Abstract
We developed a novel negative selection system for actinobacteria based on cytosine deaminase (CodA). We constructed vectors that include a synthetic gene encoding the CodA protein from Escherichia coli optimized for expression in Streptomyces species. Gene disruption and the introduction of an unmarked in-frame deletion were successfully achieved with these vectors.
Cytosine deaminase (EC 3.5.4.1), widely distributed in bacteria and fungi, catalyzes the deamination of cytosine to uracil as a part of the pyrimidine salvage pathway (20, 22). This enzyme also converts 5-fluorocytosine (5FC) into 5-fluorouracil (5FU), a highly toxic compound. This suicide effect has been exploited in negative selection procedures for eucaryotic organisms (2, 12, 20, 26).
Counterselection markers are valuable tools facilitating gene disruption based on homologous recombination. The choice of such markers is, however, limited for streptomycetes. The glucokinase gene (glkA) of Streptomyces coelicolor A3(2) can be subjected to counterselection by using resistance to 2-deoxyglucose (8). This selection must be performed with strains in which the endogenous glkA gene has been deleted (8, 16). The rpsL gene (encoding the ribosomal protein S12) confers a dominant streptomycin-sensitive phenotype in a streptomycin resistance background, the latter being obtained by spontaneous mutations of the rpsL gene (4, 14, 18, 23). Such procedures, requiring the use of specifically mutated host strains, constitute a serious limitation for many studies. So far, most gene disruption procedures include a laborious colony replication step to obtain isolates that have lost an antibiotic resistance marker. This step persists even in the recent PCR targeting procedures (10, 11).
A previous study of pyrimidine salvage pathways in Streptomyces (15) noted the absence of cytosine deaminase. Accordingly, there are no apparent codA orthologs in genomic sequences of most actinobacteria (R. Brzezinski, unpublished data). Therefore, it would be possible to use the CodA-based counterselection procedure for streptomycetes without any genetic modification of the host strain. We report here new vectors for gene disruption and replacement based on this principle.
Plasmids, strains, and culture conditions.
Strains and plasmids are detailed in the supplemental material. Escherichia coli strains were grown in Luria-Bertani broth supplemented with 100 μg of ampicillin ml−1, 500 μg of hygromycin (Hm) ml−1, 50 μg of kanamycin (Km) ml−1, or 25 μg of chloramphenicol ml−1. Standard methods were used for E. coli transformation, DNA manipulation (24), and the preparation of Streptomyces lividans TK24 protoplasts and S. lividans transformation (16). S. lividans transformants were selected after the addition of 5 mg of Hm or Km to 3 ml of soft agar overlay and purified on yeast extract or malt extract agar (16) with 250 μg of Hm or Km ml−1.
MIC of 5FC or 5FU.
5FC was prepared as a stock solution of 15 mg ml−1 in distilled water, and the solution was sterilized by filtration. 5FU was prepared as a stock solution of 5 mg ml−1 in dimethyl sulfoxide. MICs were determined by the agar dilution method (3) as detailed in the supplemental material.
Construction of genes for selection.
A cytosine deaminase gene optimized for expression in actinomycetes, codA(s), was developed from the E. coli CodA sequence (5) by reverse translation and codon optimization (9). An SphI site at the initiation codon (CGATGC, where underlining indicates the codon) was created, resulting in a Ser→Pro mutation of the second residue of CodA. The coding segment was synthesized by Geneart AG (Regensburg, Germany). The promoter from pFDNeo-S (6) was inserted upstream from the coding sequence. The codA(s) sequence has been deposited at NCBI (accession number EU099038). A D314A mutant version (19) named codA(sm) was also created by PCR-directed mutagenesis (13).
For positive selection, we used the aminoglycoside resistance gene from pFDNeo-S, providing this gene with the promoter from the aacC4 gene (1), to obtain neoA. S. lividans TK24 harboring a single copy of neoA (strain TK24 neoA) was resistant to 600 μg ml−1 of Km (data not shown).
