Abstract
Simian immunodeficiency virus (SIV) infection disseminated into the oropharyngeal tissues of rhesus macaques 6 weeks following intravenous inoculation. Severe local CD4+ T-cell depletion coincided with increases in NK cell and proinflammatory biomarkers and the disruption of growth-associated gene transcription, demonstrating the rapid establishment of pathogenesis in the oral mucosa.
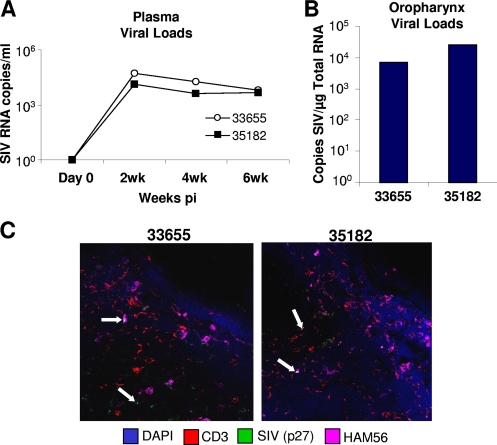
Previous studies of oral mucosal responses to simian immunodeficiency virus (SIV) infection have generally focused on analyses of infections that were initiated in the oral cavity (1, 8, 12). While evidence suggests that the mechanisms of the immune responses and the interaction between lymphocytes and the oral epithelium are impaired as a result of immunodeficiency virus infection (2), the characteristics of oral pathogenesis that result from systemic infection remain largely unknown. We have addressed some of these questions through the comparative analysis of oropharyngeal tissues from three healthy SIV-negative rhesus macaques (animals 30327, 34816, and 34249) and two SIV-infected animals (animals 33655 and 35182) 6 weeks after intravenous inoculation with 100 50% tissue culture infective doses of SIVmac251. Measurement of SIV replication by reverse transcription (RT-PCR) (5) showed that plasma viral loads peaked at about 105 to 106 RNA copies/ml at 2 weeks postinfection (p.i.) and remained relatively high at 6 weeks p.i. (Fig. 1A). Through comparisons to standardized curves generated from known SIV copy numbers, we determined that the viral loads in oral mucosal tissues were between 103 and 104 SIV copies/μg of total RNA (Fig. 1B). Immunohistochemical analysis (9, 15) showed SIV p27 expression in both macrophages and CD4+ T cells that were localized to lymphoid areas in the oral mucosa (Fig. 1C). Thus, an actively replicating viral reservoir was established in the oropharynx within 6 weeks of intravenous SIV inoculation.
FIG. 1.
SIVmac251 viral loads in plasma were determined at 2, 4, and 6 weeks p.i. (A) and those in the oropharynx were determined at 6 weeks p.i. (B) by RT-PCR. (C) Immunohistochemistry-based detection of SIV p27 in macrophages and T cells within oropharyngeal tissues at 6 weeks p.i.
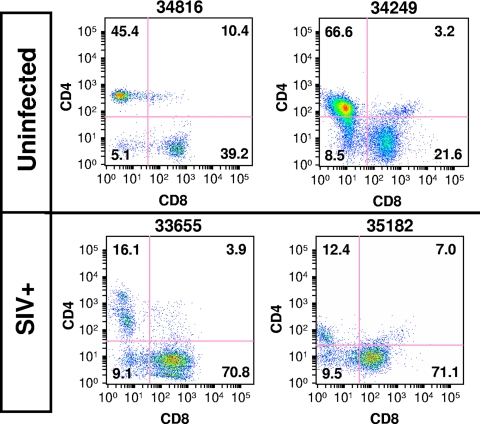
To evaluate potential alterations in oral mucosal T-cell homeostasis, we determined changes in the levels of CD4+ and CD8+ T-cell subsets in SIV-infected animals compared to those in healthy uninfected controls utilizing the flow cytometry methods described in our previous studies (5, 23). While CD4+ T cells represented 45.4% and 66.6% of the total T-cell population in the oropharynges of healthy controls (animals 34816 and 34249, respectively), their numbers appeared to be greatly reduced in SIV-infected animals (16.1% and 12.4%, respectively) (Fig. 2). As observed in studies of other mucosal compartments, CD4+ T-cell depletion coincided with a proportional increase in the percentage of CD8+ T cells (10, 17, 19, 20). Similar to reports of SIV-infected gut lymphoid tissue (7, 18, 21, 22), we found by flow cytometry increased levels of expression of activation (CD69) and proliferation (Ki67) biomarkers on the oral mucosal CD4+ T cells that had not yet been depleted from the infected animals (data not shown). Collectively, these data suggest that a massive disruption in local T-cell homeostasis is associated with the rapid dissemination of SIV into oropharyngeal tissues.
