Abstract
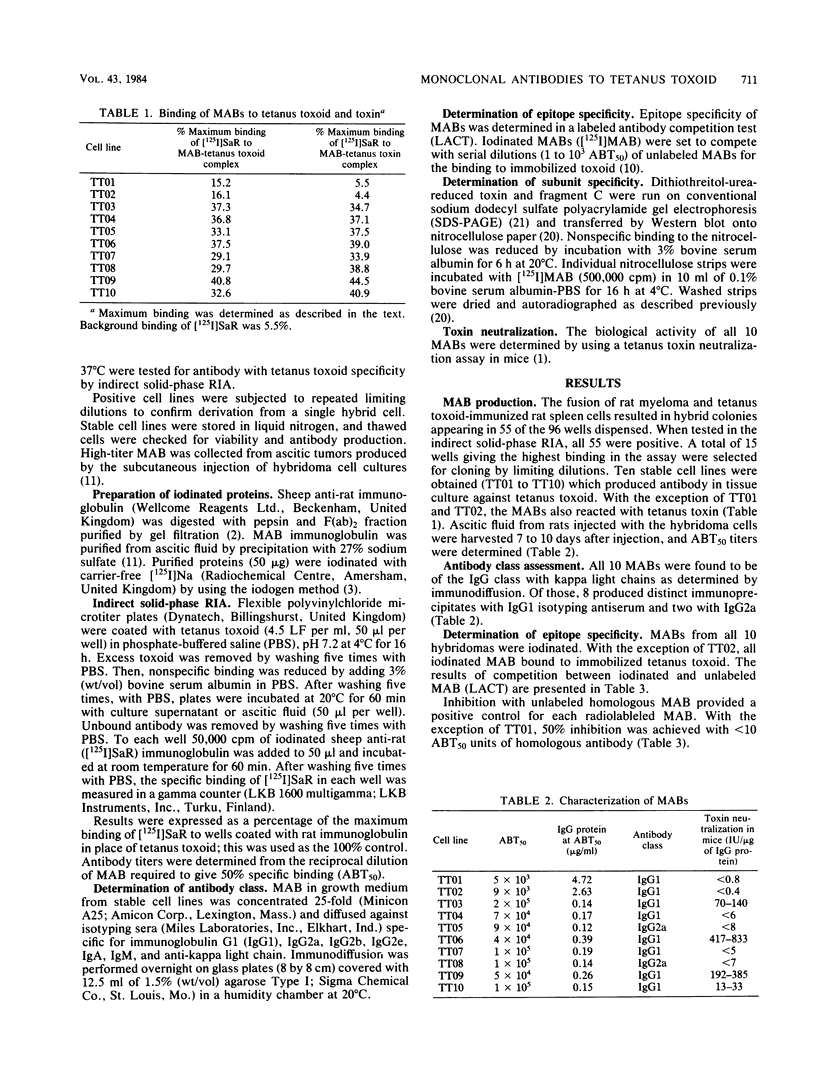
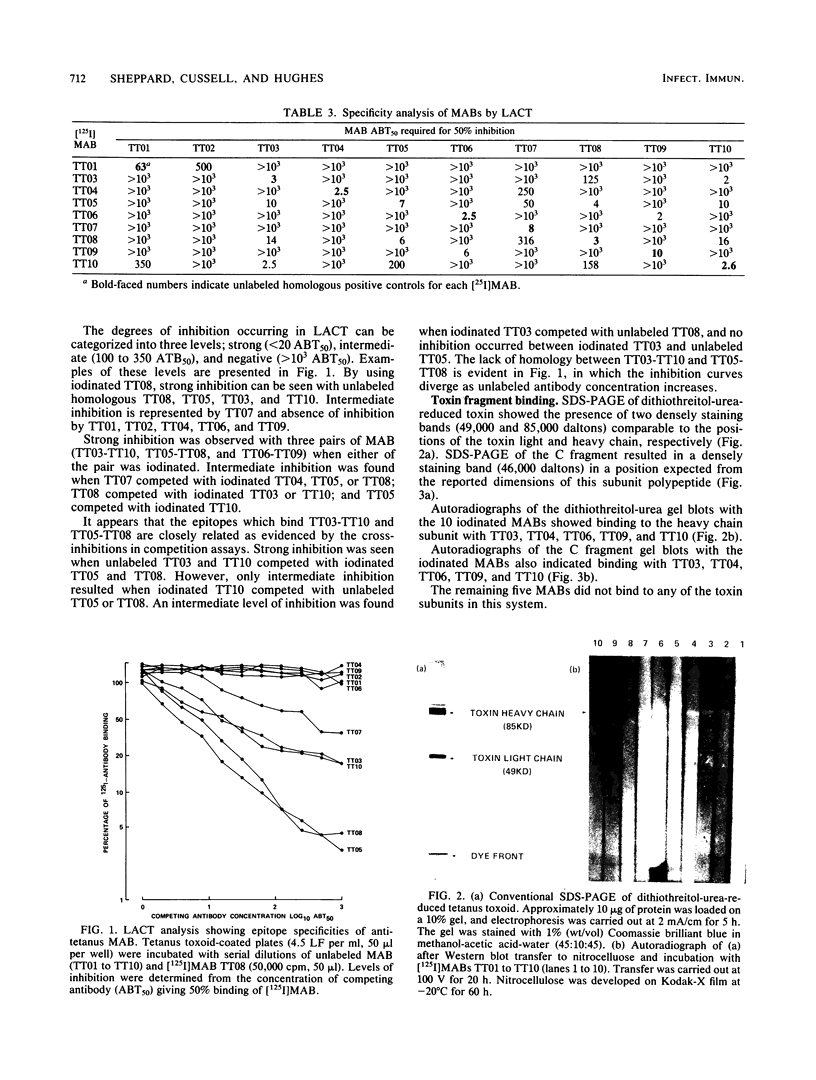
Monoclonal antibodies against tetanus toxin were produced to obtain highly specific antisera. Ten hybridoma cell lines producing monoclonal antibodies were derived from the fusion of rat myeloma cells and spleen cells from rats immunized with tetanus toxoid. Eight produced monoclonal antibodies specific for determinants on toxin and toxoid, whereas two were specific only for determinants on the toxoid. The antibodies produced by hybridomas were characterized by determination of the class of light and heavy chain components, epitope specificity, toxin neutralization, and subunit specificity. All of the antibodies contained kappa light chain, eight contained the gamma 1 heavy chain, and the remaining two contained the gamma 2a heavy chain. Five distinct epitopes were indicated by competition assay of paired monoclonal antibodies, and 4 of the 10 monoclonal antibodies neutralized the in vivo activity of tetanus toxin. The four neutralizing monoclonal antibodies and one other were specific for the C fragment of the heavy chain of the toxin molecule.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barile M. F., Hardegree M. C., Pittman M. Immunization against neonatal tetanus in New Guinea. 3. The toxin-neutralization test and the response of guinea-pigs to the toxoids as used in the immunization schedules in New Guinea. Bull World Health Organ. 1970;43(3):453–459. [PMC free article] [PubMed] [Google Scholar]
- Fey H. Light chains, Fab and F(ab')2 from bovine IgG. An easy method for their preparation as absorbents for class-specific anti-immunoglobulin. Immunochemistry. 1977 Feb;14(2):99–106. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gigliotti F., Insel R. A. Protective human hybridoma antibody to tetanus toxin. J Clin Invest. 1982 Dec;70(6):1306–1309. doi: 10.1172/JCI110730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa S., Uchida T., Mekada E., Moynihan M. R., Okada Y. Monoclonal antibody against diphtheria toxin. Effect on toxin binding and entry into cells. J Biol Chem. 1983 Apr 10;258(7):4311–4317. [PubMed] [Google Scholar]
