Abstract
The objective of this work was to elucidate if breast milk contains bifidobacteria and whether they can be transmitted to the infant gut through breastfeeding. Twenty-three women and their respective infants provided samples of breast milk and feces, respectively, at days 4 to 7 after birth. Gram-positive and catalase-negative isolates from specific media with typical bifidobacterial shapes were identified to the genus level by F6PPK (fructose-6-phosphate phosphoketolase) assays and to the species level by 16S rRNA gene sequencing. Bifidobacterial communities in breast milk were assessed by PCR-denaturing gradient gel electrophoresis (PCR-DGGE), and their levels were estimated by quantitative real-time PCR (qRTi-PCR). Bifidobacteria were present in 8 milk samples and 21 fecal samples. Bifidobacterium breve, B. adolescentis, and B. bifidum were isolated from milk samples, while infant feces also contained B. longum and B. pseudocatenulatum. PCR-DGGE revealed the presence of one to four dominant bifidobacterial bands in 22 milk samples. Sequences with similarities above 98% were identified as Bifidobacterium breve, B. adolescentis, B. longum, B. bifidum, and B. dentium. Bifidobacterial DNA was detected by qRTi-PCR in the same 22 milk samples at a range between 40 and 10,000 16S rRNA gene copies per ml. In conclusion, human milk seems to be a source of living bifidobacteria for the infant gut.
The bacterial colonization of the infant gut is a gradual process that exerts a strong influence on the health status of a host, since the members of the gut microbiota may contribute to the barrier effect against pathogens and/or to the maturation of the intestinal immune system (4). Traditionally, it has been considered that facultative anaerobic bacterial groups, such as streptococci, staphylococci, enterococci, lactobacilli, or enterobacteria, together with some strictly anaerobic ones, especially bifidobacteria, are among the first colonizers in breast-fed infants (6). In concert, they create the condition required for the proliferation of anaerobic bacteria, which become predominant after weaning (15). In contrast, the microbiota of formula-fed infants seems to be more diverse and prone to changes and contains higher counts of Bacteroides, Clostridium, and Enterobacteriaceae (2, 9, 10, 25, 31).
In recent years, culture-dependent and -independent analyses of the bacterial diversity of human milk have revealed that this biological fluid is a source of live staphylococci, streptococci, lactic acid bacteria, and enterobacteria for the infant gut (11, 17, 18, 20). Bifidobacterial DNA has also been detected in breast milk (8, 26), but to our knowledge, attempts to isolate bifidobacteria from this source have not been successful up to the present. Bifidobacteria are important members of the human gut microbiota and are believed to play a beneficial role in maintaining the health of the host. They were first isolated a century ago from infant feces and were quickly associated with a healthy infant gut because of their predominance in breast-fed infants in comparison to formula-fed ones (34). Since then, it has been widely accepted that bifidobacteria represent one of the most important bacterial groups in the infant gut. In this location, they may contribute to the maturation of the gut barrier and the gut-associated lymphoid tissue. Some studies have suggested that infants with delayed bifidobacterial colonization and/or decreased bifidobacterial numbers may be more susceptible to a variety of gastrointestinal or allergic conditions (1, 16) and that, in these cases, the exogenous administration of selected bifidobacterial strains, alone or in combination with lactic acid bacteria, can reduce the incidence of such conditions (5, 13, 27, 33).
In this context, the objective of this study was the isolation and identification of bifidobacterial strains from breast milk and the analysis of the bifidobacterial community by molecular methods.
MATERIALS AND METHODS
Subjects and sampling.