FIG. 2.
Depletion of CD4+ T cells and expansion of CD8+ T cells in the oral mucosa of rhesus macaques 6 weeks following intravenous infection with SIV. SIV+, SIV infected.
Mechanisms of host response to SIV in oral mucosa.
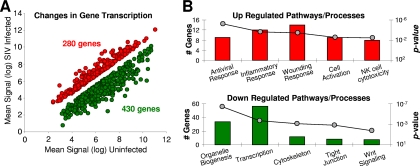
To increase our understanding of the mechanisms of the host response and elucidate the biomarkers of pathogenesis in the oral mucosa, we compared the gene expression profiles in the oropharynges of healthy control and SIV-infected animals utilizing previously reported methods (5, 6). Briefly, the mean transcription levels in the oropharyngeal tissues of healthy uninfected animals (animals 30327, 34816, and 34249) were compared to the corresponding transcription levels in the SIV-infected animals (animals 33655 and 35182). Genes whose transcription levels were altered by at least 1.5-fold (up or down) in infected animals (P ≤ 0.05 by the unpaired Student t test) were considered for further evaluation (Fig. 3A). Using these criteria, we determined that 280 genes were upmodulated and that 430 were downmodulated in the oropharynx as a result of SIV infection. The pathways and processes that were statistically overrepresented in the filtered gene set were identified (Fig. 3B). The complete data files utilized for the microarray analysis are available through the Gene Expression Omnibus database (GEO14351).
FIG. 3.
Microarray analysis of changes in gene transcription in the rhesus oropharynx after 6 weeks of SIV infection. (A) Up- and downregulated genes were identified by comparison of SIV-infected animals (n = 2) to healthy uninfected control animals (n = 3). (B) Pathways statistically overrepresented in the up- and downregulated gene list.
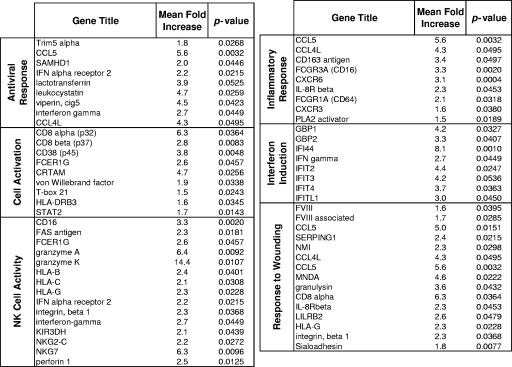
The levels of transcription of genes associated with innate immunity, inflammation, cell adhesion, and response to wounding were increased in the oropharyngeal tissues of SIV-infected animals (Fig. 3B). The innate response category included gamma interferon and genes associated with interferon induction, antiviral molecules (e.g., Trim5α), and several NK cell biomarkers (Fig. 4). While many genes regulating NK cell and CD8+ T-cell functions overlap (e.g., granzymes and perforin), the added detection of increases in the levels of expression of NKG2-C, NKG7, and KIR3DH suggest that both of these cell types may have contributed to ongoing cytolytic activity in the oropharynges of the SIV-infected animals. These data are consistent with those reported previously (3, 4, 11, 13, 14, 16) and highlight a potentially important role for NK cells in anti-SIV responses within the oral mucosa. Taken together, evidence of increased inflammation, elevated cytolytic activity, and the death of SIV-infected cells also suggested considerable potential for bystander tissue damage, a conclusion supported by the upregulation of genes mediating the response to wounding (Fig. 4).
FIG. 4.
Mean fold increases in the transcription of genes associated with upregulated pathways in the oropharynx of SIV-infected animals.
Downregulation of genes mediating growth in oropharyngeal tissue.
