Abstract
Melioidosis has been considered an emerging disease in Brazil since the first cases were reported to occur in the northeast region. This study investigated two municipalities in Ceará state where melioidosis cases have been confirmed to occur. Burkholderia pseudomallei was isolated in 26 (4.3%) of 600 samples in the dry and rainy seasons.
Melioidosis is an endemic disease in Southeast Asia and northern Australia (2, 4) and also occurs sporadically in other parts of the world (3, 7). Human melioidosis was reported to occur in Brazil only in 2003, when a family outbreak afflicted four sisters in the rural part of the municipality of Tejuçuoca, Ceará state (14). After this episode, there was one reported case of melioidosis in 2004 in the rural area of Banabuiú, Ceará (14). And in 2005, a case of melioidosis associated with near drowning after a car accident was confirmed to occur in Aracoiaba, Ceará (11).
The goal of this study was to investigate the Tejuçuoca and Banabuiú municipalities, where human cases of melioidosis have been confirmed to occur, and to gain a better understanding of the ecology of Burkholderia pseudomallei in this region.
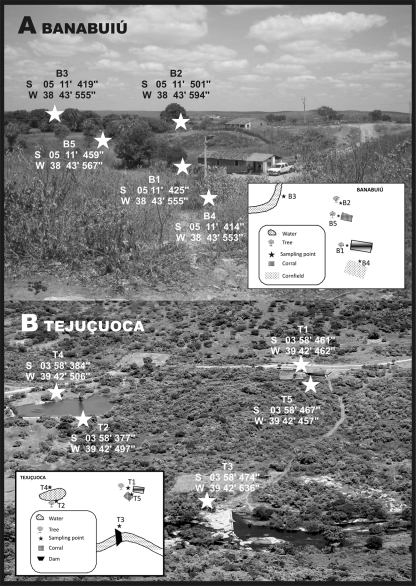
We chose as central points of the study the residences and surrounding areas of the melioidosis patients in the rural areas of Banabuiú (5°18′35″S, 38°55′14″W) and Tejuçuoca (03°59′20″S, 39°34′50′W) (Fig. 1). There are two well-defined seasons in each of these locations: one rainy (running from January to May) and one dry (from June to December). A total of 600 samples were collected at five sites in Tejuçuoca (T1, T2, T3, T4, and T5) and five in Banabuiú (B1, B2, B3, B4, and B5), distributed as follows (Fig. 2): backyards (B1 and T1), places shaded by trees (B2 and T2), water courses (B3 and T3), wet places (B4 and T4), and stock breeding areas (B5 and T5).
FIG. 1.
Municipalities of Banabuiú (5°18′35″S, 38°55′14″W) and Tejuçuoca (03°59′20″S, 39°34′50″W).
FIG. 2.
Soil sampling sites in Banabuiú and Tejuçuoca.
Once a month for 12 months (a complete dry/rainy cycle), five samples were gathered at five different depths: at the surface and at 10, 20, 30 and 40 cm (Table 1). The samples were gathered according to the method used by Inglis et al. (9). Additionally, the sample processing and B. pseudomallei identification were carried out as previously reported (1, 8, 9).
TABLE 1.
Distribution of samples with isolates by site and soil depth
| Sitesa and depth (cm) | No. of B. pseudomallei isolates in samples from:
|
||
|---|---|---|---|
| Banabuiú (n = 300) | Tejuçuoca (n = 300) | Total (n = 600) | |
| B1/T1 | 3 | ||
| Surface | 2 | ||
| 10 | |||
| 20 | 1 | ||
| 30 | |||
| 40 | |||
| B2/T2 | 1 | ||
| Surface | 1 | ||
| 10 | |||
| 20 | |||
| 30 | |||
| 40 | |||
| B3/T3 | 15 | ||
| Surface | 2 | ||
| 10 | 2 | ||
| 20 | 4 | ||
| 30 | 3 | ||
| 40 | 4 | ||
| B4/T4 | 5 | ||
| Surface | |||
| 10 | 1 | ||
| 20 | 1 | ||
| 30 | 1 | 1 | |
| 40 | 1 | ||
| B5/T5 | 2 | ||
| Surface | |||
| 10 | |||
| 20 | |||
| 30 | 2 | ||
| 40 | |||
| Total | 6 | 20 | 26 |
Sites designated with B are in Banabuiú, and sites designated with T are in Tejuçuoca. See the text for details.
