Abstract
An extracellular β-fructofuranosidase from the yeast Xanthophyllomyces dendrorhous was characterized biochemically, molecularly, and phylogenetically. This enzyme is a glycoprotein with an estimated molecular mass of 160 kDa, of which the N-linked carbohydrate accounts for 60% of the total mass. It displays optimum activity at pH 5.0 to 6.5, and its thermophilicity (with maximum activity at 65 to 70°C) and thermostability (with a T50 in the range 66 to 71°C) is higher than that exhibited by most yeast invertases. The enzyme was able to hydrolyze fructosyl-β-(2→1)-linked carbohydrates such as sucrose, 1-kestose, or nystose, although its catalytic efficiency, defined by the kcat/Km ratio, indicates that it hydrolyzes sucrose approximately 4.2 times more efficiently than 1-kestose. Unlike other microbial β-fructofuranosidases, the enzyme from X. dendrorhous produces neokestose as the main transglycosylation product, a potentially novel bifidogenic trisaccharide. Using a 41% (wt/vol) sucrose solution, the maximum fructooligosaccharide concentration reached was 65.9 g liter−1. In addition, we isolated and sequenced the X. dendrorhous β-fructofuranosidase gene (Xd-INV), showing that it encodes a putative mature polypeptide of 595 amino acids and that it shares significant identity with other fungal, yeast, and plant β-fructofuranosidases, all members of family 32 of the glycosyl-hydrolases. We demonstrate that the Xd-INV could functionally complement the suc2 mutation of Saccharomyces cerevisiae and, finally, a structural model of the new enzyme based on the homologous invertase from Arabidopsis thaliana has also been obtained.
The basidiomycetous yeast Xanthophyllomyces dendrorhous (formerly Phaffia rhodozyma) produces astaxanthin (3-3′-dihydroxy-β,β-carotene-4,4 dione [17, 25]). Different industries have displayed great interest in this carotenoid pigment due to its attractive red-orange color and antioxidant properties, which has intensified the molecular and genetic study of this yeast. As a result, several genes involved in the astaxanthin biosynthetic pathway have been cloned and/or characterized, as well as some other genes such as those encoding actin (60), glyceraldehyde-3-phosphate dehydrogenase (56), endo-β-1,3-glucanase, and aspartic protease (4). In terms of the use of carbon sources, a β-amylase (9), and an α-glucosidase (33) with glucosyltransferase activity (12), as well as a yeast cell-associated invertase (41), have also been reported.
Invertases or β-fructofuranosidases (EC 3.2.1.26) catalyze the release of β-fructose from the nonreducing termini of various β-d-fructofuranoside substrates. Yeast β-fructofuranosidases have been widely studied, including that of Saccharomyces cerevisiae (11, 14, 45, 46), Schizosaccharomyces pombe (36), Pichia anomala (40, 49), Candida utilis (5, 8), or Schwanniomyces occidentalis (2). They generally exhibit strong similarities where sequences are available, and they have been classified within family 32 of the glycosyl-hydrolases (GH) on the basis of their amino acid sequences. The catalytic mechanism proposed for the S. cerevisiae enzyme implies that an aspartate close to the N terminus (Asp-23) acts as a nucleophile, and a glutamate (Glu-204) acts as the acid/base catalyst (46). In addition, the three-dimensional structures of some enzymes in this family have been resolved, such as that of an exoinulinase from Aspergillus niger (var. awamori; 37) and the invertase from Arabidopsis thaliana (55).
As well as hydrolyzing sucrose, β-fructofuranosidases from microorganisms may also catalyze the synthesis of short-chain fructooligosaccharides (FOS), in which one to three fructosyl moieties are linked to the sucrose skeleton by different glycosidic bonds depending on the source of the enzyme (3, 52). FOS are one of the most promising ingredients for functional foods since they act as prebiotics (44), and they exert a beneficial effect on human health, participating in the prevention of cardiovascular diseases, colon cancer, or osteoporosis (28). Currently, Aspergillus fructosyltransferase is the main industrial producer of FOS (15, 52), producing a mixture of FOS with an inulin-type structure, containing β-(2→1)-linked fructose-oligomers (1F-FOS: 1-kestose, nystose, or 1F-fructofuranosylnystose). However, there is certain interest in the development of novel molecules that may have better prebiotic and physiological properties. In this context, β-(2→6)-linked FOS, where this link exits between two fructose units (6F-FOS: 6-kestose) or between fructose and the glucosyl moiety (6G-FOS: neokestose, neonystose, and neofructofuranosylnystose), may have enhanced prebiotic properties compared to commercial FOS (29, 34, 54). The enzymatic synthesis of 6-kestose and other related β-(2→6)-linked fructosyl oligomers has already been reported in yeasts such as S. cerevisiae (11) or Schwanniomyces occidentalis (2) and in fungi such as Thermoascus aurantiacus (26) or Sporotrichum thermophile (27). However, the production of FOS included in the 6G-FOS series has not been widely reported in microorganisms, probably because they are not generally produced (2, 15) or because they represent only a minor biosynthetic product (e.g., with baker's yeast invertase) (11). Most research into neo-FOS production has been carried out with Penicillium citrinum cells (19, 31, 32, 39). In this context, neokestose is the main transglycosylation product accumulated by whole X. dendrorhous cells from sucrose (30), although the enzyme responsible for this reaction remained uncharacterized.
Here, we describe the molecular, phylogenetic, and biochemical characterization of an extracellular β-fructofuranosidase from X. dendrorhous. Kinetic studies of its hydrolytic activity were performed using different substrates, and we investigated its fructosyltransferase capacity. The functionality of the gene analyzed was verified through its heterologous expression, and a structural model of this enzyme based on the homologous invertase from A. thaliana has also been obtained.
MATERIALS AND METHODS
Organisms, transformations, and culture conditions.
The X. dendrorhous strains ATCC MYA-131, ATCC 24202, and ATCC 24230 were grown at 23°C on MM medium (0.7% yeast nitrogen base; Difco) supplemented with 2% (wt/vol) maltose (MMM), glucose (MMG), or sucrose (MMS). Growth was monitored spectrophotometrically at a wavelength of 660 nm (A660). Escherichia coli DH5α competent cells were prepared, stored, and transformed by standard techniques (51). E. coli XL10-Gold ultracompetent cells (Stratagene) were used to obtain the cDNA library. Invertase-deficient S. cerevisiae SEY 2101 (MATα ura3-52 leu2-3 leu2-112 ade2-101 suc2-Δ9) was transformed by the lithium acetate method (24).
Protein purification and quantification.