A total of 23 women and their respective infants, which were fed by exclusive breastfeeding, participated in the study, and they were enrolled according to the following criteria: (i) healthy women without present or past underlying conditions; (ii) normal, full-term pregnancy; and (iii) absence of infant and/or maternal perinatal problems, including mastitis. None of the subjects enrolled in this study had received a probiotic treatment during pregnancy or after birth. All volunteers gave written informed consent to the protocol, which was approved by the Ethical Committee of Hospital Clínico (Madrid, Spain). The participants provided samples of breast milk, breast skin swabs, and infant feces between days 4 and 7 after birth. The milk samples were collected in a sterile tube by manual expression using sterile gloves. Previously, nipples and mammary areola had been cleaned with soap and sterile water and soaked in chlorhexidine (Cristalmina; Salvat, Barcelona, Spain). The first drops (∼500 μl) were discarded. Skin sampling was performed as described previously (24); briefly, a 4-cm2 area of the upper outer quarter of each breast was gently rubbed using sterile cotton swabs soaked in ST solution (0.15 M NaCl with 0.1% Tween 20). The head of each swab was aseptically cut from the handle, placed into a microcentrifuge tube containing 100 μl of ST solution, centrifuged for 5 min, and then removed. Recently, it was shown that this procedure provides a representative profile of the microbial community of human skin (7). To detect possible contamination, negative controls were prepared using cotton swabs in ST solution without any contact with skin and then subjected to the above-mentioned procedures.
All the samples were kept at 4°C until delivery to the laboratory, which occurred within 1 h after collection.
Isolation of bifidobacteria.
Proper peptone water dilutions of the milk, skin, and fecal samples were plated in triplicate onto Man-Rogosa-Sharpe (MRS; Oxoid, Basingstoke, United Kingdom) medium supplemented with l-cysteine (0.5 g/liter) (MRS-Cys) agar plates, which were incubated anaerobically (85% nitrogen, 10% hydrogen, 5% carbon dioxide) in an anaerobic workstation (MINI-MACS; DW Scientific, Shipley, United Kingdom) at 37°C for 48 h. Between 50 and 75 isolates from each sample were randomly selected, grown in MRS-Cys broth, and stored at −80°C in the presence of glycerol (30%, vol/vol).
Identification of the bacterial isolates.
The selected isolates were observed by optical microscopy to determine their morphology and Gram staining results. Additionally, they were tested for catalase, oxidase, nitrate reductase, gelatinase activities, production of indol, and production of gas from glucose. All the gram-positive and catalase-negative isolates with typical bifidobacterial shapes were identified to the genus level by demonstration of fructose-6-phosphate phosphoketolase (F6PPK) activity in cellular extracts (30) and to the species level by PCR sequencing of a 470-bp fragment of the 16S rRNA gene, using primers plb16 (5′-AGAGTTTGATCCTGGCTCAG-3′) and mlb16 (5′-GGCTGCTGGCACGTAGTTAG-3′) (positions 8 to 27 and 507 to 526 in the 16S rRNA gene sequence of Escherichia coli, respectively) (14). The PCR conditions were as follows: 96°C for 30 s, 48°C for 30 s, and 72°C for 45 s (40 cycles) and a final extension at 72°C for 4 min. The amplicons were purified using a Nucleospin Extract II kit (Macherey-Nagel, Düren, Germany) and sequenced at the Genomics Unit of the Universidad Complutense de Madrid, Spain. The resulting sequences were used to search sequences deposited in the EMBL database by using the BLAST algorithm, and the identities of the isolates were determined on the basis of the highest scores (>98%).
PCR-denaturing gradient gel electrophoresis (PCR-DGGE) and quantitative real-time PCR (qRTi-PCR) analyses of breast milk samples.
DNA was isolated from 23 samples of breast milk collected from healthy mothers by using a QIAamp DNA stool minikit (Qiagen, Hilden, Germany), following the manufacturer's instructions. Cell lysis was further optimized by performing an additional step in which pellets were homogenized in 1.4 ml of ASL buffer (Qiagen) with the aid of 0.1 mm zirconium beads (Biospec, Bartlesville, OK), using a FastPrep instrument (QBioGene, Irvine, CA) for 30 s at 5,500 rpm.
Bifidobacterium genus-specific PCR was performed using 16S rRNA gene targeting primers. To prevent a low amplicon yield, we opted for the use of a nested PCR approach as described earlier (29). This involved a first PCR in which primers Im3 (5′-CGGGTGCTICCCACTTTCATG-3′) and Im26 (5′-GATTCTGGCTCAGGATGAACG-3′) were used, followed by a second PCR with primers Bif164-F (5′-GGGTGGTAATGCCGGATG-3′) and Bif662-R (5′-CCACCGTTACACCGGGAA-3′). For DGGE analysis of PCR products, a 40-bp GC clamp was attached to the 5′ end of Bif662-R. Amplification of this fragment was successful in 22 out of 23 samples.