The data on weather and soil composition were obtained from specialized government institutions, such as FUNCEME, IPECE, and EMBRAPA. The average annual temperature in both municipalities is between 26 and 28°C. In 2007, the annual rainfall in Tejuçuoca was 496.8 mm, and that in Banabuiú was 766.8 mm. There are a range of soil types in both Tejuçuoca and Banabuiú: noncalcic brown, sodic planossolic, red-yellow podzolic, and litholic. In Banabuiú, there are also alluvial and cambisol soils. The characteristic vegetation in both municipalities is caatinga (scrublands).
There were isolates of B. pseudomallei in 26 (4.3%) of the 600 samples collected. The bacterium was isolated at a rate (3%) similar to that previously reported (9). The bacterium isolation occurred in both the dry (53.8%) and the rainy (46.2%) seasons. Tejuçuoca represented 76.9% (20/26) of the strains isolated. Four sites in Tejuçuoca (T1, T3, T4, and T5) and three in Banabuiú (B1, B2, and B4) presented isolates of the bacterium (Table 1). The isolation of the B. pseudomallei strains varied from the surface down to 40 cm. However, 17 of the 26 positive samples (65.3%) were found at depths between 20 and 40 cm (Table 1). Only two isolates were found at the surface during the dry season.
A study in Vietnam (13) and one in Australia (9) reported the presence of B. pseudomallei near the houses of melioidosis patients. In our study, the same thing happened. Site T3 (15/26; 57.6%) was located 290 m from the patient's house, as reported by the Rolim group (14).
B. pseudomallei was isolated from a sheep paddock in Australia, where animals sought shelter below mango and fig trees (17). In our study, the bacterium was isolated at site T5, a goat corral alongside the house where the outbreak occurred in Tejuçuoca. Four sites in places shaded by trees yielded positive samples (30.7%) in both Tejuçuoca (palm trees) and Banabuiú (mango trees). Additionally, B. pseudomallei was isolated on three occasions from a cornfield (site 4B) located alongside the house of the melioidosis patient in Banabuiú.
In the main areas of endemicity, the disease is more prevalent in the rainy season (4, 5, 16). The outbreak in Tejuçuoca was related to rainfall (14). Besides the association of cases of the disease with rainfall itself, the isolation of B. pseudomallei in soil and water was also demonstrated during the dry season (12, 15). An Australian study isolated strains from soil and water during the dry and rainy seasons (17). A Thai study also reported B. pseudomallei in the dry season (18). In our study, the isolation of B. pseudomallei took place either at the end of the wet season or in the dry months. Fourteen of the positive samples (53.8%) were collected during the dry season, albeit near a river or reservoir (sites T3 and B4).
Physical, biological, and chemical soil features appear to influence the survival of B. pseudomallei (6, 10). In the present study, the soil was classified as litholic with sandy or clayey textures. It is susceptible to erosion, and when there is a lack of water, it is subject to salinization. During the dry season, the clay layer becomes dried, cracked, and very hard. During the rainy season, it becomes soggy and sticky. The isolation of B. pseudomallei in the dry season is possibly related to the capacity for adaptation of this soil, since the extreme conditions of lithosols do not prevent the bacterial growth and survival.
It has been shown that B. pseudomallei is more often isolated at depths between 25 and 45 cm (17). In our study, 65.3% of the positive samples were taken at depths between 20 and 40 cm. Moreover, of these 17 samples, 10 (58.8%) were collected during the dry months. Also, unlike in other regions, two positive samples were taken from the surface in the period without rainfall.
The rainfall in Tejuçuoca and Banabuiú is generally low, and temperatures do not vary significantly during the year. Therefore, the isolation of B. pseudomallei in these places occurs outside the rainfall, temperature, and moisture conditions observed in other regions of endemicity. Our data thus suggest that peculiar environmental features, such as soil composition, might favor the multiplication of B. pseudomallei in northeast Brazil.
Acknowledgments
This work was supported by a grant from the CNPq (National Scientific and Technological Research Council; 475683/2006-4).
Footnotes
Published ahead of print on 19 December 2008.