The invertase activity secreted (2.5 U ml−1) by X. dendrorhous ATCC MYA-131 (1 liter of MMM during 60 h, A660 = 4) was concentrated through a 30000MWCO PES by using a VivaFlow 50 system (Vivascience). The active fraction (150 ml) was dialyzed in 20 mM sodium phosphate (pH 7) (buffer A), and it was applied to a DEAE-Sephacel chromatography column (20 ml) equilibrated with buffer A. The protein was eluted with a 0 to 0.2 M NaCl gradient at a flow rate of 1 ml min−1. The fractions showing invertase activity were eluted with 0.05 and 0.1 M NaCl. The 0.1 M fractions (3 ml) were pooled, dialyzed in 20 mM sodium acetate pH 5 (buffer B), and then applied to a DEAE-Sephacel column equilibrated with buffer B. The proteins were eluted as described above, and the fractions (2 ml) showing invertase activity were pooled, dialyzed, and stored at −70°C (70 U ml−1; 12 μg ml−1). All procedures were carried out at 4°C. The protein profiles were determined by column chromatography, measuring the absorbance of the eluates at 280 nm. Silver-stained (PlusOne; Amersham Biosciences) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (8% polyacrylamide) gels of the samples confirmed the purity of the invertase. Broad-range protein markers (prestained; New England Biolabs or Bio-Rad) were used as a control. When required, the samples were concentrated to the desired volume by using the Microcon YM-10 (Amicon) system. Peptide-N-glycosidase F (PNGase F; New England Biolabs) treatment was performed according to the manufacturer's protocol. Invertase activity was detected from native preparations by electrophoresis on 7% polyacrylamide gels that were subsequently stained with 1% (wt/vol) 2,3,5-triphenyltetrazolium chloride in 0.25 M NaOH as described previously (47). Invertase activity from S. cerevisiae (Novozymes) was used as a control in this test. The protein concentration was determined by using the Bio-Rad microprotein determination assay according to the manufacturer's specifications and with bovine serum albumin as a standard.
For the N-terminal amino acid sequencing, the purified protein (500 ng) was subjected to SDS-PAGE (8%) and blotted onto polyvinylidene difluoride membranes (Millipore). The membranes were stained with Coomassie brilliant blue R250, and the band obtained was excised and processed for N-terminal amino acid sequencing (Helmholtz Center for Infection Research, Germany).
Enzyme and kinetic analysis.
Unless otherwise indicated, β-fructofuranosidase activity was determined by measuring the amount of glucose liberated from different substrates (0.5% [wt/vol]) in 50 mM sodium phosphate buffer [pH 5.5]) over 10 to 20 min at 42°C. The mixture was boiled for 5 min, and the glucose was measured by using a glucose oxidase-peroxidase assay (Sigma technical bulletin 510). A calibration curve was established with a 2-mg ml−1 glucose solution. One unit of activity was defined as that corresponding to the release of 1 μmol of glucose per min under the conditions described above. The enzyme associated with the cellular fractions was assayed after the addition of pearl glass and after five cycles of agitation in a vortex mixer for 1 min as indicated previously (41).
The Michaelis-Menten kinetic constants were determined using sucrose (Merck) at 0 to 60 mM or 1-kestose (TCI Europe) at 0 to 12 mM and 0.5 U of pure enzyme (∼10 μl conveniently diluted to fit the calibration curve). The plotting and analysis of the curves was carried out using SigmaPlot software (version 7.101), and the kinetic parameters were calculated by fitting the initial rate values to the Michaelis-Menten equation.
The estimation of hydrolase activity at different pH and temperatures was carried out under the aforementioned conditions using sucrose as the substrate. The buffers used were citric acid-sodium citrate (pH 3 to 4), Na2HPO4-NaH2PO4 (pH 4 to 7), and Tris-HCl (pH 7 to 8), all at 100 mM. The thermostability was determined by incubating 0.3 U of the pure enzyme at different temperatures, removing the samples at regular intervals, and estimating the residual activity as described previously.
Production of FOS.
The invertase was added to a sucrose solution (410 g liter−1) in 0.2 M sodium acetate buffer (pH 5.6) and in a total reaction volume of 2 ml. The activity in the mixture was adjusted to 0.5 U ml−1, and the mixture was incubated at 50°C in an orbital shaker (Vortemp) at 200 rpm. At different times, 40-μl aliquots were withdrawn, diluted with 160 μl of water, and incubated for 10 min at 90°C to inactivate the enzyme. The samples were centrifuged for 5 min at 6,000 rpm in an Eppendorf centrifuge with a 0.45-μm-pore-size Durapore membrane (Millipore) and then analyzed by high-pressure liquid chromatography with a quaternary pump (Delta 600; Waters) coupled to a 5-μm Lichrosorb-NH2 column (4.6 by 250 mm; Merck) as indicated previously (2).
DNA techniques, cDNA library construction, and cloning of the X. dendrorhous invertase.
Routine recombinant DNA techniques were used throughout (51). The X. dendrorhous ATCC MYA-131 strain was grown in 100 ml of MMM at 24°C in an orbital shaker (A660 = 1.8). The cells were frozen in liquid nitrogen, total RNA was isolated by using TRI Reagent (Molecular Research Centre), and polyadenylated mRNA was enriched by using oligo(dT) cellulose chromatography (Amersham Biosciences) in accordance with the manufacturer's instructions. The X. dendrorhous cDNA library was generated with the pBluescript II XR cDNA library construction kit (Stratagene); it contained 3.3 × 105 clones with an average insert size of 1.3 kb and was stored as individual pools (1,500 to 6,000 CFU/pool) in 43% glycerol at −80°C.
The coding sequence of the extracellular invertase from X. dendrorhous was amplified by PCR using the cDNA library as the template and the universal T7 (Stratagene) and INV-Nter primers (Table 1), the latter directed against part of the N-terminal amino acid sequence (EGWMNDPMG) of the protein. The fragments were amplified with the Pwo DNA polymerase (Roche Diagnostics, Germany) under the following conditions of amplification: (i) 94°C for 120 s; (ii) 10 cycles of 94°C for 10 s, 50°C for 30 s, and 68°C for 300 s; (iii) 30 cycles of 94°C for 10 s and 50°C for 30 s; and finally (iv) 68°C for 300 s in the first cycle, which gradually increased by 12 s per cycle up to 660 s in the last one. The PCR fragment amplified (1.8 kb) was purified by agarose gel electrophoresis, it was then recovered with the QiaExII gel extraction kit (Qiagen), cloned into the pST-Blue1 vector (Invitrogen) as recommended by the supplier, and sequenced (SIDI, Universidad Autónoma de Madrid, Madrid, Spain). The GWMN, RDP, and FIN primers (Table 1) were used to complete the sequencing of the 1.8-kb fragment. The cDNA library and the T3 (Stratagene) and GWMN(−) primers (Table 1) were used to amplify and analyze the region that putatively lies upstream of the GWMN amino acid sequence.
TABLE 1.
Primers used in this study
| Primer | Sequencea |
|---|---|
| INV-Nter | GA(R)GG(N)TGGATGAA(Y)GA(Y)CC(N)ATGGG |
| GWMN | GCTGGATGAACGACCCTATGGGGTTGT |
| RDP | GAGCCCAACTTGATCGGTTTTCGAGAT |
| FIN | GCTGGCTTCCGAGTGCTTGCGTCCGA |
| FVK | GGGCGAGACGTTCTCGACGACCTT |
| GWMN(−) | CGCTGGTACAACCCCATAGGGTCGTTC |
| INVXhoI | GGGAACTCGAGAGAAACACAAACAGATGGACA |
| INVHindIII | GCGTACGCAAAGCTTCTCGACCTTCCTAATT |
| INVBamHI | GGCGTGGATGGATCCA |
| INVXbaI(MF1α) | CCCTATCTAGATGAAGAGAGAAGCTGAAGCTTTCATTGCACCTGAAGGCTGGATGAACGACCCTATG |
The MFα1 spacer region coding sequence is underlined, and the restriction sites are indicated in boldface. The restriction site in INVBamHI was not included artificially.
