Abstract
At least 20% of all arthropods and some nematode species are infected with intracellular bacteria of the genus Wolbachia. This highly diverse genus has been subdivided into eight “supergroups” (A to H) on the basis of nucleotide sequence data. Here, we report the discovery of a new Wolbachia supergroup recovered from the spider mite species Bryobia species V (Acari: Tetranychidae), based on the sequences of three protein-coding genes (ftsZ, gltA, and groEL) and the 16S rRNA gene. Other tetranychid mites possess supergroup B Wolbachia strains. The discovery of another Wolbachia supergroup expands the known diversity of Wolbachia and emphasizes the high variability of the genus. Our data also clarify the existing supergroup structure and highlight the use of multiple gene sequences for robust phylogenetic analysis. In addition to previous reports of recombination between the arthropod-infecting supergroups A and B, we provide evidence for recombination between the nematode-infecting supergroups C and D. Robust delineation of supergroups is essential for understanding the origin and spread of this common reproductive parasite and for unraveling mechanisms of host adaptation and manipulation across a wide range of hosts.
Wolbachia is a genus of endosymbiotic alphaproteobacteria infecting a wide range of arthropods and filarial nematodes. In arthropods, Wolbachia often manipulates the reproductive mode of its host, causing parthenogenesis, feminization, cytoplasmic incompatibility, or male killing (48). In nematodes, and also in some arthropods, Wolbachia is an obligate symbiont required for host fertility (11, 39). It is estimated that more than 20% of arthropod species are infected with Wolbachia, including all major insect orders and some crustaceans and chelicerates (16, 31, 33, 60). The genus Wolbachia is genetically highly diverse and is divided into eight “supergroups” (A to H) (35). Currently, all of the supergroups are thought to represent one species, Wolbachia pipientis (35). Supergroups A and B were described first and are most commonly found among arthropod species (17, 60, 61). Supergroups C and D are restricted to filarial nematodes (9). Since the description of these four supergroups, the number of host taxa investigated has grown and the accuracy of detection has improved (investigating multiple individuals per host species and using a combination of primers). This led to the discovery of new Wolbachia strains and new host species. For example, supergroup F was described in 2002 (34), yet the known diversity and geographic range of host species infected with the supergroup is rapidly expanding. It has been detected in both nematodes and several major arthropod orders (in Chelicerata, scorpions [Scorpiones]; in Hexapoda, termites [Isoptera], weevils [Coleoptera], bush crickets [Orthoptera], cimicids [Hemiptera], lice [Phthiraptera], and louse-flies [Diptera]) (6, 19, 20, 34, 38, 45). Other host taxa for which there is evidence of infection by supergroup F are cockroaches (Blattodea) and ant lions (Neuroptera) (22, 54). Supergroups E and H are less widespread, with E so far found only in springtails (Collembola) (53, 56) and H in one genus of termites (Isoptera) (14). Isolates recovered from Australian spiders (Araneae) have been assigned as supergroup G (43), although it has been argued that these strains are in fact recombinants between supergroups A and B rather than representing a distinct lineage (7). Other diverse Wolbachia strains have been detected in fleas (Siphonaptera) (25), the filarial nematode Dipetalonema gracile (Spirurida) (19), and the pseudoscorpion Cordylochernes scorpioides (Pseudoscorpionida) (63), but these strains have not yet been assigned to a supergroup.
The genotypic diversity of Wolbachia mirrors an equally impressive range of phenotypes in terms of host range and manipulative effects. Much is still unknown about the origin and spread of Wolbachia, which host taxa were first infected, and how Wolbachia was transferred between taxa. Routes of transfer are still unclear; it is, for example, unknown if arthropods or nematodes were first infected (19). Robust phylogenetic analysis and the delineation of supergroups is therefore essential for elucidating the pathways and processes by which these diverse phenotypes have evolved, and recent studies have highlighted the promise of the multiple-gene sequencing approach (4, 6, 14, 19, 44). Such datasets can also reveal evidence concerning the contribution of lateral gene transfer to the evolution and host adaptation of Wolbachia.
Here, we describe the characterization of a diverse sample of Wolbachia strains by multiple-gene sequencing of the 16S rRNA gene and three protein-coding genes (gltA, groEL, and ftsZ) that had been used earlier for supergroup descriptions (14, 19, 35). We report the discovery of a new Wolbachia supergroup (K) in a spider mite species of the genus Bryobia (Bryobia species V; Acari: Tetranychidae) and show that other tetranychid species (Bryobia praetiosa, Bryobia sarothamni, Bryobia species I, and Tetranychus urticae) harbor B-group Wolbachia. The cooccurrence of two supergroups (B and K) within a single host genus is very unusual and has previously been noted only for supergroups A and B, which often occur in the same arthropod species and are known to recombine (7, 60, 61). We further rationalize the supergroup structure by proposing that the strains recovered from fleas (Siphonaptera) (19, 25) and the filarial nematode D. gracile (Spirurida) (19) correspond to the new supergroups I and J, respectively.
MATERIALS AND METHODS
DNA isolation, amplification, and sequencing.
We analyzed Wolbachia strains from four Bryobia species (B. praetiosa, B. sarothamni, and Bryobia species I and V) and T. urticae (Table 1). The Bryobia species were sampled within Europe between 2004 and 2006, maintained as isofemale lines in the laboratory, and identified using 28S rRNA and cytochrome c oxidase subunit I sequence data (42). An infected strain of T. urticae has been maintained in the laboratory for over 10 years. DNA was extracted from single individuals using the cetyltrimethylammonium bromide method as described by Ros and Breeuwer (41).
TABLE 1.
Sampling details of tetranychid species analyzed in this study
| Code | Species | Country | Locality | Host plant name
|
Collection date | Reproductive modea | |
|---|---|---|---|---|---|---|---|
| Common | Scientific | ||||||
| NL12 | B. praetiosa | Netherlands | Amsterdam | Grasses and herbs | May 2004 | A | |
| FR16 | B. sarothamni | France | Vireux | Common Broom | Cytisus scoparius | July 2006 | S |
| NL14 | Bryobia species I | Netherlands | Nieuweschans | Vetches | Vicia sp. | May 2006 | A |
| ITA11 | Bryobia species V | Italy | S. Felice Circeo | Grasses and herbs | May 2005 | A | |
| T2 | T. urticae | Unknown | Unknown | Cucumber | Cucumis sativus | -b | S |
A, asexual; S, sexual (for details, see reference 41).
