonuclease B titers: normal values for children ages 2 to 12 in the United States. Pediatrics 10186-88. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan, E. L., F. H. Top., Jr., B. A. Dudding, and L. W. Wannamaker. 1971. Diagnosis of streptococcal pharyngitis: differentiation of active infection from the carrier state in the symptomatic child. J. Infect. Dis. 123490-501. [DOI] [PubMed] [Google Scholar]
- 11.Karmarkar, M. G., V. Venugopal, L. Joshi, and R. Kamboj. 2004. Evaluation & revaluation of upper limits of normal values of anti-streptolysin O and ant-deoxyribonuclease B in Mumbai. Indian J. Med. Res. 119(Suppl.)26-28. [PubMed] [Google Scholar]
- 12.Klein, G. C., C. N. Baker, and W. L. Jones. 1971. “Upper limits of normal” antistreptolysin O and antideoxyribonuclease B titers. Appl. Microbiol. 21999-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin, D. R., L. M. Voss, S. J. Walker, and D. Lennon. 1994. Acute rheumatic fever in Auckland, New Zealand: spectrum of associated group A streptococci different from expected. Pediatr. Infect. Dis. J. 13264-269. [DOI] [PubMed] [Google Scholar]
- 14.Negus, R. M. 1971. Rheumatic fever in western Fiji: the female preponderance. Med. J. Aust. 2251-254. [PubMed] [Google Scholar]
- 15.Nimmo, G. R., R. D. Tinniswood, N. Nuttall, G. M. Baker, and B. McDonald. 1992. Group A streptococcal infection in an aboriginal community. Med. J. Aust. 157521-522. [DOI] [PubMed] [Google Scholar]
- 16.Royston, P. 1991. Constructing time-specific reference ranges. Stat. Med. 10675-690. [DOI] [PubMed] [Google Scholar]
- 17.Sethi, S., K. Kaushik, K. Mohandas, C. Sengupta, S. Singh, and M. Sharma. 2003. Anti-streptinolysin O titres in normal healthy children aged 5-15. Indian Pediatr. 401068-1071. [PubMed] [Google Scholar]
- 18.Shet, A., and E. L. Kaplan. 2002. Clinical use and interpretation of group A streptococcal antibody tests: a practical approach for the pediatrician or primary care physician. Pediatr. Infect. Dis. J. 21420-426. [DOI] [PubMed] [Google Scholar]
- 19.Singh, P. I. P. K., J. R. Carapetis, E. M. Buadromo, P. N. Samberkar, and A. C. Steer. 2007. The high burden of rheumatic heart disease found on autopsy in Fiji. Cardiol. Young. 1862-69. [DOI] [PubMed] [Google Scholar]
- 20.Steer, A. C., M. Batzloff, M. F. Good, A. W. J. Jenney, J. Kado, E. K. Mulholland, L. Waqatakirewa, J. Hartas, G. Magor, and J. R. Carapetis. 2008. The epidemiology of group A streptococcal pyoderma in Fiji. XVII Lancefield Int. Symp. Streptococci Streptococcal Dis., p. 100.
- 21.Steer, A. C., J. R. Carapetis, T. M. Nolan, and F. Shann. 2002. Systematic review of rheumatic heart disease prevalence in children in developing countries: the role of environmental factors. J. Paediatr. Child Health 38229-234. [DOI] [PubMed] [Google Scholar]
- 22.Steer, A. C., A. J. Jenney, F. Oppedisano, M. R. Batzloff, J. Hartas, J. Passmore, F. M. Russell, J. H. Kado, and J. R. Carapetis. 2008. High burden of invasive beta-haemolytic streptococcal infections in Fiji. Epidemiol. Infect. 136621-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steer, A. C., A. W. J. Jenney, J. Kado, M. F. Good, M. Batzloff, G. Magor, R. Ritika, E. K. Mulholland, and J. R. Carapetis. Prospective surveillance of streptococcal sore throat in a tropical country. Pediatr. Infect. Dis. J., in press. [DOI] [PubMed]
- 24.Thomas, M., G. Woodfield, C. Moses, and G. Amos. 2005. Soil-transmitted helminth infection, skin infection, anaemia, and growth retardation in schoolchildren of Taveuni Island, Fiji. N. Z. Med. J. 1181-12. [PubMed] [Google Scholar]
- 25.Van Buynder, P. G., J. A. Gaggin, D. Martin, D. Pugsley, and J. D. Mathews. 1992. Streptococcal infection and renal disease markers in Australian aboriginal children. Med. J. Aust. 156537-540. [DOI] [PubMed] [Google Scholar]
- 26.Wannamaker, L. W., and E. M. Ayoub. 1960. Antibody titers in acute rheumatic fever. Circulation 21598-614. [DOI] [PubMed] [Google Scholar]
- 27.Wright, E. M., and P. Royston. 1997. A comparison of statistical methods for age-related reference intervals. J. R. Stat. Soc. A 16047-69. [Google Scholar]