To characterize the genomic DNA encoding the invertase from X. dendrorhous, total DNA from this yeast was isolated as described previously (7) and used as the template in PCR amplifications. The GWMN and FVK primers (Table 1) were used to amplify a 1.9-kb fragment that included most of this gene. Inverse PCR was used to analyze the flanking sequences of this DNA fragment (38). Briefly, genomic DNA from the yeast was digested with XhoI (an enzyme that has no restriction sites in the 1.9-kb amplified fragment); it was incubated with T4 DNA ligase (Roche Diagnostics, Germany) and then treated with BamHI (which has a single cleavage site within the 1.9-kb fragment at nucleotide 291). The digested product was used as a template in PCRs with the GWMN(−) and FIN primers (Table 1). All of the PCR products amplified were introduced into the pST-Blue1 vector and sequenced.
To express the hypothetical invertase from X. dendrorhous in a heterologous system, the 1,788-nucleotide fragment was amplified from the cDNA library using the INVHindIII and INVXhoI primers (Table 1). This fragment started at the TTC codon, it terminated at the TAA stop codon, and it was introduced into the pBluescript SK(+) plasmid (Stratagene). The resulting INV-BS construct was then used as a template to fuse an ATG codon, followed by the MFα1 spacer region (KREAEA) using the INVBamHI and INVXbaI(MF1α) primers (Table 1), thereby generating a sequence encoding the putative extracellular protein. The INVBS-MF construct generated was verified by sequencing and digested with XbaI and XhoI, and the 1,809-nucleotide fragment obtained was introduced into the pVT103-L plasmid (57) under the control of the ADH1 promoter. The resulting pINV-PVT plasmid was used to transform S. cerevisiae SEY 2101.
Phylogenetic analysis and molecular modeling.
The amino acid sequence encoded by the β-fructofuranosidase gene from X. dendrorhous (GenBank accession no. FJ539193) was analyzed by using the BLAST server against the Swiss-Prot protein database (http://www.expasy.org/tools/blast/), and the sequences were aligned with the CLUSTALW interface in MEGA4.0 (http://www.megasoftware.net/; pairwise alignment gap opening penalty, 10; gap extension penalty, 0.1; multiple alignment gap opening penalty, 10; gap extension penalty, 0.2). The bootstrap test of phylogeny was used with the tree obtained.
A structure-based alignment of X. dendrorhous invertase and A. thaliana invertase (PDB identifier 2ac1) was performed with MUSCLE (10), and the resulting alignment was further refined manually. This alignment was used to build a structural model with MODELLER9V4 (50).
Nucleotide sequence accession numbers.
The sequences encoding the invertase from X. dendrorhous have been assigned EMBL accession no. FJ539193.
RESULTS
Biochemical characterization of a β-fructofuranosidase activity from X. dendrorhous.
The yeast X. dendrorhous is able to consume sucrose (17), and a cell-associated invertase activity has already been reported in this organism (41). In an attempt to characterize this enzyme, the yeast was grown in liquid media (33), and the invertase activity was determined from samples taken at different growth times. In these conditions, maximum levels of activity (approximately 2 to 4 U ml−1) were detected in the culture filtrates at the beginning of the stationary phase (A660 = 4), and these levels were maintained for at least 80 h of growth (Fig. 1). However, and contrary to previous reports (41), only low levels of activity (≤0.8 U ml−1) were found in the cell-associated fraction during this period. In addition, no activity was detected when glucose was used as a carbon source (data not shown), pointing to the catabolic repression of the enzyme analyzed. It was notable that similar activity levels and profiles were obtained using three strains of this particular yeast (ATCC MYA-131, ATCC 24202, and ATCC 24230; data not shown).
FIG. 1.

Extracellular invertase activity. Inocula from X. dendrorhous ATCC MYA-131 were grown (⋄) in 250-ml flasks containing 50 ml of MMM. The invertase activity was measured in 0.01 ml of culture filtrates at the times indicated using sucrose (▴) as the substrate. Each point represents the average of three independent measurements with a standard deviation of ±5%. Similar results were obtained for two other different cultures (data not shown).
To purify the invertase activity from X. dendrorhous, the culture was collected and processed as described in Materials and Methods. The overall yield of the purification was 30% (data not shown), and only one band of ∼160 kDa was evident when assayed by SDS-PAGE (Fig. 2A). Treatment with PNGase F resulted in a shift in the apparent molecular mass of this protein to ∼66 kDa (Fig. 2B). Thus, presuming that the glycosylated and unglycosylated forms behave similarly in the gel, N-linked oligosaccharides appear to represent ca. 60% (94 kDa) of the total protein mass.
FIG. 2.
SDS-PAGE analysis of the purified invertase and PNGase F treatment. (A) Purification. The concentrated culture filtrate from X. dendrorhous ATCC MYA-131 expressing the invertase activity was subjected to SDS-PAGE before (lane 2) or after DEAE-Sephacel column chromatography at pH 7 (lane 3) and pH 5 (lane 4). Lane 1, protein standards. (B) Purified invertase digested (5 μg, lane +) with 0.2 U of PNGase F for 90 min at 37°C or undigested (10 μg, lane −). (C) Purified invertase activity was revealed in situ (lane 2), and the S. cerevisiae enzyme was used as a control (lane 3). Lane 1, protein standards. The positions of the molecular mass markers are indicated (in kilodaltons) at the left of panels A and C.
The purified enzyme yielded a smeared band greater than 200 kDa in activity-staining gels (Fig. 2C). In this assay the invertase from S. cerevisiae was used as a control, and it also produced a smeared band with a molecular mass greater than 200 kDa (Fig. 2C), which probably corresponded to the 270-kDa glycosylated, functionally active homodimer described previously (14). This correlation suggested that the active enzyme from X. dendrorhous was also likely to function as a dimmer.
The biochemical properties of the enzyme purified from X. dendrorhous were characterized, including the active pH range and optimal temperatures, as well as its thermostability and substrate specificity. This enzyme displayed maximum activity at pH 5.0 to 6.5 (Fig. 3A) and a temperature of 65 to 70°C, with ca. 90% of its activity maintained in the range of 60 to 75°C (Fig. 3B). In general, two properties should be considered in association with high-temperature adaptation: the thermophilicity, the ability of an enzyme to exhibit activity at high temperatures, and the thermostability, the ability to remain stable and/or active after storage at high temperature. To determine the thermostability of the X. dendrorhous invertase, the purified enzyme was preincubated for different periods of time prior to substrate addition at temperatures in the range of 40 to 85°C. Only minor inactivation of the enzyme (<10%) was detected after 4 days at 40 to 50°C, whereas incubation for 24 h at 60°C decreased its activity by 50%, and it was completely inactivated within 10 min at 85°C (data not shown). The enzyme was then preincubated at 60 to 85°C and for 10 to 120 min. Under these conditions, a 50% loss of activity (T50) was produced in the 66 to 71°C range (Fig. 3C).
FIG. 3.