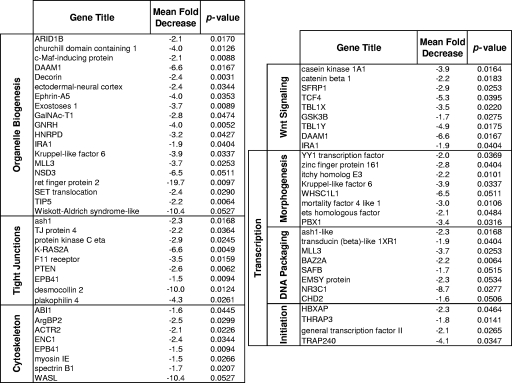
In contrast to the increased levels of expression of genes controlling defense responses and inflammation, genes mediating biogenesis pathways, transcriptional regulation, cytoskeletal organization, tight junction formation, and Wnt pathway signaling were expressed at considerably lower levels in the SIV-infected animals than in the healthy uninfected control animals (Fig. 3B). More than two dozen genes associated with biogenesis were transcriptionally repressed in the SIV-infected animals. These included genes involved in B- and T-cell differentiation (c-Maf-inducing protein, Kruppel-like factor 6), cytoskeletal development (actin, tubulin) epithelial development (decorin, calmodulin-like 5), muscle development (myosins, troponins), and neuronal development (BAIAP2, neurotrimin). A number of genes involved in Wnt signaling were also downmodulated, highlighted by strong (more than fivefold) reductions in the levels of expression of casein kinase 1A1, PPP2R5E, DAAM1, Y-linked transducin, and TCF-4 (Fig. 5).
FIG. 5.
Mean fold decreases in the transcription of genes associated with downregulated pathways in the oropharynx of SIV-infected animals.
The cumulative loss of transcription of genes regulating biogenesis may underscore a reduced capacity for oropharyngeal tissue regeneration during SIV infection. Logically, an inability to repair early damage to the oral mucosa could set the stage for continuing deterioration throughout the course of infection and may ultimately contribute to an impaired efficacy of the host response to challenge from secondary opportunistic pathogens. It is important to note, however, that our findings are based on analyses with a small number of animals and should therefore be considered preliminary until larger groups of animals can be evaluated.
Acknowledgments
We thank Andreas Baumler for supplying oropharyngeal tissues and the pathologists and animal technicians at the California National Primate Research Center for their support.
This study was completed through funding (grants 1R21DE018097 and R01DK43183) from the National Institutes of Health.
Footnotes
Published ahead of print on 17 December 2008.
REFERENCES
- 1.Abel, K., B. Pahar, K. K. Van Rompay, L. Fritts, C. Sin, K. Schmidt, R. Colon, M. McChesney, and M. L. Marthas. 2006. Rapid virus dissemination in infant macaques after oral simian immunodeficiency virus exposure in the presence of local innate immune responses. J. Virol. 806357-6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Challacombe, S. J., and S. P. Sweet. 2002. Oral mucosal immunity and HIV infection: current status. Oral Dis. 8(Suppl. 2)55-62. [DOI] [PubMed] [Google Scholar]
- 3.Couedel-Courteille, A., R. Le Grand, M. Tulliez, J. G. Guillet, and A. Venet. 1997. Direct ex vivo simian immunodeficiency virus (SIV)-specific cytotoxic activity detected from small intestine intraepithelial lymphocytes of SIV-infected macaques at an advanced stage of infection. J. Virol. 711052-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genesca, M., P. J. Skinner, J. J. Hong, J. Li, D. Lu, M. B. McChesney, and C. J. Miller. 2008. With minimal systemic T-cell expansion, CD8+ T cells mediate protection of rhesus macaques immunized with attenuated simian-human immunodeficiency virus SHIV89.6 from vaginal challenge with simian immunodeficiency virus. J. Virol. 8211181-11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George, M. D., E. Reay, S. Sankaran, and S. Dandekar. 2005. Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. J. Virol. 792709-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George, M. D., D. Verhoeven, Z. McBride, and S. Dandekar. 2006. Gene expression profiling of gut mucosa and mesenteric lymph nodes in simian immunodeficiency virus-infected macaques with divergent disease course. J. Med. Primatol. 35261-269. [DOI] [PubMed] [Google Scholar]
- 7.Kaur, A., C. L. Hale, S. Ramanujan, R. K. Jain, and R. P. Johnson. 2000. Differential dynamics of CD4+ and CD8+ T-lymphocyte proliferation and activation in acute simian immunodeficiency virus infection. J. Virol. 748413-8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu, F. X., and R. S. Jacobson. 2007. Oral mucosal immunity and HIV/SIV infection. J. Dent. Res. 86216-226. [DOI] [PubMed] [Google Scholar]