DGGE analysis of PCR amplicons was performed as described previously (22), using the DCode system (Bio-Rad Laboratories, Hercules, CA). Polyacrylamide gels consisted of 8% (vol/vol) polyacrylamide (37.5:1 acrylamide-bisacrylamide) in 0.5× Tris-acetate-EDTA. A denaturing acrylamide of 100% was defined as 7 M urea and 40% formamide. The gels were poured from the top by using a gradient maker and a pump (Econopump; Bio-Rad, La Jolla, CA) set at a speed of 4.5 ml/min, and gradients of 45 to 55% were used for the separation of the generated amplicons. Immediately after the denaturing gel was poured (28-ml gradient volume), a 7.5-ml stacking gel without denaturing chemicals was added and the appropriate comb was inserted. Electrophoresis was performed for 16 h at 85 V with a 0.5× Tris-acetate-EDTA buffer at a constant temperature of 60°C. Gels were stained with AgNO3 according to the method of Sanguinetti et al. (28).
PCR amplicons generated with primers Bif164-F and Bif662-R were used for constructing clone libraries, as described previously (16). Briefly, the PCR products were purified with a Nucleo Spin Extract II kit (Macherey-Nagel, Düren, Germany) according to the manufacturer's instructions and were cloned in E. coli XL-1 blue competent cells (Stratagene, La Jolla, CA) by using a pGEM-T easy cloning kit (Promega Corp., Madison, WI). Plasmids containing an insert of the appropriate size were screened by DGGE analysis. Representative clones corresponding to a specific banding position were selected for sequence analysis at the genomic facilities of BaseClear (Leiden, The Netherlands) and Parque Científico de Madrid (UCM, Madrid, Spain).
For qRTi-PCR assays, SYBR green PCR amplifications were performed with an iCycler iQ real-time detection system (Bio-Rad) associated with the iCycler Optical System Interface software program (version 2; Bio-Rad). The total bacterial copy number was determined with primers 1369F and 1492R (32) and, for bifidobacteria specifically, with primers Bif164F and Bif662R (26). All PCR experiments were carried out in triplicate with a reaction volume of 25 μl, using iCycler iQ 96-well optical-grade PCR plates (Bio-Rad) covered with iCycler optical-quality sealing film (Bio-Rad). The reaction mixture contained 2× SYBR green PCR mixture (Bio-Rad), 0.5 μM of each primer, and 5 μl of either the template or water. The amplification program consisted of 1 cycle at 95°C for 3 min and then 40 cycles at 95°C for 30 s, 62°C for 40 s, and 72°C for 1 min. The fluorescent product was detected at the last step of each cycle. Following amplification, melting temperature analysis of PCR products was performed to determine the specificity of the PCR. The melting curves were obtained by slow heating at 0.5°C/s increments from 62 to 95°C, with continuous fluorescence collection. For the determination of the number of bifidobacterial species present in each sample, fluorescent signals detected from two or three serial dilutions in the linear range of the assay were averaged and compared to a standard curve generated with standard DNA in the same experiment. Standard curves for all experiments were generated as follows. Bifidobacterium longum genomic DNA was subjected to PCR using the 27F and 1492R primers. The conditions were as follows: 95°C for 2 min; followed by 35 cycles at 95°C for 30 s, 52°C for 40 s, and 72°C for 90 s; and a final extension for 5 min at 72°C. The amplicons were purified using DNA Clean and Concentrator-5 (ZymoResearch, Orange, CA) according to the manufacturer's instructions, and the yield was quantified with a Nanodrop-1000 spectrophotometer (Nanodrop, Wilmington, DE). The 16S rRNA gene content, expressed as numbers of copies/ml, was calculated from the spectrophotometric results, using the average molecular weight of a nucleotide and standard solutions serially diluted from 108 to 10 and used in each quantitative PCR run. B. longum genomic DNA was selected to construct the standard curve because a previous study indicated that DNA from this species was dominant in breast milk samples (8).
Nucleotide sequence accession numbers.
The sequences obtained in this study were deposited in the GenBank database under accession numbers FJ441215 to FJ441240.
RESULTS
Isolation and identification of bifidobacteria from breast milk samples and infant feces.