REFERENCES
- 1.Ashdown, L. R. 1979. Identification of Pseudomonas pseudomallei in the clinical laboratory. J. Clin. Pathol. 32:500-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaowagul, W. N. J., D. A. White, Y. Dance, P. Wattanagoon, T. M. Naigowit, S. Davis, S. Looareesuwan, and N. Pitakwatchara. 1989. Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J. Infect. Dis. 159:890-899. [DOI] [PubMed] [Google Scholar]
- 3.Cheng, A. C., and B. J. Currie. 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 18:383-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Currie, B. J., D. A. Fisher, D. M. Howard, J. N. Burrow, D. Lo, S. Selva-Nayagam, N. M. Anstey, S. E. Huffam, P. L. Snelling, P. J. Marks, D. P. Stephens, G. D. Lum, S. P. Jacups, and V. L. Krause. 2000. Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature. Clin. Infect. Dis. 31:981-986. [DOI] [PubMed] [Google Scholar]
- 5.Currie, B. J., and S. P. Jacups. 2003. Intensity of rainfall and severity of melioidosis, Australia. Emerg. Infect. Dis. 9:1538-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dance, D. A. 2000. Ecology of Burkholderia pseudomallei and the interactions between environmental Burkholderia spp. and human-animal hosts. Acta Trop. 74:159-168. [DOI] [PubMed] [Google Scholar]
- 7.Dance, D. A. 2000. Melioidosis as an emerging global problem. Acta Trop. 74:115-119. [DOI] [PubMed] [Google Scholar]
- 8.Howard, K., and T. J. Inglis. 2003. Novel selective medium for isolation of Burkholderia pseudomallei. J. Clin. Microbiol. 41:3312-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inglis, T. J., N. F. Foster, D. Gal, K. Powell, M. Mayo, R. Norton, and B. J. Currie. 2004. Preliminary report on the northern Australian melioidosis environmental surveillance project. Epidemiol. Infect. 132:813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inglis, T. J., and J. L. Sagripanti. 2006. Environmental factors that affect the survival and persistence of Burkholderia pseudomallei. Appl. Environ. Microbiol. 72:6865-6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inglis, T. J., D. B. Rolim, and A. Q. Sousa. 2006. Melioidosis in the Americas. Am. J. Trop. Med. Hyg. 75:947-954. [PubMed] [Google Scholar]
- 12.Nachiangmai, N. P., B. Patamasucon, A. Tipayamonthein, and S. Nakaviroj. 1985. Pseudomonas pseudomallei in southern Thailand. Southeast Asian J. Trop. Med. Public Health 16:83-87. [PubMed] [Google Scholar]
- 13.Parry, C. M. V., N. T. Wuthiekanun, T. S. Hoa, L. T. Diep, P. V. Thao, B. A. Loc, J. Wills, T. T. Hien, N. J. White, and J. J. Farrar. 1999. Melioidosis in Southern Vietnam: clinical surveillance and environmental sampling. Clin. Infect. Dis. 29:1323-1326. [DOI] [PubMed] [Google Scholar]
- 14.Rolim, D. B., D. C. Vilar, A. Q. Sousa, I. S. Miralles, D. C. de Oliveira, G. Harnett, L. O'Reilly, K. Howard, I. Sampson, and T. J. Inglis. 2005. Melioidosis, northeastern Brazil. Emerg. Infect. Dis. 11:1458-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strauss, J. M., M. G. Groves, M. Mariappan, and D. W. Ellison. 1969. Melioidosis in Malaysia. II. Distribution of Pseudomonas pseudomallei in soil and surface water. Am. J. Trop. Med. Hyg. 18:698-702. [PubMed] [Google Scholar]
- 16.Suputtamongkol, Y. A. J., D. A. Dance, W. Chaowagul, A. Rajchanuvong, M. D. Smith, and N. J. White. 1994. The epidemiology of melioidosis in Ubon Ratchatani, northeast Thailand. Int. J. Epidemiol. 23:1082-1090. [DOI] [PubMed] [Google Scholar]
- 17.Thomas, A. D., J. Forbes-Faulkner, and M. Parker. 1979. Isolation of Pseudomonas pseudomallei from clay layers at defined depths. Am. J. Epidemiol. 110:515-521. [DOI] [PubMed] [Google Scholar]
- 18.Wuthiekanun, V., M. D. Smith, D. A. Dance, and N. J. White. 1995. Isolation of Pseudomonas pseudomallei from soil in north-eastern Thailand. Trans. R. Soc. Trop. Med. Hyg. 89:41-43. [DOI] [PubMed] [Google Scholar]