Vector construction.
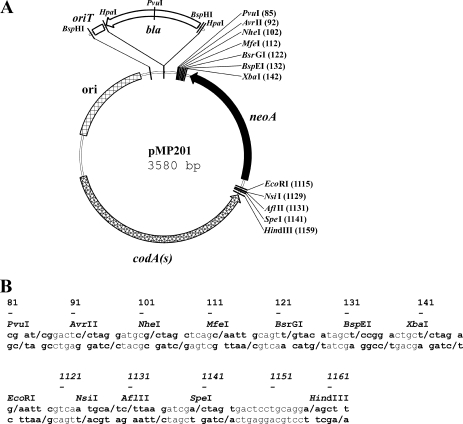
The codA(s) cassette was inserted into Litmus 38i (7). We then added neoA and deleted the Apr gene and the origin of replication of M13. Finally, we introduced two newly designed polylinkers, obtaining the vector pMP201 (Fig. 1). Polylinker HindIII-EcoRI was introduced between codA(s) and neoA, while polylinker XbaI-PvuI was inserted downstream from neoA (Fig. 1). Both polylinkers will accommodate DNA fragments in insertional gene disruption procedures, while only the polylinker XbaI-PvuI will be used for cloning the fragments involved in gene replacement, including in-frame deletion fragments. Replacing codA(s) by the mutated version codA(sm), we obtained pMG201M (see Fig. S1 in the supplemental material).
FIG. 1.
Vector pMP201 and derivatives. (A) Map of the pMP201 vector with polylinkers XbaI-PvuI and HindIII-EcoRI. neoA, aminoglycoside resistance gene with the promoter from the aacC4 gene; codA(s), synthetic version of the cytosine deaminase gene; ori, origin of replication for E. coli. The positions of oriT (origin of conjugative transfer), present in pMP301 and pMP302, and bla (ampicillin resistance gene), present in pMP302, are also shown. Note that the PvuI site in the polylinker is not unique in derivatives containing the bla gene. (B) DNA sequences of polylinkers in pMP201 and derivatives (coordinates in both panels are from the pMP201 sequence, and those in panel B correspond to the first boldface base pair for each restriction enzyme). Boldface letters and dashes indicate the restriction sites and the respective cleavage sites.
The conjugative vector pMP301 [Kmr codA(s) oriT] was obtained by inserting the transfer origin oriT from pIJ773 (10) into pMP201. To allow the use of our method with Kmr E. coli donor strains, an Apr derivative, pMP302 [Apr Kmr codA(s) oriT], was constructed. Construction is detailed in the supplemental material.
Resistance to 5FC is frequent among actinobacteria.
The growth of CodA-positive E. coli is inhibited at 20 μg ml−1 of 5FC in minimal medium (21). In contrast, concentrations as high as 400 to 800 μg ml−1 were necessary to inhibit the growth of tested actinobacteria (Table 1). Most strains were very sensitive to 5FU. The absence of cytosine deaminase, observed in S. coelicolor and Streptomyces griseus (15), seems to be frequent and is perhaps generalized among actinobacteria. The CodA-based counterselection system could then be applicable to a vast array of species.
TABLE 1.