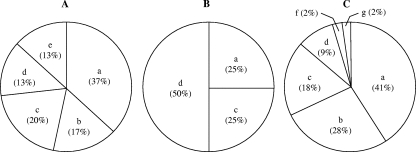
All the gram-positive and catalase-negative isolates with typical bifidobacterial shapes were identified to the genus level by the F6PPK test. In addition, none of these isolates produced either gas from glucose or indol, and all were negative for oxidase, nitrate reductase, and gelatinase activities. Globally, bifidobacteria were isolated from 8 milk samples and 21 fecal samples (Table 1), but in contrast, they could not be isolated from any breast skin swab. Isolates belonging to three bifidobacterial species were isolated from milk samples: B. breve (four milk samples), B. adolescentis (two samples), and B. bifidum (two samples) (Fig. 1). The species more frequently isolated from infant feces were B. adolescentis (11 samples), B. bifidum (6 samples), B. longum (5 samples), B. breve (4 samples), and B. pseudocatenulatum (4 samples) (Table 1 and Fig. 1).
TABLE 1.
Bifidobacterial species isolated from the biological samples
| Mother/child pair no. | Sample type | Bifidobacterial species |
|---|---|---|
| 1 | Milk | B. adolescentis |
| Infant feces | B. adolescentis | |
| 2 | Milk | |
| Infant feces | B. adolescentis | |
| 3 | Milk | |
| Infant feces | B. adolescentis | |
| 4 | Milk | B. adolescentis |
| Infant feces | B. adolescentis | |
| 5 | Milk | |
| Infant feces | B. adolescentis | |
| 6 | Milk | |
| Infant feces | B. bifidum, B. longum | |
| 7 | Milk | |
| Infant feces | B. adolescentis, B. pseudocatenulatum, B. dentium, B. angulatum | |
| 8 | Milk | |
| Infant feces | B. adolescentis, B. pseudocatenulatum | |
| 9 | Milk | |
| Infant feces | B. adolescentis, B. bifidum, B. longum | |
| 10 | Milk | |
| Infant feces | B. adolescentis | |
| 11 | Milk | B. breve |
| Infant feces | B. breve, B. pseudocatenulatum | |
| 12 | Milk | |
| Infant feces | B. adolescentis, B. longum | |
| 13 | Milk | B. breve |
| Infant feces | B. breve | |
| 14 | Milk | |
| Infant feces | B. longum | |
| 15 | Milk | |
| Infant feces | B. dentium | |
| 16 | Milk | B. breve |
| Infant feces | B. breve, B. bifidum | |
| 17 | Milk | B. bifidum |
| Infant feces | B. bifidum | |
| 18 | Milk | B. bifidum |
| Infant feces | B. bifidum, B. pseudocatenulatum | |
| 19 | Milk | |
| Infant feces | B. adolescentis, B. bifidum | |
| 20 | Milk | B. breve |
| Infant feces | B. breve | |
| 21 | Milk | |
| Infant feces | B. longum | |
| 22 | Milk | |
| Infant feces | ||
| 23 | Milk | |
| Infant feces |
FIG. 1.
Relative abundances (percentages) of the different bifidobacterial species isolated from infant feces (A) and breast milk samples (B) or detected in the clone library obtained from breast milk samples (C). a, B. adolescentis; b, B. longum; c, B. bifidum; d, B. breve; e, B. pseudocatenulatum; f, B. dentium; g, Bifidobacterium spp.
PCR-DGGE and qRTi-PCR analyses of breast milk samples.