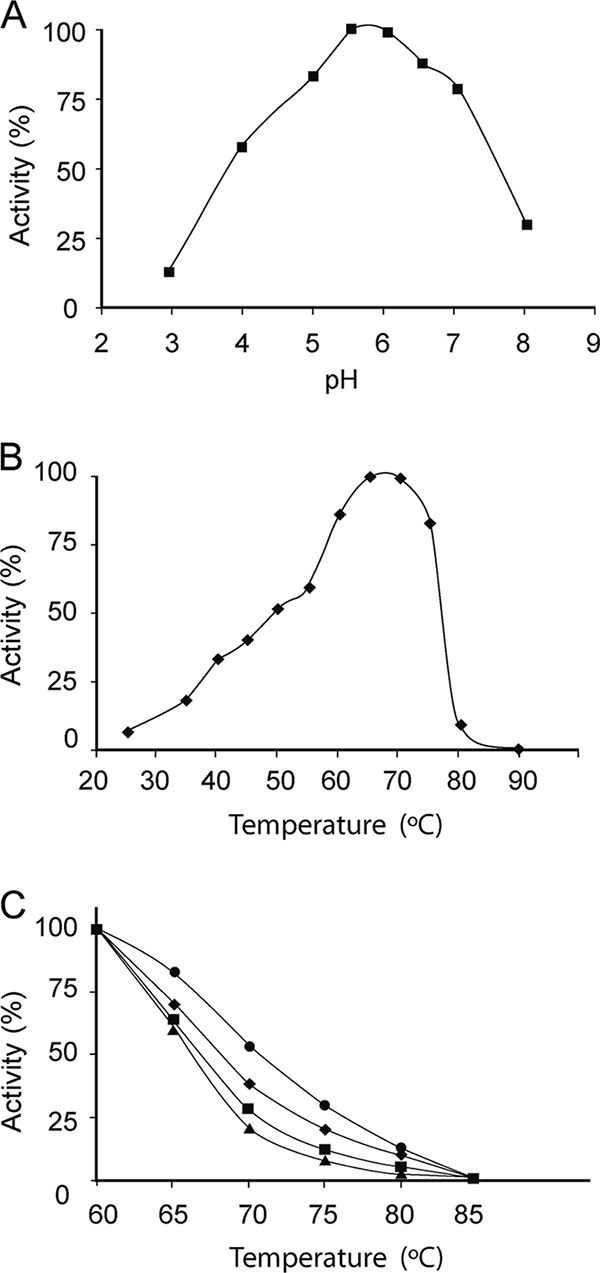
Temperature, pH dependence, and thermostability profiles. The effect of pH (A) and temperature (B) on the X. dendrorhous invertase activity was evaluated at 42°C and at pH 5.5, respectively. (C) The purified invertase was incubated for 10 (•), 20 (⧫), 60 (▪), and 120 (▴) min at temperatures in the range of 60 to 85°C in 50 mM sodium phosphate buffer (pH 5.5) prior to the addition of the substrate. The remaining activity was determined at 42°C as described in Materials and Methods. Each point represents the mean of four independent measurements with a standard deviation of ±4%.
Substrate specificity of the enzyme and kinetic properties.
The enzyme purified from X. dendrorhous was able to liberate glucose from fructosyl-β-(2→1)-linked nonreducing carbohydrates such as sucrose [α-d-glucopyranosyl-(1→2)-β-d-fructofuranose], 1-kestose [α-d-glucopyranosyl-(1→2)-β-d-fructofuranosyl-(1→2)-β-d-fructofuranose], or nystose [α-d-glucopyranosyl-(1→2)-β-d-fructofuranosyl-(1→2)-β- d-fructofuranosyl-(1→2)-β-d-fructofuranose], as well as from palatinose [α-d-glucopyranosyl-(1→6)-d-fructofuranose]. However, while a specific activity of ∼5,200 mU μg−1 was quantified for sucrose, only 1,200 mU μg−1 was measured for 1-kestose, and very weak activity was observed for nystose (220 mU μg−1) and palatinose (90 mU μg−1). The enzyme was not active on maltose [α-d-glucopyranosyl-(1→4)-d-glucopyranose], lactose [β-d-galactopyranosyl-(1→4)-d-glucopyranose], or leucrose [α-d-glucopyranosyl-(1→5)-d-fructofuranose], indicating that it only recognizes carbohydrates containing fructosyl-β-(2-1) or -(6-1)-linked bonds. This enzyme displayed Michaelis kinetics toward sucrose and 1-kestose (results not shown), and the kinetic parameters obtained are presented in Table 2. The Km value of 4 mM sucrose was similar to that obtained with the Schwanniomyces occidentalis enzyme (4.9 mM [2]), about twofold that found for the C. utilis enzyme (1 to 2 mM [5]) and less than that measured for the invertase from P. anomala (16 mM [49]) or S. cerevisiae (26.1 mM [45]). In addition, the catalytic efficiency defined by the kcat/Km ratio showed that the X. dendrorhous fructofuranosidase hydrolyzes sucrose ∼4.2 times more efficiently than 1-kestose.
TABLE 2.
Kinetic analysis of the β-fructofuranosidase from X. dendrorhous
| Substrate | Mean ± SEa
|
||
|---|---|---|---|
| kcat (min−1) | Km (mM) | kcat/Km (min−1 mM−1) | |
| Sucrose | 341 ± 14 | 4.0 ± 0.5 | 85.3 ± 6.8 |
| 1-Kestose | 90.8 ± 7.3 | 4.5 ± 0.7 | 20.2 ± 2.3 |
Reaction rate measurements were performed in triplicate. Values of kcat were calculated from the Vmax considering a protein molecular mass of 64.63 kDa. The kcat/Km standard errors were obtained by fitting the normalized Michaelis-Menten equation as: v = (kcat/Km)[S]/(1 + [S]/Km).
Transfructosylating activity.
The transfructosylating activity of the X. dendrorhous enzyme was assayed with sucrose under the conditions indicated in Materials and Methods, and the analysis of the reaction products showed that the enzyme possessed this activity (Fig. 4A). The amount of fructose detected was slightly less than that detected for glucose, a finding indicative of the fructosyltransferase activity of the enzyme at high sucrose concentrations. A blank reaction in the absence of enzyme was also assessed, and peaks 4 and 5 were not evident (data not shown).
FIG. 4.
(A) High-pressure liquid chromatogram corresponding to the reaction of sucrose with the β-fructofuranosidase from X. dendrorhous. Peaks: 1, fructose; 2, glucose; 3, sucrose; 4, neokestose; 5, 1-kestose; 6 to 8, tetrasaccharides. (B) Schematic view of the transfructosylation reactions.
Based on their chromatographic mobility, the compounds corresponding to peaks 4 and 5 were identified as neokestose (6G-FOS series) and 1-kestose (1F-FOS series), respectively. Figure 5 shows the reaction profile using a 410-g liter−1 (41% [wt/vol]) sucrose solution. The neokestose/1-kestose ratio varied during the reaction, with values between 2/1 and 3/1. At the point of maximum FOS production (48 h), the reaction mixture contained 132 g of fructose, 179 g of glucose, 49 g of sucrose, 40 g of neokestose, 18 g of 1-kestose, and 8 g of tetrasaccharides (mainly nystose) per liter. The total FOS production is shown in Fig. 5B. The maximum FOS concentration achieved was 65.9 g liter−1; the 15.8% FOS percentage referred to the total amount of sugars in the mixture.
FIG. 5.
(A) Time course of neo-FOS and FOS production catalyzed by the β-fructofuranosidase from X. dendrorhous. Experimental conditions: 410 g of sucrose liter−1, 0.5 U ml−1, 0.2 M sodium acetate buffer (pH 5.6), 50°C. (B) Formation of total FOS.
Molecular characterization of the fructofuranosidase from X. dendrorhous.