Maintained in the laboratory on bean (Phaseolus vulgaris) for over 10 years.
FtsZ was amplified and sequenced using the primer sets ftsZ1 (5′-CCGTATGCCGATTGCAGAGCTTG-3′) and ftsZ2 (5′-GCCATGAGTATTCACTTGGCT-3′) (30) and ftsZf1 (5′-GTTGTCGCAAATACCGATGC-3′) and ftsZr1 (5′-CTTAAGTAAGCTGGTATATC-3′) (61). groEL was amplified using the primers groEL-F (5′-CAACRGTRGSRRYAACTGCDGG-3′) and groEL-R (5′-GATADCCRCGRTCAAAYTGC-3′), hand designed from available Wolbachia and Rickettsia genome sequences (2, 37, 62) and tested on isolates representative of supergroups A and B (samples kindly donated by Robert Butcher, University of Bath). gltA was amplified and sequenced as described by Casiraghi et al. (19).
PCR amplifications were performed in 25-μl reaction mixtures containing 2.5 μl 10× Super Taq buffer (HT BioTechnology, Cambridge, United Kingdom), 1.25 μl bovine serum albumin (10 mg/ml), 5 μl deoxynucleotide triphosphate mixture (1 mM of each nucleotide), 0.2 μl of each primer (20 μM each), 0.2 μl of super Taq (5 U/μl) (HT BioTechnology), 13.15 μl water, and 2.5 μl of DNA extract. The PCR cycling profile for ftsZ was 35 cycles of 30 s at 95°C, 30 s at 51°C, and 1 min at 72°C, and for groEL it was 35 cycles of 1 min at 95°C, 1 min at 49°C, and 1.5 min at 72°C. The PCR products were purified using a DNA extraction kit (Fermentas, St. Leon-Rot, Germany). The purified products were directly sequenced using the ABI Prism BigDye Terminator Sequence Kit (Applied Biosystems, Nieuwerkerk a/d IJssel, The Netherlands) according to the manufacturer's instructions but diluted 16 times. Both strands of the products were sequenced using the same primers used in the PCR amplification. Sequences were run on an ABI 3700 automated DNA sequencer.
Amplification and cloning of the 16S rRNA gene.
To check for multiple infections (by different Wolbachia strains or by other bacteria), part of the 16S rRNA gene was amplified and cloned for the four Bryobia samples. DNA was extracted from five pooled individuals per isofemale line following the cetyltrimethylammonium bromide method (41). Part of the 16S rRNA gene was amplified using the primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1513R (5′-ACGGYTACCTTGTTACGACTT-3′) (59) in a 25-μl reaction mixture containing 2.5 μl 10× Super Taq buffer, 5 μl deoxynucleotide triphosphate mixture (1 mM of each nucleotide), 0.5 μl of each primer (10 μM each), 0.2 μl of super Taq (5 U/μl), 13.3 μl water, and 3 μl of DNA extract. The PCR cycling profile was 35 cycles of 1 min at 94°C, 1 min at 54°C, and 1 min at 72°C. The PCR products were purified using the method of Boom et al. (13). The purified products were ligated into a vector (pGEM-T Easy Vector System) and then used to transform JM109 competent cells, according to the manufacturer's instructions (Promega, Madison WI). Plasmids were recovered for 10 colonies per sample, using minipreparation procedures (46). The plasmids were sequenced using the M13 forward primer and the internal 16S rRNA gene primers 704f (5′-GTAGAGGTRAAATTCGTAAA-3′) and 765r (5′-CTGTTTGCTCCCCACGCYTTG-3′) (modified from the method of Bandi et al. [10]) as described above. For T. urticae, the 16S rRNA gene was sequenced directly using the same primers and PCR protocol.
Data assembly and phylogenetic analyses.
The sequences obtained in this study were combined with sequences obtained from GenBank representing all currently known supergroups of Wolbachia (see Table S1 in the supplemental material). We analyzed alignments of 636 bp for gltA, 489 bp for ftsZ, 491 bp for groEL, and 1,255 bp for the 16S rRNA gene. Sequences for which at least two of the three protein-coding genes were available were included. The 16S rRNA gene data set contained a few additional samples (for which data on the mentioned protein-coding genes are unknown) to explore the full range of Wolbachia diversity. Sequences were identified by the name of the host species. The sequences were aligned using ClustalX version 1.8.0 with default settings (52). Phylogenetic analyses were performed using neighbor-joining (NJ), maximum likelihood (ML), and Bayesian methods for each locus separately and for a concatenated data set of the three protein-coding genes. PAUP* version 4.0b10 (50) was used to select the optimal evolution model by critically evaluating the selected parameters (51) using the Akaike Information Criterion (1). For the protein-coding genes, we tested if the likelihood of models could be further improved by incorporating specific rates for each codon position (47). This approach suggested the following models: 16S rRNA gene (HKY + I + G), gltA (TIM + G), ftsZ (TrN with site-specific rates for each codon position), groEL (submodel of GTR with rate class “a b c c d c” and site-specific rates), and the concatenated data set (submodel of GTR + I + G with rate class “a b c c d e”). Under the selected models, the parameters and tree topology were optimized using the successive-approximations approach (49). NJ analyses (p distances) and ML analyses (heuristic search, random addition of sequences with 10 replicates, and TBR branch swapping) were performed in PAUP. The robustness of nodes was assessed with 100 bootstrap replicates. However, as PAUP does not allow site-specific rates in bootstrap analysis, ML bootstrapping for ftsZ and groEL was performed with gamma distributed rates with 100 bootstrap replicates. Bootstrap values were then plotted on the phylogeny obtained with the original model with site-specific rates. Bayesian analyses were performed as implemented in MrBayes 3.1.2 (40). The models used were GTR + I + G (16S rRNA gene), GTR + G (gltA), and GTR with separate rates for each codon position (ftsZ and groEL). For the concatenated data set, the same models were used for each gene partition. Analyses were initiated from random starting trees. Two separate Markov chain Monte Carlo (MCMC) runs, each composed of four chains (one cold and three heated), were run for 6,000,000 generations. The cold chain was sampled every 100 generations, and the first 15,000 generations were later discarded (a burn-in of 25%). Posterior probabilities were computed from the remaining trees. We checked whether the MCMC analyses ran long enough by using the program AWTY (http://ceb.csit.fsu.edu/awty). Stationarity (stable topologies and posterior probabilities with increasing run time) was assumed when there was convergence between the two MCMC runs and when the cumulative posterior probabilities of splits stabilized; in all analyses, 6,000,000 generations proved sufficient.