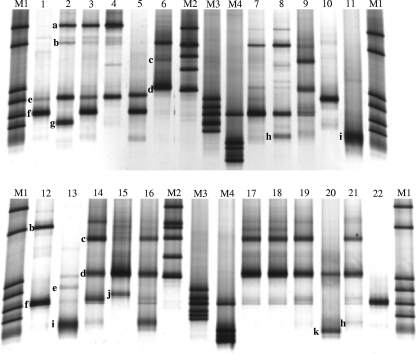
DNA isolation and subsequent PCR-DGGE analysis of 16S rRNA genes were successful for 22 out of 23 samples (Fig. 2). The samples showed between one and four dominant bands. Sequence analysis of unique clones obtained from the samples containing the highest levels of diversity was used for further identification of the DGGE bands. Sequences with similarities above 98% were identified as Bifidobacterium breve, B. adolescentis, B. longum, B. bifidum, and B. dentium upon comparison with sequences of 16S rRNA genes of cultured bacterial isolates deposited in the NCBI database. Most breast milk samples were dominated by B. adolescentis, which was present in 18 out of 22 samples. Sequences related to B. longum, B. bifidum, and B. breve were retrieved from 12, 8, and 4 samples, respectively. Clone j, related to B. dentium, was found in only one sample, as was clone g, Bifidobacterium spp., which did not reach the 98% threshold but had 97% similarity to B. adolescentis (Fig. 1 and 2). Bifidobacterial DNA was detected by qRTi-PCR in 22 out of 23 breast milk samples at a range between 40 and 10,000 16S rRNA gene copies per ml (Table 2). The percentages of bifidobacterial DNA among total bacterial DNA were ≤16% (Table 2).
FIG. 2.
Bifidobacterium species diversity in breast milk samples (lanes 1 to 22) as determined by DGGE. Dominant bands were identified as corresponding to B. adolescentis (a), B. adolescentis (b), B. bifidum (c), B. longum (d), B. adolescentis (e), B. adolescentis (f), Bifidobacterium spp. (97% B. adolescentis) (g), B. longum (h), B. breve (i), B. dentium (j), and B. breve (k). M1 to M4 are markers.
TABLE 2.
Counts of bacteria and bifidobacteria in breast milk samples as determined by qRTi-PCR
| Mother/child pair no. | Total no. of bacteriaa | No. of bifidobacteriaa | %b |
|---|---|---|---|
| 1 | 1.14E + 05 | 4.04E + 03 | 3.54 |
| 2 | 4.04E + 05 | 1.16E + 04 | 2.87 |
| 3 | 2.08E + 05 | 1.08E + 04 | 5.19 |
| 4 | 1.48E + 05 | 9.40E + 03 | 6.35 |
| 5 | 1.43E + 05 | 5.20E + 03 | 3.64 |
| 6 | 1.17E + 05 | 1.04E + 02 | 0.09 |
| 7 | 9.00E + 07 | 8.68E + 02 | 0.00 |
| 8 | 2.48E + 05 | 6.88E + 03 | 2.77 |
| 9 | 2.40E + 02 | 0.00E + 00 | 0.00 |
| 10 | 1.08E + 05 | 3.15E + 03 | 2.92 |
| 11 | 4.56E + 04 | 7.32E + 03 | 16.05 |
| 12 | 7.72E + 04 | 2.01E + 03 | 2.60 |
| 13 | 6.68E + 04 | 5.04E + 03 | 7.54 |
| 14 | 1.25E + 05 | 2.13E + 02 | 0.17 |
| 15 | 1.36E + 05 | 4.32E + 01 | 0.03 |
| 16 | 1.61E + 05 | 1.00E + 03 | 0.62 |
| 17 | 8.40E + 06 | 9.36E + 02 | 0.01 |
| 18 | 1.16E + 05 | 6.00E + 02 | 0.52 |
| 19 | 1.27E + 05 | 1.74E + 03 | 1.37 |
| 20 | 2.25E + 05 | 1.72E + 02 | 0.08 |
| 21 | 1.20E + 05 | 1.50E + 02 | 0.13 |
| 22 | 4.44E + 04 | 2.42E + 03 | 5.45 |
| Mean | 4.60E + 06 | 3.35E + 03 | 2.82 |
Expressed as the number of copies of the 16S rRNA gene.
Bifidobacterial load expressed as the percentage of total bacteria.
DISCUSSION
In recent years, breast milk has been shown to be a continuous source of commensal, mutualistic, and/or probiotic bacteria for the infant gut, including staphylococci, streptococci, and lactic acid bacteria (11, 20). These bacterial groups may also play an important role in the reduction of the incidence and severity of infections in the breast-fed infant. In fact, some of the lactic acid bacteria strains isolated from this biological fluid have the ability to inhibit the growth of a wide spectrum of pathogenic bacteria by competitive exclusion and/or through the production of antimicrobial compounds, such as bacteriocins, organic acids, or hydrogen peroxide (3, 21, 23).