MICs
| Species and/or strain | MIC (μg ml−1) of:
|
|
|---|---|---|
| 5-FC | 5-FU | |
| Amycolatopsis orientalis subsp. orientalis ATCC 19795 | 800 | 5 |
| Mycobacterium smegmatis MC2155 | >800 | 20 |
| “Nocardia lactamdurans” NRRL 3802 | 800 | 10 |
| Nocardioides sp. strain N106 | >800 | 0.4 |
| Saccharopolyspora erythraea ATCC 11635 | 400 | <0.1 |
| Streptomyces antibioticus Tü 1718 (SKB) | >800 | 40 |
| Streptomyces avermitilis ATCC 31267 | >800 | >40 |
| Streptomyces avidinii ATCC 27419 | >800 | 40 |
| Streptomyces clavuligerus NRRL 3585 | 800 | <0.1 |
| S. coelicolor A3(2) M145 | 800 | <0.1 |
| Streptomyces fradiae | 400 | <0.1 |
| S. griseus B2682 | 800 | <0.1 |
| S. lividans TK24 | 800 | <0.1 |
| Streptomyces melanosporofaciens EF-76 | >800 | <0.1 |
| Streptomyces scabiei EF-35 | >800 | 0.8 |
| Streptomyces venezuelae ATCC 15439 | 800 | <0.1 |
| S. lividans TK24 mutant strains | ||
| TK24 neoA | 800 | ≤0.1 |
| TK24 codA(s) | 200 | ≤0.1 |
| TK24 codA(sm) | 20 | ≤0.1 |
| TK24 ΔcsnA::neoA | 800 | ≤0.1 |
| TK24 Δ2657h | 800 | ≤0.1 |
Cytosine deaminase increases 5FC sensitivity in S. lividans.
We synthesized de novo the entire coding sequence for CodA, improving its codon adaptation index (25) for S. lividans from 0.271 to 0.991. S. lividans TK24 harboring a single codA(s) gene [strain TK24 codA(s)] was four times more sensitive to 5FC than its parent strain (Table 1). Its growth and sporulation phenotypes remained unchanged.
The D314A mutant form of CodA has 17-fold lower activity against native cytosine, while its activity against 5FC is 2-fold higher (19). S. lividans TK24 harboring one copy of the mutated codA(sm) gene [strain TK24 codA(sm)] was 10 times more sensitive to 5FC than TK24 codA(s) and 40 times more sensitive than the native TK24 (Table 1), allowing counterselection at lower 5FC concentrations.
codA(s)-based counterselection.
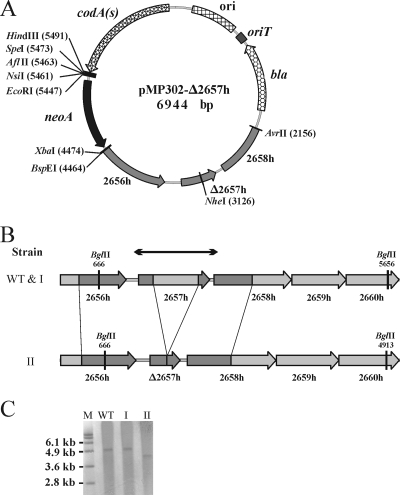
We first introduced an in-frame deletion into the S. lividans homolog of the SCO2657 gene (designated 2657h) encoding a putative ROK family protein (27). Disruption cassettes amplified from the S. lividans genome were cloned into pMP302, and E. coli ET12567(pUZ8002) was then transformed with the resulting plasmid. After conjugation with S. lividans spores, one single-crossover Kmr clone for each cell/spore ratio (1:1 and 40:1) was obtained, resulting in a recombination frequency of 5 × 10−7. In the counterselection step, the plating of 20,000 spores onto MM with 200 μg of 5FC ml−1 yielded 18 resistant colonies, resulting in a second-crossover frequency of ∼10−3. Figure 2B summarizes the recombination events. Of 12 purified clones, 11 regained the original gene configuration while 1 harbored the in-frame deletion, as shown by Southern blotting (Fig. 2C).
FIG. 2.