Analysis of recombination.
Alignments were checked for signs of intragenic recombination using the software package RDP3 (36) and by visual inspection. The programs used in the RDP3 software package were RDP, Geneconv, Bootscan, MaxChi, Chimaera, and Sister Scanning. Analyses were run with default settings, except for window and step sizes, which were varied during independent analyses. Analyses were performed for total data sets and reduced data sets with one or two strains representing each supergroup. We have included only those cases of recombination that were detected by at least two of the listed programs and supported by visual inspection.
Nucleotide sequence accession numbers.
All new sequences were deposited in the GenBank database under accession numbers EU499315 to EU499334 (see Table S1 in the supplemental material).
RESULTS
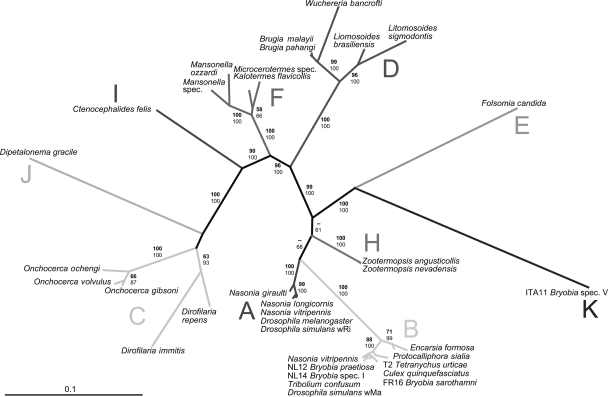
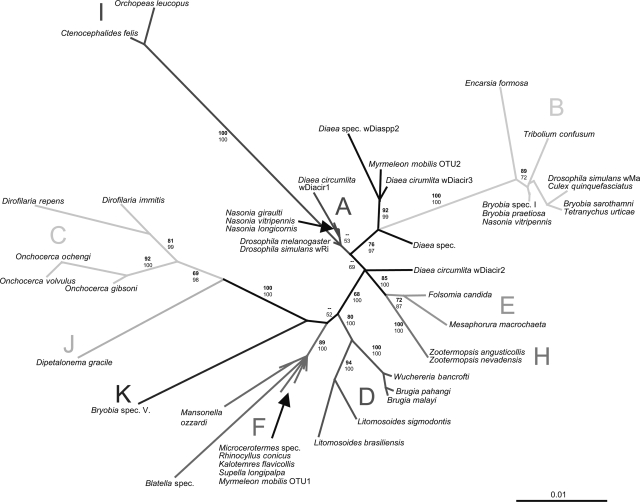
Phylogenetic analysis of four Wolbachia loci (a total of 2,871 bp) showed the existence of two highly divergent Wolbachia strains within the family Tetranychidae (Fig. 1 and 2). The species Bryobia species V harbors a Wolbachia strain that does not cluster with any of the previously described supergroups. It forms a distinct phylogenetic lineage, highly divergent from all known supergroups (2.8 to 5.5% pairwise difference of the 16S rRNA gene). The three other Bryobia species and T. urticae harbor (different) Wolbachia strains that cluster with Wolbachia supergroup B.
FIG. 1.
Concatenated phylogenetic tree (ML, unrooted) based on Wolbachia sequences of three protein-coding genes (gltA, ftsZ, and groEL) (1,616 bp). Strains are characterized by the names of their host species. ML bootstrap values (top numbers, in boldface) based on 100 replicates and Bayesian posterior probabilities (bottom numbers) are depicted (only values larger than 50 are indicated). Values for recently diverged taxa within supergroups are sometimes excluded for clarity of presentation. The bar indicates a branch length of 10% likelihood distance.
FIG. 2.
Unrooted ML phylogenetic tree of Wolbachia 16S rRNA gene sequences (1,255 bp). Strains are characterized by the names of their host species. ML bootstrap values (top numbers, in boldface) based on 100 replicates and Bayesian posterior probabilities (bottom numbers) are depicted (only values larger than 50 are indicated). Values for recently diverged taxa within supergroups are sometimes excluded for clarity of presentation. The bar indicates a branch length of 1% likelihood distance.
Cloning of the 16S rRNA gene showed that each Bryobia mite is infected with a single Wolbachia lineage, and no double infections with other bacteria were found. The 10 clones sequenced per species showed minimal sequence differences (<1%), and those differences that were detected may reflect cloning artifacts (9). For each sample, a majority-rule consensus sequence was created. Among the 10 clones sequenced for Bryobia species I, one clone was identified as belonging to the genus Streptococcus (“oral clone”). This clone was considered contamination and discarded.
Our analysis confirms supergroups A to F and H as distinct, coherent phylogenetic clades, supported by high bootstrap and posterior probability values (Fig. 1 and 2). These clades were consistently recovered for each locus separately (data not shown), although support for supergroup A is low in the 16S rRNA gene phylogeny (Fig. 2). Apart from these known supergroups, three further distinct Wolbachia lineages were observed: a lineage found in Ctenocephalides felis and Orchopeas leucopus (both Siphonaptera) (19, 25), a lineage found in D. gracile (Spirurida) (19), and a lineage in Bryobia species V (Tetranychidae; this study). These lineages are highly distinct from all other supergroups for all analyzed loci. We propose to name these lineages supergroups I, J, and K, respectively (Fig. 1 and 2; see Table S1 in the supplemental material).