Up to the present, the descriptions of bacterial diversity in breast milk have been based almost exclusively on the use of culture media that are suitable for the growth of lactic acid bacteria, streptococci, staphylococci, and closely related gram-positive bacteria (11, 20). This implies that the presence of additional bacterial species that are not cultivable or difficult to cultivate may have been overlooked. The recent application of culture-independent molecular techniques, particularly those based on 16S rRNA genes, has allowed a complementary assessment of the biodiversity of the human milk microbiota (8, 17) and has confirmed the strong influence of this biological fluid on the bacterial colonization of the neonatal gut (18). However, the number of molecular microbiology studies focused on human milk is still low, and their progressive incorporation will open new perspectives in this field.
The results of this work confirmed that the presence of bifidobacterial DNA is a common event in breast milk (8, 26). In addition, this is, to our knowledge, the first report describing the physical isolation of bifidobacteria from this biological fluid. Bifidobacteria were isolated from only 8 of the 23 milk samples. Among them, B. breve was isolated from the four samples in which DNA corresponding to this species was detected by PCR-DGGE and qRTi-PCR. Isolation of other bifidobacterial species whose DNA was present in the milk samples was more difficult (B. adolescentis and B. bifidum) or even impossible (B. longum and B. pseudocatenulatum). This may reflect the fastidious growth requirements of some bifidobacterial species that could be also present in the samples and/or their relatively low concentrations in this biological fluid. In this context, the fact that bifidobacteria were isolated from a higher number of infant feces samples (than from the corresponding breast milk ones) may be a consequence of their rather different concentrations (>107 CFU/g in feces versus <103 CFU/ml in breast milk).
The elucidation of the origin of the bacteria present in breast milk will be an attractive research target in the future. Traditionally, it was considered that they are acquired by skin contamination. Obviously, sampling of breast milk for microbiological analysis must take into account that skin contamination is almost unavoidable and that doubts as to the original location (internal mammary gland or skin) of the isolated bifidobacteria may arise; however, it has been pointed out that since bifidobacteria belongs to a strictly anaerobic genus, the latter source seems very unlikely (8). Such observation is in accordance with the results of this study since no bifidobacteria could be isolated from skin swabs obtained from women that provided the milk samples from which bifidobacteria were isolated. Although it has been shown that, under certain conditions, Bifidobacterium boum and Bifidobacterium thermophilum are able to grow in the presence of oxygen (12), these species were not detected in our study. In addition, none of the bifidobacterial isolates obtained from milk samples or feces in this study grew in aerobic conditions. Previously, it was reported that lactobacilli and enterococcal isolates present in human milk are genotypically different from those isolated in the skin, within the same bacterial species and the same host (20). The suggestions that the origin of the live bacteria found in breast milk could be the maternal gut and that the bacteria arrive at the mammary gland through an endogenous route (the so-called entero-mammary pathway) involving maternal dendritic cells and macrophages (19) has recently been confirmed (26). These authors showed that fresh human milk contains a number (<3 log CFU/ml) of viable bacteria and a wide range of free bacterial DNA signatures, including bifidobacterial DNA, which may program the neonatal immune system.
In conclusion, the results of this study indicate that breast milk is a source of bifidobacteria for the infant gut. Work is in progress to characterize some of the bifidobacterial strains isolated in this study and to further investigate the role of milk bifidobacteria in the development of the infant gut microbiota.
Acknowledgments
This study was partly supported by the FUN-C-FOOD (Consolider-Ingenio 2010) and AGL2007-62042 projects of the Ministerio de Educación y Ciencia (Spain).
Footnotes
Published ahead of print on 16 December 2008.