- 9.Macal, M., S. Sankaran, T.-W. Chun, E. Reay, J. Flamm, T. J. Prindiville, and S. Dandekar. 2008. Effective CD4+ T-cell restoration in gut associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 1475-488. [DOI] [PubMed] [Google Scholar]
- 10.Mattapallil, J. J., E. Reay, and S. Dandekar. 2000. An early expansion of CD8alphabeta T cells, but depletion of resident CD8alphaalpha T cells, occurs in the intestinal epithelium during primary simian immunodeficiency virus infection. AIDS 14637-646. [DOI] [PubMed] [Google Scholar]
- 11.McChesney, M. B., J. R. Collins, and C. J. Miller. 1998. Mucosal phenotype of antiviral cytotoxic T lymphocytes in the vaginal mucosa of SIV-infected rhesus macaques. AIDS Res. Hum Retrovir. 14(Suppl. 1)S63-S66. [PubMed] [Google Scholar]
- 12.Milush, J. M., K. Stefano-Cole, K. Schmidt, A. Durudas, I. Pandrea, and D. L. Sodora. 2007. Mucosal innate immune response associated with a timely humoral immune response and slower disease progression after oral transmission of simian immunodeficiency virus to rhesus macaques. J. Virol. 816175-6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphey-Corb, M., L. A. Wilson, A. M. Trichel, D. E. Roberts, K. Xu, S. Ohkawa, B. Woodson, R. Bohm, and J. Blanchard. 1999. Selective induction of protective MHC class I-restricted CTL in the intestinal lamina propria of rhesus monkeys by transient SIV infection of the colonic mucosa. J. Immunol. 162540-549. [PubMed] [Google Scholar]
- 14.Quigley, M. F., K. Abel, B. Zuber, C. J. Miller, J. K. Sandberg, and B. L. Shacklett. 2006. Perforin expression in the gastrointestinal mucosa is limited to acute simian immunodeficiency virus infection. J. Virol. 803083-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sankaran, S., M. D. George, E. Reay, M. Guadalupe, J. Flamm, T. Prindiville, and S. Dandekar. 2008. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J. Virol. 82538-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitz, J. E., R. S. Veazey, M. J. Kuroda, D. B. Levy, A. Seth, K. G. Mansfield, C. E. Nickerson, M. A. Lifton, X. Alvarez, A. A. Lackner, and N. L. Letvin. 2001. Simian immunodeficiency virus (SIV)-specific cytotoxic T lymphocytes in gastrointestinal tissues of chronically SIV-infected rhesus monkeys. Blood 983757-3761. [DOI] [PubMed] [Google Scholar]
- 17.Smit-McBride, Z., J. J. Mattapallil, M. McChesney, D. Ferrick, and S. Dandekar. 1998. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4+ T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J. Virol. 726646-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sopper, S., U. Sauer, J. G. Muller, C. Stahl-Hennig, and V. ter Meulen. 2000. Early activation and proliferation of T cells in simian immunodeficiency virus-infected rhesus monkeys. AIDS Res. Hum Retrovir. 16689-697. [DOI] [PubMed] [Google Scholar]
- 19.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280427-431. [DOI] [PubMed] [Google Scholar]
- 20.Veazey, R. S., P. A. Marx, and A. A. Lackner. 2003. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. J. Infect. Dis. 187769-776. [DOI] [PubMed] [Google Scholar]
- 21.Veazey, R. S., I. C. Tham, K. G. Mansfield, M. DeMaria, A. E. Forand, D. E. Shvetz, L. V. Chalifoux, P. K. Sehgal, and A. A. Lackner. 2000. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 7457-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verhoeven, D., S. Sankaran, and S. Dandekar. 2007. Simian immunodeficiency virus infection induces severe loss of intestinal central memory T cells which impairs CD4+ T-cell restoration during antiretroviral therapy. J. Med. Primatol. 36219-227. [DOI] [PubMed] [Google Scholar]
- 23.Verhoeven, D., S. Sankaran, M. Silvey, and S. Dandekar. 2008. Antiviral therapy during primary simian immunodeficiency virus infection fails to prevent acute loss of CD4+ T cells in gut mucosa but enhances their rapid restoration through central memory T cells. J. Virol. 824016-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]