The 16S rRNA gene phylogenetic tree includes additional samples of interest. These represent newly described supergroups or were recovered from host species only recently discovered to harbor Wolbachia (21, 22, 25, 34, 54). Our analysis also includes isolates assigned to supergroup G (43) (Fig. 2; see Table S1 in the supplemental material). The phylogeny of the 16S rRNA gene is less well resolved than the phylogenies of the protein-coding genes. Supergroup A lacks support, and samples from the spider genus Diaea (Araneae) and the ant lion Myrmeleon mobilis (Neuroptera) fall between or within supergroups A and B. This finding is therefore consistent with a previous suggestion by Baldo and Werren (7) that the Wolbachia strains from Diaea are supergroup A/B recombinants rather than forming an independent lineage (supergroup G), as proposed by Rowley et al. (43). These observations also challenge the common assumption that the 16S rRNA gene is a reliable phylogenetic marker that is recalcitrant to recombination. Our data reveal at least one clear case of recombination in the 16S rRNA gene (Fig. 3). Recombination has occurred between Wolbachia strains from M. mobilis and isolates from supergroups B (B. praetiosa) and F (Kalotermes flavicollis) (P < 0.05 for MaxChi, Chimaera, Bootscan, and Siscan) (Fig. 3a). Exact breakpoints could not be determined, and it remains unclear which sequence represents the recombinant. A second, less evident, recombination event was revealed between strains from supergroups E (Folsomia candida), I (C. felis), and B (B. praetiosa) (P < 0.05 for MaxChi and Chimaera) (Fig. 3b). In this case, recombination may have occurred at several places along the sequences and between all strains. This could reflect recombination events in the distant past. Clear recombination is also evident in the striking mosaic structure observed within gltA between strains from supergroups C and D (Fig. 4) (P < 0.001 in all cases). The sequence of Wuchereria bancrofti (supergroup D) is a combination of the sequence of Brugia malayi (D) and Onchocerca gibsoni (C) (Fig. 4). Strains of these two supergroups infect filarial nematodes but are highly divergent (Fig. 1 and 2). Within gltA, recombination was also detected within and between strains from supergroups A and B, similar to earlier findings of Baldo et al. (3). No well-supported signs of recombination were detected within groEL or ftsZ.
FIG. 3.
Two cases of recombination between Wolbachia 16S rRNA gene sequences. Only polymorphic sites are shown (the position in the alignment is given on top). Sequences are named by their GenBank accession numbers, supergroups (in boldface), and host species names. Different shadings indicate possible recombinant regions (see Results). Differences and identities (dots) compared to the middle sequence are shown.
FIG. 4.
Overview of recombination between Wolbachia gltA sequences of filarial nematodes (supergroups C and D). Only polymorphic sites are shown (the position in the alignment is given on top). Sequences are named by their GenBank accession numbers, supergroups (in boldface), and host species names. Different shadings indicate the similarity of the recombinant strain in W. bancrofti to each of the other strains.
DISCUSSION
We describe the discovery of a new Wolbachia supergroup, named K, within Bryobia species V. The other Wolbachia strains from B. praetiosa, B. sarothamni, Bryobia species I, and T. urticae clustered within supergroup B. While supergroups A and B frequently infect species of a single genus and have even been found coinfecting the same species (17, 60, 61), thus providing the opportunity for recombination (5, 7), other supergroups have rarely been found coinfecting the same host genus. The discovery of another Wolbachia supergroup expands the known diversity of Wolbachia, and we consider it very likely that more supergroups remain to be discovered as screening techniques improve and a wider variety of potential host species are examined. The detection of supergroup K within Bryobia also underscores the fact that little is known about Wolbachia diversity even within known host species and that the analysis of multiple individuals within single host species will further increase the chance of detection of new strains or even supergroups. The design of more pairs of degenerate primers utilizing the known range of sequence diversity within the Wolbachia will expedite this process, as detection may be biased by primer choice and previous screening studies have almost certainly underestimated the abundance of Wolbachia. In order to spread the net as wide as possible, we suggest that screening studies should utilize a combination of validated primers based on conserved sequences spanning the broad range of diversity currently described for Wolbachia. A more fully representative nucleotide data set is required before a universal primer set can be identified which reliably encompasses the full range of Wolbachia diversity.
Wolbachia has been found in several species of the spider mite family Tetranychidae (16, 27, 28, 57, 58). Prior to this study, all isolates recovered from spider mites have corresponded to supergroup B (16, 31). Wolbachia infection in Bryobia is known to cause parthenogenesis in B. praetiosa (57), and there is good evidence that many other Bryobia species, including Bryobia species I and Bryobia species V, are asexual (42). Possibly, Wolbachia is widespread within the genus Bryobia and is responsible for the parthenogenetic reproduction. The fact that two different supergroups are detected in this genus suggests at least two independent infections within Bryobia. Independent infections could explain our finding that asexual species do not form a monophyletic clade, and asexuality originated more than once (42). We also note that Wolbachia infection is not restricted to asexually reproducing Bryobia species, as it is also detected in the sexually reproducing species B. sarothamni. In sexual species of other spider mite genera (Tetranychus, Panonychus, and Oligonychus), including T. urticae, which is included in this study, Wolbachia causes cytoplasmic incompatibility (15, 26, 27, 28, 55). However, in other studies, no detectable effect of Wolbachia infection was found (24, 27, 28). The effect of Wolbachia in B. sarothamni remains to be investigated.
Even though a strict definition of a supergroup is lacking, the generation of data from multiple genes clearly can be used to delineate well-supported clades within Wolbachia. Depending on how one views the phylogenetic status of a “supergroup,” the multilocus sequencing approach for defining Wolbachia supergroups falls somewhere between multilocus sequence typing, in which the alleles at a set of housekeeping genes define strains and clonal groups within a named species, and multilocus sequence analysis, in which the same procedure provides evidence concerning the boundaries between closely related “species” (23). The level of diversity within each supergroup varies (e.g., supergroup C is very diverse) (Fig. 1 and 2), but all currently recognized supergroups are well supported (A to F and H), and the levels of divergence between the supergroups are generally more akin to inter- rather than intraspecies differences (Table 2). Similarly, the newly defined supergroups I, J, and K are highly divergent from all other supergroups and are well supported by all analyzed loci. More strikingly, for all four analyzed genes, the distances between these new supergroups and any current supergroups are equal to (in one case, between I and F for groEL) or larger than (in all other cases) the minimum distance between current supergroups. Moreover, the maximum observed distance between all supergroups for all genes involves either one or both of the new supergroups, indicating the high diversity of these groups. Therefore the putative new supergroups more than match the criteria for sequence “distinctness” on which current supergroups are based.