REFERENCES
- 1.Arvola, T., T. Ruuska, J. Keränen, H. Hyöty, S. Salminen, and E. Isolauri. 2006. Rectal bleeding in infancy: clinical, allergological, and microbiological examination. Pediatrics 117:e760-e768. [DOI] [PubMed] [Google Scholar]
- 2.Balmer, S. E., and B. A. Wharton. 1989. Diet and faecal flora in the newborn: breast milk and infant formula. Arch. Dis. Child. 64:1672-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beasley, S. S., and P. E. J. Saris. 2004. Nisin-producing Lactococcus lactis strains isolated from human milk. Appl. Environ. Microbiol. 70:5051-5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cebra, J. J. 1999. Influences of microbiota on intestinal immune system development. Am. J. Clin. Nutr. 69:1046S-1051S. [DOI] [PubMed] [Google Scholar]
- 5.Correa, N. B., L. A. Peret Filho, F. J. Penna, F. M. Lima, and J. R. Nicoli. 2005. A randomised formula controlled trial of Bifidobacterium lactis and Streptococcus thermophilus for prevention of antibiotic-associated diarrhea in infants. J. Clin. Gastroenterol. 39:385-389. [DOI] [PubMed] [Google Scholar]
- 6.Favier, C. F., W. M. De Vos, and A. D. Akkermans. 2003. Development of bacterial and bifidobacterial communities in feces of newborn babies. Anaerobe 9:219-229. [DOI] [PubMed] [Google Scholar]
- 7.Grice, E. A., H. H. Kong, G. Renaud, A. C. Young, G. G. Bouffard, R. W. Blakesley, T. G. Wolfsberg, M. L. Turner, J. A. Segre, et al. 2008. A diversity profile of the human skin microbiota. Genome Res. 18:1043-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gueimonde, M., K. Laitinen, S. Salminen, and E. Isolauri. 2007. Breast milk: a source of bifidobacteria for infant gut development and maturation? Neonatology 92:64-66. [DOI] [PubMed] [Google Scholar]
- 9.Haarman, M., and J. Knol. 2005. Quantitative real-time PCR assays to identify and quantify fecal Bifidobacterium species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 71:2318-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harmsen, H. J., A. C. Wildeboer-Veloo, A. A. Raangs, N. Wagendorp, J. G. Klijn, J. G. Bindels, and G. W. Welling. 2000. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 30:61-67. [DOI] [PubMed] [Google Scholar]
- 11.Heikkilä, M. P., and P. E. J. Saris. 2003. Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. J. Appl. Microbiol. 95:471-478. [DOI] [PubMed] [Google Scholar]
- 12.Kawasaki, S., T. Mimura, T. Satoh, K. Takeda, and Y. Niimura. 2006. Response of the microaerophilic Bifidobacterium species, B. boum and B. thermophilum, to oxygen. Appl. Environ. Microbiol. 72:6854-6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirjavainen, P. V., T. Arvola, S. Salminen, and E. Isolauri. 2002. Aberrant composition of gut microbiota of allergic infants: a target for bifidobacterial therapy at weaning? Gut 51:51-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kullen, M. J., R. B. Sanozky-Dawes, D. C. Crowell, and T. R. Klaenhammer. 2000. Use of the DNA sequence of variable regions of the 16S rRNA gene for rapid and accurate identification of bacteria in the Lactobacillus acidophilus complex. J. Appl. Microbiol. 89:511-516. [DOI] [PubMed] [Google Scholar]
- 15.Mackie, R. I., A. Sghir, and H. R. Gaskins. 1999. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 69:1035S-1045S. [DOI] [PubMed] [Google Scholar]
- 16.Mah, K. W., B. Björkstén, B. W. Lee, H. P. van Bever, L. P. Shek, T. N. Tan, Y. K. Lee, and K. Y. Chua. 2006. Distinct pattern of commensal gut microbiota in toddlers with eczema. Int. Arch. Allergy Immunol. 140:157-163. [DOI] [PubMed] [Google Scholar]
- 17.Martín, R., H. G. Heilig, E. G. Zoetendal, E. Jiménez, L. Fernández, H. Smidt, and J. M. Rodríguez. 2007. Cultivation-independent assessment of the bacterial diversity of breast milk among healthy women. Res. Microbiol. 158:31-37. [DOI] [PubMed] [Google Scholar]