Inactivation of an S. lividans SCO2657 homolog gene (2657h) via replacement by an in-frame deletion. (A) Map of the pMP302 vector derivative (pMP302-Δ2657h) harboring PCR-amplified S. lividans genome fragments for homologous recombination. Positions of restriction sites are listed in parentheses. (B) Diagrams showing the wild-type (WT) genomic section of S. lividans and its configuration after the second crossing over. I, strain with a reversion to the wild-type configuration after two recombination events involving the same PCR-amplified fragment; II, strain with an in-frame deletion obtained by recombination events occurring alternatively in both amplified segments; 2656h to 2660h, S. lividans homologs of the respective S. coelicolor A3(2) genes; arrows, DNA fragment used as a probe in Southern blotting. Positions of restriction sites are given. (C) Southern blotting analysis of the 2657h gene area before (WT) and after (I or II) selection steps. Genomic DNA from the host strain (WT) and two PCR-preselected clones (I and II) resistant to 5FC was digested with BglII and analyzed by Southern blotting. M, molecular size markers.
We used pMG201M for the disruption of the S. lividans TK24 chitosanase gene (csnA), an ortholog of SCO0677 from S. coelicolor A3(2) encoding an endochitosanase (17). S. lividans TK24 was transformed with the plasmid pMG201M-ΩcsnA (see the supplemental material). A Kmr colony was picked up, and its spores were plated onto MM with Km (250 μg ml−1) and 5FC (50 μg ml−1). 5FC-resistant colonies appeared at a 7 × 10−3 frequency. Fragments of the expected lengths were observed by on-colony PCR performed with two 5FC-resistant colonies, indicating the successful disruption of the csnA gene (data not shown).
Conclusion.
The cytosine deaminase gene can be efficiently used as a negative selection marker for gene disruption and replacement in Streptomyces. The method can be used for any actinobacterial species naturally resistant to 5FC while sensitive to 5FU. It should be a valuable addition to the actinobacterial genetic toolbox.
Supplementary Material
Acknowledgments
We thank Susan E. Jensen, David H. Sherman, Luc Gaudreau, and Gilles P. van Wezel for providing strains, Alain Fleury for bioinformatic analysis, and Nathalie Côté for preliminary MIC determination.
This work was supported by Discovery grants from the Natural Science and Engineering Research Council of Canada to R.B. and C.B. M.-P.D. is the recipient of a doctoral student fellowship from NSERC and FQRNT.
Footnotes
Published ahead of print on 19 December 2008.
REFERENCES
- 1.Blondelet-Rouault, M.-H., J. Weiser, A. Lebrihi, P. Branny, and J. L. Pernodet. 1997. Antibiotic resistance gene cassettes derived from the Ω interposon for use in E. coli and Streptomyces. Gene 190:315-317. [DOI] [PubMed] [Google Scholar]
- 2.Braks, J. A. M., B. Franke-Fayard, H. Kroeze, C. J. Janse, and A. P. Waters. 2006. Development and application of a positive-negative selectable marker system for use in reverse genetics in Plasmodium. Nucleic Acids Res. 34:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 7th ed., vol 26. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Coëffet-Le Gal, M.-F., L. Thurston, P. Rich, V. Mao, and R. H. Baltz. 2006. Complementation of daptomycin dptA and dptD deletion mutations in trans and production of hybrid lipopeptide antibiotics. Microbiology 152:2993-3001. [DOI] [PubMed] [Google Scholar]
- 5.Danielsen, S., M. Kilstrup, K. Barilla, B. Jochimsen, and J. Neuhard. 1992. Characterization of the Escherichia coli codBA operon encoding cytosine permease and cytosine deaminase. Mol. Microbiol. 6:1335-1344. [DOI] [PubMed] [Google Scholar]
- 6.Denis, F., and R. Brzezinski. 1991. An improved aminoglycoside resistance gene cassette for use in Gram-negative bacteria and Streptomyces. FEMS Microbiol. Lett. 81:261-264. [DOI] [PubMed] [Google Scholar]