TABLE 2.
Minimum, maximum, and average p distances within and between Wolbachia supergroups for each of the analyzed genes
| Distance parameter | Valueb
|
||||
|---|---|---|---|---|---|
| gltA | ftsZ | groEL | Concatenated | 16S rRNA genec | |
| Within | |||||
| Minimuma | 0.0032 (H) | 0.0021 (H) | 0 (H) | 0.0019 (H) | 0 (H) |
| Maximum | 0.0907 (C) | 0.0732 (C) | 0.0536 (C) | 0.0675 (C) | 0.0173 (C) |
| Avg | 0.0425 | 0.0273 | 0.0256 | 0.0331 | 0.0084 |
| Between | |||||
| Minimum | 0.0613 (AB) | 0.0556 (AH) | 0.0672 (IF) | 0.0716 (AH) | 0.0135 (AE) |
| Maximum | 0.2484 (IK) | 0.1396 (CK) | 0.1711 (JK) | 0.2248 (EJ) | 0.0658 (IJ) |
| Avg | 0.1764 | 0.1120 | 0.1276 | 0.1477 | 0.0361 |
Excluding supergroups with only one strain.
The supergroup(s) between or within which the minimum or maximum distance was found is indicated in parentheses.
Excluding strains that were not assigned to a supergroup.
In our opinion, this validates the designation of these supergroups and the possible promotion of Wolbachia supergroups to species status. The occurrence of occasional gene transfer between supergroups does not preclude such a reevaluation, as by this token, many closely related named bacterial species should be lumped together. Although there is currently no universal conceptual basis for species (or supergroup) assignment, the equivalence of supergroups and species would help to bring Wolbachia systematics into line with other bacterial taxa and would encourage debate as to the evolutionary and ecological significance of these major Wolbachia clades.
The 16S rRNA gene data set was supplemented with recently identified strains of interest. Within the ant lion M. mobilis (Neuroptera), two distinct strains were detected (22), one of which clusters with supergroup F. The other strain clusters with samples previously identified as supergroup G (see below). Thus, two divergent strains infect this ant lion species, although this should be further investigated using other genes as our data and previous studies point to the possibility of recombination at the 16S rRNA gene. Furthermore, Wolbachia strains from two cockroach species, Blatella sp. and Supella longipalpa (Blattodea), and a weevil, Rhinocyllus conicus (Coleoptera), cluster with supergroup F based on 16S rRNA gene data (34, 54). This shows that, like supergroups A and B, supergroup F is also widespread among different arthropod orders (Table 3). Supergroup G was described for Wolbachia strains from two species of the spider genus Diaea (Araneae) (43). We included these samples in our 16S rRNA gene analysis, and Fig. 2 shows that these samples fall in between supergroups A and B. Based on analyses of wsp sequences, Baldo and Werren (7) showed that Wolbachia strains defined as supergroup G are recombinants between strains of supergroups A and B. Recombination is apparently also present at the 16S rRNA gene (Fig. 3). This is illustrated for the sequence from M. mobilis, which clusters with samples from the two Diaea species in the 16S rRNA gene phylogenetic tree (Fig. 2). This sequence shows signs of recombination events with strains from supergroups B (B. praetiosa) and F (K. flavicollis) (Fig. 3a). Only part of the 16S rRNA gene is sequenced for the Diaea samples, making exact inference of relationships impossible. A more detailed investigation including other genes should be performed to infer the exact relationship of these samples.
TABLE 3.
Distribution of Wolbachia supergroups across nematode and arthropod orders
| Taxon | Name | Supergroup(s) |
|---|---|---|
| Phylum | Arthropoda | |
| Subphylum | Chelicerata | |
| Class | Arachnida | |
| Ordera | Acarinab | B,K |
| Araneae | A,B,(G)d | |
| Pseudoscorpionida | ?c | |
| Scorpiones | F | |
| Subphylum | Crustaceae | |
| Class | Ostracoda | |
| Order | Podocopida | A,B |
| Class | Malacostraca | |
| Order | Amphipoda | B |
| Isopoda | B | |
| Subphylum | Hexapoda | |
| Class | Entognatha | |
| Order | Collembola | B,E |
| Class | Insecta | |
| Order | Blattodea | F |
| Coleoptera | A,B,F | |
| Dermaptera | ? | |
| Diptera | A,B,F | |
| Hemiptera | A,B,F | |
| Hymenoptera | A,B | |
| Isoptera | A,B,F,H | |
| Lepidoptera | A,B | |
| Mecoptera | ? | |
| Neuroptera | F,? | |
| Odonata | B | |
| Orthoptera | B,F | |
| Phthiraptera | F | |
| Psocoptera | A,B | |
| Siphonaptera | I | |
| Thysanoptera | B,F | |
| Thysanura | B | |
| Phylum | Nematoda | |
| Class | Secernentea | |
| Order | Spirurida | C,D,F,J |
| Strongylida | ? |
Only orders in which Wolbachia was found are listed.
Oribatida, Prostigmata, Mesostigmata.
?, Wolbachia was detected, but the supergroup was undetermined.
The existence of supergroup G is disputed; see text.
While horizontal transfer and recombination between arthropod-infecting supergroups A and B are common, they are considered entirely absent in or between nematode-infecting supergroups (3, 4, 5, 32). So far, phylogenies of filarial nematodes and their Wolbachia parasites were found congruent, suggesting coevolution between nematodes and Wolbachia for nearly 100 million years (9, 18). We illustrate a clear case of recombination between nematode supergroups C and D. Although Wolbachia-nematode associations are found to be highly specific, this apparently does not exclude occasional horizontal transfer and recombination. Possibly, there are ecological opportunities (e.g., sharing of the same nematode host or arthropod vector species) allowing strains of different supergroups to recombine.