To isolate the gene encoding the β-fructofuranosidase from X. dendrorhous, the enzyme was initially processed for amino acid sequencing as indicated in Materials and Methods. The N terminus of the extracellular mature protein was determined to be FIAPEGWMNDPMGL, which already included part of the β-fructosidase NDPN motif, and it aligned with part of the amino acid sequences from yeast and fungal invertases in BLAST searches. A cDNA library of this yeast was constructed and used as the template for PCRs, including an oligonucleotide targeted to the N-terminal amino acid sequence of this protein (for details, see Materials and Methods). In this way, an open reading frame (ORF) of 1,788 bp was identified (Xd-INV), corresponding to a 595-amino-acid polypeptide. A molecular mass of 64.62 kDa was calculated for the polypeptide derived from this sequence, with no signal peptide, and this was in accordance with the apparent molecular mass of the 66 kDa for the purified unglycosylated enzyme. The analysis of the sequences flanking the 1,788-bp ORF showed that the first in-frame ATG codon was located at position 210 upstream of the TTC codon that encodes the initial Phe of the mature protein. This suggests the presence of a putative signal peptide of 70 amino acids that will not be present in the mature protein. In silico analysis of this putative peptide provided evidence of a potential secretion signal (6) and a predicted cleavage site between positions 17 and 18 (AYA-AEL). In addition, the sequence of the mature protein had a predicted pI of 4.4, and it contained 17 putative N-linked glycosylation sites (N-X-S/T), as well as another four such sites located between amino acids 24 and 57 of the presumptive signal peptide. Comparing the cDNA sequence with that of the genomic DNA identified three introns of 92, 95, and 117 bp, all located in the first third of the sequence encoding the protein analyzed.
The deduced protein sequence of Xd-INV was very similar to that of the β-fructofuranosidases from basidiomyceta yeasts and Aspergillus spp. Indeed, it was most similar to invertases from Uromyces fabae (41% identity over a 295-amino-acid overlap), A. niger 20611 (later reclassified as A. japonicus ATCC 20611; FopA: 33% over a 223-amino-acid overlap), A. niger (SucA: 33% over a 211-amino-acid overlap), and A. sydowii (31% over a 175-amino-acid overlap). Lower similarities were observed for proteins from the yeasts S. cerevisiae or C. utilis, as well as for other bacteria and plant proteins (Fig. 6A). Xd-INV contained most of the elements characteristic of β-fructofuranosidases and, indeed, the six domains that are well conserved among the microbial β-fructofuranosidases (A to F [8]) were all essentially present in the X. dendrorhous protein (data not shown). Multiple-sequence alignment of the GH families 32, 43, 62, and 68 revealed the presence of three conserved domains (A, D, and E), each containing a key acidic residue that is implicated in substrate binding and hydrolysis (43), and these residues were also present in the enzyme isolated from X. dendrorhous (Fig. 6B).
FIG. 6.
Phylogenetic analysis of the fructofuranosidase from X. dendrorhous and a comparison of the catalytic residues. (A) The radical tree was constructed from the alignment of the amino acid sequences by using the CLUSTALW program. The GenBank accession numbers are indicated. (B) Alignment of conserved sequences including the A, D, and E domains of fructosylhydrolases. Underlined residues indicate the positions of the acidic residues implicated in substrate binding and hydrolysis. The sequence identifiers are in accordance with the nomenclature in Swiss-Prot.
To prove the functionality of the Xd-INV gene isolated, we fused the 1,788-bp ORF to the ATG codon and an S. cerevisiae MFα1 spacer region in the pVT103-L expression vector. The pINV-PVT plasmid generated was used to transform a S. cerevisiae strain unable to ferment sucrose as a sole carbon source. The growth of transformants (leu+) carrying either the pVT103-L vector or the pINV-PVT plasmid was no different on a glucose-based medium (MMG). However, only the pINV-PVT was able to complement the invertase-negative phenotype of the S. cerevisiae strain on a sucrose-based medium (MMS). Invertase activity was detected in positive transformants, but only weak activity (10 mU ml−1) was quantified from the cellular fraction. Together, theses data provide direct evidence that Xd-INV gene truly acts as an invertase.
DISCUSSION
In contrast to previous studies that indicated the presence of an invertase exclusively associated with the cell fraction of X. dendrorhous (41), we have purified an extracellular activity from this yeast that is able to liberate glucose from sucrose. The enzyme was glycosylated, and it presented a molecular mass of 160 kDa that was derived from a 66-kDa unglycosylated monomer. Its active form probably represents a homooligomeric protein with an apparent molecular mass greater than 200 kDa as judged from its mobility in seminative acrylamide gels. Similarly, invertases described in a number of yeasts are also dimeric or multimeric enzymes generated from unglycosylated monomeric peptides with an average molecular mass of 60 to 65 kDa, including that of S. cerevisiae, S. pombe, P. anomala, or C. utilis (Table 3). The maximum activity of the enzyme from X. dendrorhous was reached at pH 5.0 to 6.5, which is also in accordance with the data for other yeast invertases such as that of P. anomala, and it is only a slightly higher range than that found for S. cerevisiae or C. utilis (Table 3). However, the thermophilicity (maximum activity at 65 to 70°C) and thermostability (T50 in the range 66 to 71°C) of this enzyme were above that exhibited by most yeast invertases, the optimal temperatures of which are generally around 40 to 50°C and that are rather unstable at higher values (Table 3). Nevertheless, an invertase from Rhodotorula sp. that is very stable at temperatures just below 66°C was recently described (20), and another from C. utilis has an optimum temperature of 70°C, but its thermostability remains to be defined (5).
TABLE 3.
Properties of β-fructofuranosidases from different sources
| Source | Molecular mass (kDa)
|
Optimum
|
Transglycosylation main product(s) | Source or reference(s) | ||
|---|---|---|---|---|---|---|
| Glycosylateda | Native | pH | Temp (°C) | |||
| Aspergillus aculeatus | 65 | 134 | 5.0-7.0 | 60 | 1-Kestose | 15 |
| Aspergillus foetidus | 90 | 180 | NR | NR | 1-Kestose | 47 |
| Aspergillus niger (SucB) | 75 | NRb | 5.0 | 40°C | 1-Kestose, nystose | 18 |
| Aspergillus japonicus ATCC 20611 | 100 | 340 | 5.0 | 50 | 1-Kestose, nystose | 22, 23 |
| Candida utilis | 150 | 300 | 4.4 | 70 | NR | 5 |
| Pichia anomala | 86 | 254 | 4.0-6.5 | 38 | NR | 49 |
| Saccharomyces cerevisiae | 135 | 270 | 3.5-5.5 | 50 | 6-Kestose, 6β-fructofuranosylglucose | 11, 14, 36 |
| Schizosaccharomyces pombe | 205 | 1,070 | NR | NR | NR | 36 |
| Schwanniomyces occidentalis | 85 | 85,175 | 5.5 | 45-55 | 6-Kestose | 2 |
| Uromyces fabae | 118 | NR | 4.6 | 40 | NR | 59 |
| Xanthophyllomyces dendrorhous | 160 | >200 | 5.0-6.5 | 65-70 | Neokestose | This study |
Approximate molecular mass obtained from SDS-PAGE for the monomeric form.
NR, not reported.
The enzyme purified from X. dendrorhous is a β-fructofuranosidase that can hydrolyze fructosyl-β(2→1)-linked carbohydrates (sucrose, 1-kestose, and nystose) and palatinose [α-d-Glc-(1→6)-d-Fru]. The enzyme hydrolyzes sucrose very efficiently, and it has transfructosylating activity. In contrast to other microbial β-fructofuranosidases that produce mainly 1F-FOS and little or no 6G-FOS (Table 3) (19, 52), the main transglycosylation product of the X. dendrorhous enzyme is neokestose (6G-FOS), followed by 1-kestose. The maximum FOS concentration of 65.9 g liter−1 from a concentration of 410 g liter−1 sucrose corresponded to 15.8% (wt/wt) of the total sugar composition in the mixture. This yield could be improved by increasing the initial sucrose concentration favoring the transglycosylation activity (42). In this context, 49.4 g of neo-FOS liter−1 (8.2% wt/wt in the sugar composition) was obtained with intact immobilized P. citrinum cells and a 600-g liter−1 sucrose solution (31), and the neo-FOS production increased to 108.4 g liter−1 (18% [wt/wt] of the total sugar composition) by coimmobilization of P. citrinum cells and their neofructosyltransferase (32). Neokestose is a bifidogenic substance with prebiotic effects that may surpass those of commercial FOS (29, 34, 54). In addition, the branched structure of the neo-FOS confers enhanced chemical stability in comparison to conventional FOS (32), and for this reason the new enzyme characterized here could be of considerable biotechnological value.