- 7.Evans, P. D., S. N. Cook, P. D. Riggs, and C. J. Noren. 1995. LITMUS: multipurpose cloning vectors with a novel system for bidirectional in vitro transcription. BioTechniques 19:130-135. [PubMed] [Google Scholar]
- 8.Fisher, S. H., C. J. Bruton, and K. F. Chater. 1987. The glucose kinase gene of Streptomyces coelicolor and its use in selecting spontaneous deletions for desired regions of the genome. Mol. Gen. Genet. 206:35-44. [DOI] [PubMed] [Google Scholar]
- 9.Grote, A., K. Hiller, M. Scheer, R. Münch, B. Nörtemann, D. C. Hempel, and D. Jahn. 2005. JCat: a novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res. 33:W526-W531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gust, B., G. L. Challis, K. Fowler, T. Kieser, and K. F. Chater. 2003. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. USA 100:1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gust, B., G. Chandra, D. Jakimowicz, T. Yuqing, C. J. Bruton, and K. F. Chater. 2004. λ Red-mediated genetic manipulation of antibiotic-producing Streptomyces. Adv. Appl. Microbiol. 54:107-128. [DOI] [PubMed] [Google Scholar]
- 12.Hartzog, P. E., B. P. Nicholson, and J. H. McCusker. 2005. Cytosine deaminase MX cassettes as positive/negative selectable markers in Saccharomyces cerevisiae. Yeast 22:789-798. [DOI] [PubMed] [Google Scholar]
- 13.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 14.Hosted, T. J., and R. H. Baltz. 1997. Use of rpsL for dominance selection and gene replacement in Streptomyces roseosporus. J. Bacteriol. 179:180-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes, L. E., D. A. Beck, and G. A. O'Donovan. 2005. Pathways of pyrimidine salvage in Streptomyces. Curr. Microbiol. 50:8-10. [DOI] [PubMed] [Google Scholar]
- 16.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, United Kingdom.
- 17.Li, H., P.-É. Jacques, M. G. Ghinet, R. Brzezinski, and R. Morosoli. 2005. Determining the functionality of putative Tat-dependent signal peptides in Streptomyces coelicolor A3(2) by using two different reporter proteins. Microbiology 151:2189-2198. [DOI] [PubMed] [Google Scholar]
- 18.Lindahl, L., and J. M. Zengel. 1986. Ribosomal genes in E. coli. Annu. Rev. Genet. 20:297-326. [DOI] [PubMed] [Google Scholar]
- 19.Mahan, S. D., G. C. Ireton, B. L. Stoddard, and M. E. Black. 2004. Alanine-scanning mutagenesis reveals a cytosine deaminase mutant with altered substrate preference. Biochemistry 43:8957-8964. [DOI] [PubMed] [Google Scholar]
- 20.Mullen, C. A., M. Kilstrup, and R. M. Blaese. 1992. Transfer of the bacterial gene for cytosine deaminase to mammalian cells confers lethal sensitivity to 5-fluorocytosine—a negative selection system. Proc. Natl. Acad. Sci. USA 89:33-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neuhard, J., and P. Nygaard. 1987. Purines and pyrimidines, p. 445-473. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, vol. 1. American Society for Microbiology, Washington, DC. [Google Scholar]
- 22.O'Donovan, G. A., and J. Neuhard. 1970. Pyrimidine metabolism in microorganisms. Bacteriol. Rev. 34:278-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell, C. B., and F. W. Dahlquist. 1989. Exchange of chromosomal and plasmid alleles in Escherichia coli by selection for loss of a dominant antibiotic sensitivity marker. J. Bacteriol. 171:2614-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 25.Sharp, P. M., and W.-H. Li. 1987. The codon adaptation index—a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 15:1281-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stougaard, J. 1993. Substrate-dependent negative selection in plants using a bacterial cytosine deaminase gene. Plant J. 3:755-761. [Google Scholar]
- 27.Titgemeyer, F., J. Reizer, A. Reizer, and M. H. Saier, Jr. 1994. Evolutionary relationships between sugar kinases and transcriptional repressors in bacteria. Microbiology 140:2349-2354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.