Some supergroups have taxonomically widespread host ranges, while others are restricted to a single taxon (Table 3). Supergroups A and B are found in the three major arthropod subphyla (Chelicerata, Crustacea, and Hexapoda). Supergroup F is found in the subphyla Chelicerata and Hexapoda and also in the phylum Nematoda, indicating interhost transmission of Wolbachia between arthropods and nematodes (19, 38). Transmission of supergroups A, B, and F between the subphyla is also thought to be common. In contrast, supergroups E, H, I, J, and K are each currently restricted to a single order or even a single species, although there is very little evidence concerning the true distribution of these supergroups. When all subphyla are considered, the Hexapoda contain the highest diversity of Wolbachia strains: all supergroups are found except C, D, J (restricted to the phylum Nematoda), and K (found only in the order Prostigmata [Acarina]). Within the subphylum Crustacea, only supergroups A and B are found. The diversity within the subphylum Chelicerata is much greater: so far, supergroups B (spiders [Araneae], mites, and ticks [Acarina]), A (spiders), F (scorpions [Scorpiones]), and K (mites) and possible new strains (pseudoscorpions [Pseudoscorpionida] and spiders) have been reported (6, 16, 29, 43, 63). This suggests multiple independent infections of the Chelicerata with Wolbachia. Most of these findings were only recently reported, and we expect that future investigations within these and other taxa will further enlarge the known diversity of Wolbachia.
In conclusion, the combined analysis of the three protein-coding genes gltA, ftsZ, and groEL give a congruent and well-supported representation of the current supergroup designation. This agrees with the findings of Casiraghi et al. (19) and Bordenstein and Rosengaus (14). The phylogeny obtained from 16S rRNA gene sequences is less well resolved, and recombination is observed within this region, rendering it less suitable for supergroup assignment (34). Although a single intragenic recombination event was found for gltA, this does not affect the supergroup classification. Sets of primers are available that work well across all known supergroups, and these should help in refining the structure further and for uncovering more diversity in the future.
Supplementary Material
Acknowledgments
We thank Betsie Voetdijk and Sangeeta Jessurun for assistance with cloning and sequencing and Jan van Arkel for help with the figures. Thanks are due to Robert Butcher and Tim Karr for useful discussions and to Steph Menken for useful discussions and valuable comments on the manuscript.
This research was funded by a grant from The Netherlands Organization for Scientific Research (NWO; ALW4PJ/03-25).
Footnotes
Published ahead of print on 19 December 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Akaike, H. 1974. A new look at the statistical model identification. IEEE Trans. Autom. Contr. 19:716-723. [Google Scholar]
- 2.Andersson, S. G. E., A. Zomorodipour, J. O. Andersson, T. Sicheritz-Ponten, U. C. M. Alsmark, R. M. Podowski, A. K. Naslund, A. S. Eriksson, H. H. Winkler, and C. G. Kurland. 1998. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396:133-140. [DOI] [PubMed] [Google Scholar]
- 3.Baldo, L., S. Bordenstein, J. J. Wernegreen, and J. H. Werren. 2006. Widespread recombination throughout Wolbachia genomes. Mol. Biol. Evol. 23:437-449. [DOI] [PubMed] [Google Scholar]
- 4.Baldo, L., J. C. D. Hotopp, K. A. Jolley, S. R. Bordenstein, S. A. Biber, R. R. Choudhury, C. Hayashi, M. C. J. Maiden, H. Tettelin, and J. H. Werren. 2006. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 72:7098-7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldo, L., N. Lo, and J. H. Werren. 2005. Mosaic nature of the Wolbachia surface protein. J. Bacteriol. 187:5406-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldo, L., L. Prendini, A. Corthals, and J. H. Werren. 2007. Wolbachia are present in Southern African scorpions and cluster with supergroup F. Curr. Microbiol. 55:367-373. [DOI] [PubMed] [Google Scholar]
- 7.Baldo, L., and J. H. Werren. 2007. Revisiting Wolbachia supergroup typing based on wsp: spurious lineages and discordance with MLST. Curr. Microbiol. 55:81-87. [DOI] [PubMed] [Google Scholar]
- 8.Reference deleted.
- 9.Bandi, C., T. J. C. Anderson, C. Genchi, and M. L. Blaxter. 1998. Phylogeny of Wolbachia in filarial nematodes. Proc. R. Soc. Lond. B 265:2407-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandi, C., G. Damiani, L. Magrassi, A. Grigolo, R. Fani, and L. Sacchi. 1994. Flavobacteria as intracellular symbionts in cockroaches. Proc. R. Soc. Lond. B 257:43-48. [DOI] [PubMed] [Google Scholar]
- 11.Bandi, C., A. M. Dunn, G. D. D. Hurst, and T. Rigaud. 2001. Inherited microorganisms, sex-specific virulence and reproductive parasitism. Trends Parasitol. 17:88-94. [DOI] [PubMed] [Google Scholar]
- 12.Reference deleted.