Plants contain different forms of invertases that can be distinguished by their subcellular localization, as well as through their biochemical characteristics. Formation of neokestose by plant fructosyltransferases (6G-FFT) that catalyze the transfer of a fructose residue from 1-kestose to the C6 of the glucose moiety of sucrose has been well studied in liliaceous species such as onion and asparagus (13, 53, 58). In this context, a comparative amino acid sequence analysis of theses proteins might help to clarify their different properties (thermostabilities, specificities, regioselectivities, etc.) and to understand the unusual behavior of the X. dendrorhous enzyme.
We have isolated and characterized the Xd-INV gene that encodes the invertase from X. dendrorhous after determining the 14 N-terminal amino acids of the purified protein. This sequence was also found in the deduced amino acid sequence of the cloned gene and, furthermore, this Xd-INV encoded an invertase when it was expressed in an invertase-deficient yeast strain. This provides convincing evidence that the gene analyzed encodes the enzyme characterized from X. dendrorhous. The amino acid sequence of the protein encoded by Xd-INV revealed close similarity to other β-fructofuranosidases within the GH32 enzyme family, which includes invertases, inulinases, levanases, and fructosyltransferases. Indeed, a structural model of this enzyme based on the homologous invertase from A. thaliana (55) has been obtained (Fig. 7). These two proteins only share 19% sequence identity but, nevertheless, the enzyme from X. dendrorhous has the fivefold propeller and β-sandwich motifs characteristic of many family 32 glycoside hydrolases. The complete β-fructosidase motif, also known as the NDPN box (16), was reduced to NDP in this new protein. A similar change was also found in the enzyme from U. fabae (59), as well as in the putative enzyme from U. maydis and in other related fungal β-fructofuranosidases. Similarly, the entire ECP/V box (16) could not be identified in any of these proteins, including Xd-INV. Nevertheless, all of the proteins analyzed share a common acidic residue in these two boxes, which has been previously identified experimentally in S. cerevisiae invertase (46), A. awamori exoinulinase (37), or T. maritima β-fructosidase (1). This residue appears to form part of the catalytic machinery responsible for the cleavage of glycosidic bonds. On the basis of our multiple sequence alignment and our structural model, we propose Asp80 and Glu303 (Asp10 and Glu233 in the mature protein) to be the two presumptive catalytic residues in the X. dendrorhous enzyme. The predicted Xd-INV sequence also contains the conserved RDP motif, and we speculate that, as in the A. awamori enzyme (37), Arg220 and Asp221 (Arg150 and Asp151 in the mature protein) within this motif could also participate in substrate recognition.
FIG. 7.
Molecular model of X. dendrorhous fructofuranosidase. Ribbon representation of the overall structure showing the catalytic residues: D10 (nucleophile) and E233 (catalytic acid/base) catalytic residues. The profile-profile derived alignment of X. dendrorhous fructofuranosidase (INV-Xd) and A. thaliana invertase (2ac1) is presented in the supplemental material.
The main industrial FOS producers are currently enzymes from Aspergillus which generally provide a mixture of molecules of the inulin-type β (2→1) structure 1F-FOS. Despite the fact that catalytic specificity may be dependent on experimental conditions, β-fructofuranosidase SucB of A. niger and FopA of A. japonicus ATCC 20611 (previously A. niger ATCC 20611) produce 1-kestose and nystose, whereas that of A. sydowii IAM 2544 produces some high-molecular-weight polymers (21) (Table 3). In addition, the fructosyltransferase from A. foetidus, which clusters in a separate branch of the phylogenetic tree (Fig. 6A) to the other fungal proteins, produces 1-kestose (Table 3). None of these enzymes produces 6G-FOS (neokestose), such as the X. dendrorhous enzyme (Table 3). Furthermore, and as far as we know, no transferase activity has been reported for the β-fructofuranosidase SucA of A. niger, A. oryzae, U. fabae, U. maydis, P. anomala, C. utilis, and S. pombe (Table 3). Remarkably, although the enzyme from X. dendrorhous and those of A. niger and A. sydowii cluster in the same branch of the phylogenetic tree, their enzymatic activities appear to be fairly different. However, the invertase from S. cerevisiae clusters in a different branch even though it produces FOS with a levan-type β (2→6) structure, mainly 6-kestose (6F-FOS), with neokestose (6G-FOS) being a side product of the reaction (11). Penicillium citrinum cells also produced some neokestose from sucrose (19) but, unfortunately, no protein responsible for this biosynthetic reaction has yet been identified and characterized.
The overall amino acid sequence similarity between the enzyme from X. dendrorhous and that of the plant fructosyltransferases that produce neokestose or fructans with a higher degree of polymerization (13, 53, 58) was low (<26% over an ∼100-amino-acid overlap). Indeed, all of these proteins cluster as a distinct group in the phylogenetic tree. Based on the sequence comparisons and enzymatic properties, fructosyltransferases from plants are thought to evolve from vacuolar invertases that lack transferase activity. In this context, replacing 33 amino acids that correspond to the N terminus of the mature onion vacuolar invertase with the corresponding region of onion 6G-FFT led to a shift in activity from the hydrolysis of sucrose toward a transferase reaction (48). In addition, site-directed mutagenesis studies have revealed that positions relatively far from the N terminus are involved in fructosyl transfer reactions of levansucrases (GH68) from Zymomonas mobilis (61) or Bacillus subtilis (35). In general, the structural motives required for transferase activity of the β-fructofuranosidases are poorly defined. Further research into these structure-specificity relationships should shed additional light on the determinants responsible for fructosyltransferase activity within the GH32 enzyme family.
Supplementary Material
Acknowledgments
This study was supported by grants from the Plan Nacional CICYT (BIO2004-03773-C04-01/03 and BIO2007-67708-C04-01/03), by Genoma España (National Foundation for Promoting Genomics and Proteomics), and by an institutional grant from the Fundación Ramón Areces to the Centro de Biología Molecular Severo Ochoa. D.L. was supported by a Spanish FPU fellowship from the Ministerio de Educación y Ciencia.
We thank Antonio Ballesteros (ICP-CSIC) for his support during this research. We also thank Rita Getzlaff (HZI, Germany) for protein sequence analyses, Manuel Ferrer (ICP-CSIC) for support in the protein blotting, and David Abia for help with the protein modeling.