- 18.Martín, R., H. G. Heilig, E. G. Zoetendal, H. Smidt, and J. M. Rodríguez. 2007. Diversity of the Lactobacillus group in breast milk and vagina of healthy women and potential role in the colonization of the infant gut. J. Appl. Microbiol. 103:2638-2644. [DOI] [PubMed] [Google Scholar]
- 19.Martín, R., S. Langa, C. Reviriego, E. Jiménez, M. L. Marín, M. Olivares, J. Boza, J. Jiménez, L. Fernández, J. Xaus, and J. M. Rodríguez. 2004. The commensal microflora of human milk: new perspectives for food bacteriotherapy and probiotics. Trends Food Sci. Technol. 15:121-127. [Google Scholar]
- 20.Martín, R., S. Langa, C. Reviriego, E. Jiménez, M. L. Marín, J. Xaus, L. Fernández, and J. M. Rodríguez. 2003. Human milk is a source of lactic acid bacteria for the infant gut. J. Pediatr. 143:754-758. [DOI] [PubMed] [Google Scholar]
- 21.Martín, R., M. Olivares, M. L. Marín, L. Fernández, J. Xaus, and J. M. Rodríguez. 2005. Probiotic potential of 3 lactobacilli strains isolated from breast milk. J. Hum. Lact. 21:8-17. [DOI] [PubMed] [Google Scholar]
- 22.Muyzer, G., E. C. de Waal, and G. A. Uitterlinden. 1993. Profiling of complex populations by denaturating gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivares, M., M. P. Díaz-Ropero, R. Martín, J. M. Rodríguez, and J. Xaus. 2006. Antimicrobial potential of four Lactobacillus strains isolated from breast milk. J. Appl. Microbiol. 101:72-79. [DOI] [PubMed] [Google Scholar]
- 24.Paulino, L. C., C. H. Tseng, B. E. Strober, and M. J. Blaser. 2006. Molecular analysis of fungal microbiota in samples from healthy human skin and psoriatic lesions. J. Clin. Microbiol. 44:2933-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penders, J., C. Vink, C. Driessen, N. London, C. Thijs, and E. E. Stobberingh. 2005. Quantification of Bifidobacterium spp., Escherichia coli and Clostridium difficile in faecal samples of breast-fed and formula-fed infants by real-time PCR. FEMS Microbiol. Lett. 243:141-147. [DOI] [PubMed] [Google Scholar]
- 26.Perez, P. F., J. Doré, M. Leclerc, F. Levenez, J. Benyacoub, P. Serrant, I. Segura-Roggero, E. J. Schiffrin, and A. Donnet-Hughes. 2007. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics 119:e724-e732. [DOI] [PubMed] [Google Scholar]
- 27.Saavedra, J. M., N. A. Bauman, I. Oung, J. A. Perman, and R. H. Yolken. 1994. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet 344:1046-1049. [DOI] [PubMed] [Google Scholar]
- 28.Sanguinetti, C. J., E. Dias Nieto, and A. J. G. Simpson. 1994. Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. BioTechniques 17:914-921. [PubMed] [Google Scholar]
- 29.Satokari, R. M., E. E. Vaughan, A. D. L. Akkermans, M. Saarela, and W. M. de Vos. 2001. Bifidobacterial diversity in human feces detected by genus-specific PCR and denaturating gradient gel electrophoresis. Appl. Environ. Microbiol. 67:504-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scardovi, V. 1986. Genus Bifidobacterium Orla-Jensen 1924, 472AL, p. 1418-1434. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. Williams and Wilkins, Baltimore, MD. [Google Scholar]
- 31.Stark, P. L., and A. Lee. 1982. The microbial ecology of the large bowel of breast-fed and formula-fed infants during the first year of life. J. Med. Microbiol. 15:189-203. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki, M. T., O. Beja, L. T. Taylor, and E. F. Delong. 2001. Phylogenetic analysis of ribosomal RNA operons from uncultivated coastal marine bacterioplankton. Environ. Microbiol. 3:323-331. [DOI] [PubMed] [Google Scholar]
- 33.Thibault, H., C. Aubert-Jacquin, and O. Goulet. 2004. Effects of long-term consumption of a fermented infant formula (with Bifidobacterium breve c50 and Streptococcus thermophilus 065) on acute diarrhea in healthy infants. J. Pediatr. Gastroenterol. Nutr. 39:147-152. [DOI] [PubMed] [Google Scholar]
- 34.Tissier, H. 1906. Traitement des infections intestinales par la méthode de la flore bactérienne de l'intestin. C. R. Soc. Biol. 60:359-361. [Google Scholar]