- 13.Boom, R., C. J. A. Sol, M. M. M. Salimans, C. L. Jansen, P. M. E. Wertheim-van Dillen, and J. Van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bordenstein, S., and R. B. Rosengaus. 2005. Discovery of a novel Wolbachia supergroup in Isoptera. Curr. Microbiol. 51:393-398. [DOI] [PubMed] [Google Scholar]
- 15.Breeuwer, J. A. J. 1997. Wolbachia and cytoplasmic incompatibility in the spider mites Tetranychus urticae and T. turkestani. Heredity 79:41-47. [Google Scholar]
- 16.Breeuwer, J. A. J., and G. Jacobs. 1996. Wolbachia: Intracellular manipulators of mite reproduction. Exp. Appl. Acarol. 20:421-434. [DOI] [PubMed] [Google Scholar]
- 17.Breeuwer, J. A. J., R. Stouthamer, S. M. Barns, D. A. Pelletier, W. G. Weisburg, and J. H. Werren. 1992. Phylogeny of cytoplasmic incompatibility micro-organisms in the parasitoid wasp genus Nasonia (Hymenoptera: Pteromalidae) based on 16S ribosomal DNA sequences. Insect Mol. Biol. 1:25-36. [DOI] [PubMed] [Google Scholar]
- 18.Casiraghi, M., T. J. C. Anderson, C. Bandi, C. Bazzocchi, and C. Genchi. 2001. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology 122:93-103. [DOI] [PubMed] [Google Scholar]
- 19.Casiraghi, M., S. R. Bordenstein, L. Baldo, N. Lo, T. Beninati, J. J. Wernegreen, J. H. Werren, and C. Bandi. 2005. Phylogeny of Wolbachia pipientis based on gltA, groEL and ftsZ gene sequences: clustering of arthropod and nematode symbionts in the F supergroup, and evidence for further diversity in the Wolbachia tree. Microbiology 151:4015-4022. [DOI] [PubMed] [Google Scholar]
- 20.Covacin, C., and S. C. Barker. 2007. Supergroup F Wolbachia bacteria parasitise lice (Insecta: Phthiraptera). Parasitol. Res. 100:479-485. [DOI] [PubMed] [Google Scholar]
- 21.Czarnetzki, A. B., and C. C. Tebbe. 2004. Detection and phylogenetic analysis of Wolbachia in Collembola. Environ. Microbiol. 6:35-44. [DOI] [PubMed] [Google Scholar]
- 22.Dunn, A. K., and E. V. Stabb. 2005. Culture-independent characterization of the microbiota of the ant lion Myrmeleon mobilis (Neuroptera: Myrmeleontidae). Appl. Environ. Microbiol. 71:8784-8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gevers, D., F. M. Cohan, J. G. Lawrence, B. G. Spratt, T. Coenye, E. J. Feil, E. Stackebrandt, Y. Van de Peer, P. Vandamme, F. L. Thompson, and J. Swings. 2005. Re-evaluating prokaryotic species. Nat. Rev. Microbiol. 3:733-739. [DOI] [PubMed] [Google Scholar]
- 24.Gomi, K., T. Gotoh, and H. Noda. 1997. Wolbachia having no effect on reproductive incompatibility in Tetranychus kanzawai Kishida (Acari: Tetranychidae). Appl. Entomol. Zool. 32:485-490. [Google Scholar]
- 25.Gorham, C. H., Q. Q. Fang, and L. A. Durden. 2003. Wolbachia endosymbionts in fleas (Siphonaptera). J. Parasitol. 89:283-289. [DOI] [PubMed] [Google Scholar]
- 26.Gotoh, T., H. Noda, T. Fujita, K. Iwadate, Y. Higo, S. Saito, and S. Ohtsuka. 2005. Wolbachia and nuclear-nuclear interactions contribute to reproductive incompatibility in the spider mite Panonychus mori (Acari: Tetranychidae). Heredity 94:237-246. [DOI] [PubMed] [Google Scholar]
- 27.Gotoh, T., H. Noda, and X. Y. Hong. 2003. Wolbachia distribution and cytoplasmic incompatibility based on a survey of 42 spider mite species (Acari: Tetranychidae) in Japan. Heredity 91:208-216. [DOI] [PubMed] [Google Scholar]
- 28.Gotoh, T., J. Sugasawa, H. Noda, and Y. Kitashima. 2007. Wolbachia-induced cytoplasmic incompatibility in Japanese populations of Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 42:1-16. [DOI] [PubMed] [Google Scholar]
- 29.Hartelt, K., R. Oehme, H. Frank, S. O. Brockmann, D. Hassler, and P. Kimmig. 2004. Pathogens and symbionts in ticks: prevalence of Anaplasma phagocytophilum (Ehrlichia sp.), Wolbachia sp., Rickettsia sp., and Babesia sp. in Southern Germany. Int. J. Med. Microbiol. 293:86-92. [DOI] [PubMed] [Google Scholar]
- 30.Holden, P. R., P. Jones, and J. F. Y. Brookfield. 1993. Evidence for a Wolbachia symbiont in Drosophila melanogaster. Genet. Res. 62:23-29. [DOI] [PubMed] [Google Scholar]
- 31.Jeyaprakash, A., and M. A. Hoy. 2000. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol. Biol. 9:393-405. [DOI] [PubMed] [Google Scholar]
- 32.Jiggins, F. M. 2002. The rate of recombination in Wolbachia bacteria. Mol. Biol. Evol. 19:1640-1643. [DOI] [PubMed] [Google Scholar]
- 33.Jiggins, F. M., J. K. Bentley, M. E. N. Majerus, and G. D. D. Hurst. 2001. How many species are infected with Wolbachia? Cryptic sex ratio distorters revealed to be common by intensive sampling. Proc. R. Soc. Lond. B 268:1123-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo, N., M. Casiraghi, E. Salati, C. Bazzocchi, and C. Bandi. 2002. How many Wolbachia supergroups exist? Mol. Biol. Evol. 19:341-346. [DOI] [PubMed] [Google Scholar]
- 35.Lo, N., C. Paraskevopoulos, K. Bourtzis, S. L. O'Neill, J. H. Werren, S. R. Bordenstein, and C. Bandi. 2007. Taxonomic status of the intracellular bacterium Wolbachia pipientis. Int. J. Syst. Evol. Microbiol. 57:654-657. [DOI] [PubMed] [Google Scholar]
- 36.Martin, D. P., C. Williamson, and D. Posada. 2005. RDP2: recombination detection and analysis from sequence alignments. Bioinformatics 21:260-262. [DOI] [PubMed] [Google Scholar]
- 37.McLeod, M. P., X. Qin, S. E. Karpathy, J. Gioia, S. K. Highlander, G. E. Fox, T. Z. McNeill, H. Y. Jiang, D. Muzny, L. S. Jacob, A. C. Hawes, E. Sodergren, R. Gill, J. Hume, M. Morgan, G. W. Fan, A. G. Amin, R. A. Gibbs, C. Hong, X. J. Yu, D. H. Walker, and G. M. Weinstock. 2004. Complete genome sequence of Rickettsia typhi and comparison with sequences of other rickettsiae. J. Bacteriol. 186:5842-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panaram, K., and J. L. Marshall. 2007. F supergroup Wolbachia in bush crickets: what do patterns of sequence variation reveal about this supergroup and horizontal transfer between nematodes and arthropods? Genetica 130:53-60. [DOI] [PubMed] [Google Scholar]