Footnotes
Published ahead of print on 16 December 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Alberto, F., E. Jordi, B. Henrissat, and M. Czjzek. 2006. Crystal structure of inactivated Thermotoga maritima invertase in complex with the trisaccharide substrate raffinose. Biochem. J. 395:457-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Álvaro-Benito, M., M. A. de Abreu, L. Fernández-Arrojo, F. J. Plou, J. Jiménez-Barbero, A. Ballesteros, J. Polaina, and M. Fernández-Lobato. 2007. Characterization of a β-fructofuranosidase from Schwanniomyces occidentalis with transfructosylating activity yielding the prebiotic 6-kestose. J. Biotechnol. 132:75-81. [DOI] [PubMed] [Google Scholar]
- 3.Antosova, M., and M. Polakovic. 2001. Fructosyltransferases: the enzymes catalyzing production of fructooligosaccharides. Chem. Pap.-Chem. Zvesti. 55:350-358. [Google Scholar]
- 4.Bang, M. L., I. Villadsen, and T. Sandal. 1999. Cloning and characterization of an endo-β-1,3(4)glucanase and an aspartic protease from Phaffia rhodozyma CBS 6938. Appl. Microbiol. Biotechnol. 51:215-222. [DOI] [PubMed] [Google Scholar]
- 5.Belcarz, A., G. Ginalska, J. Lobarzewski, and C. Penel. 2002. The novel non-glycosylated invertase from Candida utilis (the properties and the conditions of production and purification). Biochim. Biophys. Acta 1594:40-53. [DOI] [PubMed] [Google Scholar]
- 6.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 7.Castillo, A., and V. Cifuentes. 1994. Presence of double-stranded RNA and virus-like particles in Phaffia rhodozyma. Curr. Genet. 26:364-368. [DOI] [PubMed] [Google Scholar]
- 8.Chávez, F. P., T. Pons, J. M. Delgado, and L. Rodriguez. 1998. Cloning and sequence analysis of the gene encoding invertase (INV1) from the yeast Candida utilis. Yeast 14:1223-1232. [DOI] [PubMed] [Google Scholar]
- 9.Díaz, A., C. Sieiro, and T. G. Villa. 2003. Production and partial characterization of a beta-amylase by Xanthophyllomyces dendrorhous. Lett. Appl. Biotechnol. 36:203-207. [DOI] [PubMed] [Google Scholar]
- 10.Edgar, R. C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farine, S., C. Versluis, P. J. Bonnici, A. Heck, C. L'Homme, A. Puigserver, and A. Biagini. 2001. Application of high performance anion exchange chromatography to study invertase-catalysed hydrolysis of sucrose and formation of intermediate fructan products. Appl. Microbiol. Biotechnol. 55:55-60. [DOI] [PubMed] [Google Scholar]
- 12.Fernández Arrojo, L., D. Marín, A. Gómez de Segura, D. Linde, M. Alcalde, P. Gutiérrez Alonso, F. J. Plou, M. Fernández-Lobato, and A. Ballesteros. 2007. Transformation of maltose into prebiotic isomaltooligosaccharides by a novel alpha-glucosidase from Xanthophyllomyces dendrorhous. Process Biochem. 42:1530-1536. [Google Scholar]
- 13.Fujishima, M., H. Sakai, K. Ueno, N. Takahashi, S. Onodera, N. Benkeblia, and N. Shiomi. 2005. Purification and characterization of a fructosyltransferase from onion bulbs and its key role in the synthesis of fructo-oligosaccharides in vivo. New Phytol. 165:513-524. [DOI] [PubMed] [Google Scholar]
- 14.Gascón, S., N. P. Neumann, and J. O. Lampen. 1968. Comparative study of the properties of the purified internal and external invertases from yeast. J. Biol. Chem. 243:1573-1577. [PubMed] [Google Scholar]
- 15.Ghazi, I., L. Fernández-Arrojo, H. Garcia-Arellano, F. J. Plou, and A. Ballesteros. 2007. Purification and kinetic characterization of a fructosyltransferase from Aspergillus aculeatus. J. Biotechnol. 128:204-211. [DOI] [PubMed] [Google Scholar]
- 16.Goetz, M., and T. Roitsch. 1999. The different pH optima and substrate specificities of extracellular and vacuolar invertases from plants are determined by a single amino-acid substitution. Plant J. 20:707-711. [DOI] [PubMed] [Google Scholar]
- 17.Golubev, W. I. 1995. Perfect state of Rhodomyces dendrorhous (Phaffia rhodozyma). Yeast 11:101-110. [DOI] [PubMed] [Google Scholar]
- 18.Goosen, C., X. L. Yuan, J. M. van Munster, A. F. Ram, M. J. van der Maarel, and L. Dijkhuizen. 2007. Molecular and biochemical characterization of a novel intracellular invertase from Aspergillus niger with transfructosylating activity. Eukaryot. Cell 6:674-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi, S., T. Yoshiyama, N. Fujii, and S. Shinohara. 2000. Production of a novel syrup containing neofructo-oligosaccharides by the cells of Penicillium citrinum. Biotechnol. Lett. 22:1465-1469. [Google Scholar]
- 20.Hernalsteens, S., and F. Maugeri. 2008. Purification and characterisation of a fructosyltransferase from Rhodotorula sp. Appl. Microbiol. Biotechnol. 79:589-596. [DOI] [PubMed] [Google Scholar]
- 21.Heyer, A. G., and R. Wendenburg. 2001. Gene cloning and functional characterization by heterologous expression of the fructosyltransferase of Aspergillus sydowi IAM 2544. Appl. Environ. Microbiol. 67:363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hidaka, H., M. Hirayama, and N. Sumi. 1988. A fructooligosaccharides-producing enzyme from Aspergillus niger ATCC 20611. Agric. Biol. Chem. 52:1181-1187. [Google Scholar]
- 23.Hirayama, M., N. Sumi, and H. Hidaka. 1989. Purification and properties of a fructooligosaccharide-producing beta-fructofuranosidase from Aspergillus niger ATCC-20611. Agric. Biol. Chem. 53:667-673. [Google Scholar]
- 24.Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, E. A. 2003. Phaffia rhodozyma: colorful odyssey. Int. Microbiol. 6:169-174. [DOI] [PubMed] [Google Scholar]
- 26.Katapodis, P., and P. Christakopoulos. 2004. Induction and partial characterization of intracellular β-fructofuranosidase from Thermoascus aurantiacus and its application in the synthesis of 6-kestose. World J. Microbiol. Biotechnol. 20:667-672. [Google Scholar]
- 27.Katapodis, P., E. Kalogeris, D. Kekos, B. J. Macris, and P. Christakopoulos. 2004. Biosynthesis of fructo-oligosaccharides by Sporotrichum thermophile during submerged batch cultivation in high sucrose media. Appl. Microbiol. Biotechnol. 63:378-382. [DOI] [PubMed] [Google Scholar]
- 28.Kaur, N., and A. K. Gupta. 2002. Applications of inulin and oligofructose in health and nutrition. J. Biosci. 27:703-714. [DOI] [PubMed] [Google Scholar]
- 29.Kilian, S., S. Kritzinger, C. Rycroft, G. Gibson, and J. du Preez. 2002. The effects of the novel bifidogenic trisaccharide, neokestose, on the human colonia microbiota. 2002. World J. Microbiol. Biotechnol. 18:637-644. [Google Scholar]
- 30.Kritzinger, S. M., S. G. Kilian, M. A. Potgieter, and J. C. du Preez. 2003. The effect of production parameters on the synthesis of the prebiotic trisaccharide, neokestose, by Xanthophyllomyces dendrorhous (Phaffia rhodozyma). Enz. Microbial Technol. 32:728-737. [Google Scholar]
- 31.Lim, J. S., S. W. Park, J. W. Lee, et al. 2005. Immobilization of Penicillium citrinum by entrapping cells in calcium alginate for the production of neo-fructooligosaccharides. J. Microbiol. Biotechnol. 15:1317-1322. [Google Scholar]
- 32.Lim, J. S., J. H. Lee, S. W. Kang, S. W. Park, and S. W. Kim. 2007. Studies on production and physical properties of neo-FOS produced by co-immobilized Penicillium citrinum and neo-fructosyltransferase. Eur. Food Res. Technol. 225:457-462. [Google Scholar]
- 33.Marín, D., D. Linde, and M. Fernández-Lobato. 2006. Purification and biochemical characterization of a Xanthophyllomyces dendrorhous alpha-glucosidase. Yeast 23:117-125. [DOI] [PubMed] [Google Scholar]
- 34.Marx, S. P., S. Winkler, and W. Hartmeier. 2000. Metabolization of beta-(2,6)-linked fructose-oligosaccharides by different bifidobacteria. FEMS Microbiol. Lett. 182:163-169. [DOI] [PubMed] [Google Scholar]
- 35.Meng, G., and K. Fütterer. 2008. Donor substrate recognition in the raffinose-bound E342A mutant of fructosyltransferase Bacillus subtilis levansucrase. BMC Struct. Biol. 8:16-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreno, S., Y. Sánchez, and L. Rodríguez. 1990. Purification and characterization of the invertase from Schizosaccharomyces pombe. Biochem. J. 267:697-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagem, R. A. P., A. L. Rojas, A. M. Golubev, O. S. Korneeva, E. V. Eneyskaya, A. A. Kulminskaya, K. N. Neustroev, and I. Polikarpov. 2004. Crystal structure of exo-inulinase from Aspergillus awamori: the enzyme fold and structural determinants of substrate recognition. J. Mol. Biol. 344:471-480. [DOI] [PubMed] [Google Scholar]
- 38.Ochman, H., A. S. Gerber, and D. L. Hatl. 1988. Genetic applications of an inverse polymerase chain reaction. Genetics 120:621-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park, M. C., J. S. Lim, J. C. Kim, S. W. Park, and S. W. Kim. 2005. Continuous production of neo-fructooligosaccharides by immobilization of whole cells of Penicillium citrinum. Biotechnol. Lett. 27:127-130. [DOI] [PubMed] [Google Scholar]
- 40.Pérez, J. A., J. Rodríguez, L. Rodríguez, and T. Ruiz. 1996. Cloning and sequence analysis of the invertase gene INV1 from the yeast Pichia anomala. Curr. Genet. 29:234-240. [DOI] [PubMed] [Google Scholar]
- 41.Persike, S. D., T. M. B. Bonfirm, M. R. H. Santos, S. M. O. Ling, M. D. Chiarello, and J. D. Fontana. 2002. Invertase and urease activities in carogenogenetic yeast Xanthophyllomyces dendrorhous (formerly Phaffia rhodozyma). Bioresour. Technol. 82:79-85. [DOI] [PubMed] [Google Scholar]
- 42.Plou, F. J., A. Gómez de Segura, and A. Ballesteros. 2007. Application of glycosidases and transglycosidases for the synthesis of oligosaccharides., p. 141-157. In J. Polaina and A. P. MacCabe (ed.), Industrial enzymes: structure, function, and applications. Springer, New York, NY.
- 43.Pons, T., D. G. Naumoff, C. Martínez-Fleites, and L. Hernández. 2004. Three acidic residues are at the active site of a beta-propeller architecture in glycoside hydrolase families 32, 43, 62, and 68. Proteins 54:424-432. [DOI] [PubMed] [Google Scholar]
- 44.Rao, A. V. 1999. Dose-response effects of inulin and oligofructose on intestinal bifidogenesis effects. J. Nutr. 129(Suppl. 7):1442S-1445S. [DOI] [PubMed] [Google Scholar]
- 45.Reddy, V. A., and F. Maley. 1990. Identification of an active-site residue in yeast invertase by affinity labelling and site-directed mutagenesis. J. Biol. Chem. 265:10817-10820. [PubMed] [Google Scholar]
- 46.Reddy, A., and F. Maley. 1996. Studies on identifying the catalytic role of Glu-204 in the active site of yeast invertase. J. Biol. Chem. 271:13953-13957. [DOI] [PubMed] [Google Scholar]
- 47.Rehm, J., L. Willmitzer, and A. G. Heyer. 1998. Production of 1-kestose in transgenic yeast expressing a fructosyltransferase from Aspergillus foetidus. J. Bacteriol. 180:1305-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ritsema, T., L. Hernández, A. Verhaar, D. Altenbach, T. Boller, A. Wiemken, and S. Smeekens. 2006. Developing fructan-synthesizing capability in a plant invertase via mutations in the sucrose-binding box. Plant J. 48:228-237. [DOI] [PubMed] [Google Scholar]
- 49.Rodríguez, J., J. A. Pérez, T. Ruiz, and L. Rodríguez. 1995. Characterization of the invertase from Pichia anomala. Biochem. J. 306:235-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sali, A., and T. L. Blundell. 1993. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234:779-815. [DOI] [PubMed] [Google Scholar]
- 51.Sambrook, K. J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 52.Sangeetha, P. T., M. N. Ramesh, and S. G. Prapulla. 2005. Fructooligosaccharide production using fructosyl transferase obtained from recycling culture of Aspergillus oryzae CFR 202. Process Biochem. 40:1085-1088. [Google Scholar]
- 53.Ueno, K., S. Onodera, A. Kawakami, M. Yoshida, and N. Shiomi. 2005. Molecular characterization and expression of a cDNA encoding fructan:fructan 6G-fructosyltransferase from asparagus (Asparragus officinalis). New Phytol. 165:813-824. [DOI] [PubMed] [Google Scholar]
- 54.Van der Westhuizen, R. J. 2008. The potential of neokestose as a prebiotic for broiler chickens. Magister's thesis. University of the Free State, Bloemfontein, South Africa.
- 55.Verhaest, M., W. Lammens, K. Le Roy, B. De Coninck, C. J. De Ranter, A. Van Laere, W. Van den Ende, and A. Rabijns. 2006. X-ray diffraction structure of a cell-wall invertase from Arabidopsis thaliana. Biol. Crystallogr. 62:1555-1563. [DOI] [PubMed] [Google Scholar]
- 56.Verdoes, J. C., J. Wery, T. Boekhout, and A. J. Van Ooyen. 1997. Molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase gene of Phaffia rhodozyma. Yeast 13:1231-1242. [DOI] [PubMed] [Google Scholar]
- 57.Vernet, T., D. Dignard, and D. Y. Thomas. 1987. A family of yeast expression vectors containing the phage f1 intergenic region. Gene 52:225-233. [DOI] [PubMed] [Google Scholar]
- 58.Vijn, I., and S. Smeekens. 1999. Fructan: more than a reserve carbohydrate? Plant Physiol. 120:351-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Voegele, R. T., S. Wirsel, U. Möll, M. Lechner, and K. Mendgen. 2006. Cloning and characterization of a novel invertase from the obligate biotroph Uromyces fabae and analysis of expression patterns of host and pathogen invertases in the course of infection. Mol. Plant-Microbe Interact. 19:625-634. [DOI] [PubMed] [Google Scholar]
- 60.Wery, J., M. J. Dalderup, J. Ter Linde, T. Boekhout, A. J. Van Ooyen. 1996. Structural and phylogenetic analysis of the actin gene from the yeast Phaffia rhodozyma. Yeast 12:641-651. [DOI] [PubMed] [Google Scholar]
- 61.Yanase, H., M. Maeda, E. Hagiwara, H. Yagi, K. Taniguchi, and K. Okamoto. 2002. Identification of functionally important amino acid residues in Zymomonas mobilis levansucrase. J. Biochem. 132:565-572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