- 39.Pannebakker, B. A., B. Loppin, C. P. H. Elemans, L. Humblot, and F. Vavre. 2007. Parasitic inhibition of cell death facilitates symbiosis. Proc. Natl. Acad. Sci. USA 104:213-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ronquist, F., and J. P. Huelsenbeck. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572-1574. [DOI] [PubMed] [Google Scholar]
- 41.Ros, V. I. D., and J. A. J. Breeuwer. 2007. Spider mite (Acari: Tetranychidae) mitochondrial COI phylogeny reviewed: host plant relationships, phylogeography, reproductive parasites and barcoding. Exp. Appl. Acarol. 42:239-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ros, V. I. D., J. A. J. Breeuwer, and S. B. J. Menken. 2008. Origins of asexuality in Bryobia mites (Acari: Tetranychidae). BMC Evol. Biol. 8:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowley, S. M., R. J. Raven, and E. A. McGraw. 2004. Wolbachia pipientis in Australian spiders. Curr. Microbiol. 49:208-214. [DOI] [PubMed] [Google Scholar]
- 44.Roy, V., and M. Harry. 2007. Diversity of Wolbachia isolated from the Cubitermes sp. affinis subarquatus complex of species (Termitidae), revealed by multigene phylogenies. FEMS Microbiol. Lett. 274:102-111. [DOI] [PubMed] [Google Scholar]
- 45.Sakamoto, J. M., J. Feinstein, and J. L. Rasgon. 2006. Wolbachia infections in the Cimicidae: museum specimens as an untapped resource for endosymbiont surveys. Appl. Environ. Microbiol. 72:3161-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 47.Shapiro, B., A. Rambaut, and A. J. Drummond. 2006. Choosing appropriate substitution models for the phylogenetic analysis of protein-coding sequences. Mol. Biol. Evol. 23:7-9. [DOI] [PubMed] [Google Scholar]
- 48.Stouthamer, R., J. A. J. Breeuwer, and G. D. D. Hurst. 1999. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 53:71-102. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan, J., Z. Abdo, P. Joyce, and D. L. Swofford. 2005. Evaluating the performance of a successive-approximations approach to parameter optimization in maximum-likelihood phylogeny estimation. Mol. Biol. Evol. 22:1386-1392. [DOI] [PubMed] [Google Scholar]
- 50.Swofford, D. L. 2002. PAUP*, Phylogenetic Analysis Using Parsimony (*and Other Methods). Sinauer Associates, Sunderland, MA.
- 51.Swofford, D. L., and J. Sullivan. 2003. Phylogeny inference based on parsimony and other methods using PAUP*, p. 160-206. In M. Salemi and A.-M. Vandamme (ed.), The phylogenetic handbook. A practical approach to DNA and protein phylogeny. Cambridge University Press, Cambridge, United Kingdom.
- 52.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Timmermans, M. J. T. N., J. Marien, D. Roelofs, N. M. van Straalen, and J. Ellers. 2004. Evidence for multiple origins of Wolbachia infection in springtails. Pedobiologia 48:469-475. [Google Scholar]
- 54.Vaishampayan, P. A., D. P. Dhotre, R. P. Gupta, P. Lalwani, H. Ghate, M. S. Patole, and Y. S. Shouche. 2007. Molecular evidence and phylogenetic affiliations of Wolbachia in cockroaches. Mol. Phyl. Evol. 44:1346-1351. [DOI] [PubMed] [Google Scholar]
- 55.Vala, F., J. A. J. Breeuwer, and M. W. Sabelis. 2000. Wolbachia-induced ‘hybrid breakdown’ in the two-spotted spider mite Tetranychus urticae Koch. Proc. R. Soc. Lond. B 267:1931-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vandekerckhove, T. T. M., S. Watteyne, A. Willems, J. G. Swing, J. Mertens, and M. Gillis. 1999. Phylogenetic analysis of the 16S rDNA of the cytoplasmic bacterium Wolbachia from the novel host Folsomia candida (Hexapoda, Collembola) and its implications for wolbachial taxonomy. FEMS Microbiol. Lett. 180:279-286. [DOI] [PubMed] [Google Scholar]
- 57.Weeks, A. R., and J. A. J. Breeuwer. 2001. Wolbachia-induced parthenogenesis in a genus of phytophagous mites. Proc. R. Soc. Lond. B 268:2245-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weeks, A. R., R. Velten, and R. Stouthamer. 2003. Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. Proc. R. Soc. Lond. B 270:1857-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16s ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Werren, J. H., D. Windsor, and L. R. Guo. 1995. Distribution of Wolbachia among neotropical arthropods. Proc. R. Soc. Lond. B 262:197-204. [Google Scholar]
- 61.Werren, J. H., W. Zhang, and L. R. Guo. 1995. Evolution and phylogeny of Wolbachia—reproductive parasites of arthropods. Proc. R. Soc. Lond. B 261:55-63. [DOI] [PubMed] [Google Scholar]
- 62.Wu, M., L. V. Sun, J. Vamathevan, M. Riegler, R. Deboy, J. C. Brownlie, E. A. McGraw, W. Martin, C. Esser, N. Ahmadinejad, C. Wiegand, R. Madupu, M. J. Beanan, L. M. Brinkac, S. C. Daugherty, A. S. Durkin, J. F. Kolonay, W. C. Nelson, Y. Mohamoud, P. Lee, K. Berry, M. B. Young, T. Utterback, J. Weidman, W. C. Nierman, I. T. Paulsen, K. E. Nelson, H. Tettelin, S. L. O'Neill, and J. A. Eisen. 2004. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PloS Biol. 2:327-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeh, D. W., J. A. Zeh, and M. M. Bonilla. 2005. Wolbachia, sex ratio bias and apparent male killing in the harlequin beetle riding pseudoscorpion. Heredity 95:41-49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.