- Helting T. B., Zwisler O. Structure of tetanus toxin. I. Breakdown of the toxin molecule and discrimination between polypeptide fragments. J Biol Chem. 1977 Jan 10;252(1):187–193. [PubMed] [Google Scholar]
- Helting T. B., Zwisler O., Wiegandt H. Structure of tetanus toxin. II. Toxin binding to ganglioside. J Biol Chem. 1977 Jan 10;252(1):194–198. [PubMed] [Google Scholar]
- Hernandez R., Just M., Bürgin-Wolff A. Immunoglobulin classes of human antitoxin after tetanus vaccination studied by immunofluorescence with agarose bound tetanus toxoid. Z Immunitatsforsch Exp Klin Immunol. 1973 Jun;145(4):376–384. [PubMed] [Google Scholar]
- Hughes M., Thomson R. O., Knight P., Stephen J. The immunopurification of tetanus toxoid. J Appl Bacteriol. 1974 Dec;37(4):603–621. doi: 10.1111/j.1365-2672.1974.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Ivanyi J., Davies P. Monoclonal antibodies against human growth hormone. Mol Immunol. 1980 Feb;17(2):287–290. doi: 10.1016/0161-5890(80)90082-6. [DOI] [PubMed] [Google Scholar]
- Ivanyi J. Study of antigenic structure and inhibition of activity of human growth hormone and chorionic somatomammotropin by monoclonal antibodies. Mol Immunol. 1982 Dec;19(12):1611–1618. doi: 10.1016/0161-5890(82)90272-3. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Hara T., Yoneda M. Proceedings: Structure-function relationship of tetanus toxin: studies on "complementary" polypeptide fragments of the toxin. Jpn J Med Sci Biol. 1975 Oct-Dec;28(5-6):326–328. [PubMed] [Google Scholar]
- Matsuda M., Yoneda M. Antigenic substructure of tetanus neurotoxin. Biochem Biophys Res Commun. 1977 Jul 11;77(1):268–274. doi: 10.1016/s0006-291x(77)80192-7. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Yoneda M. Isolation and purification of two antigenically active, "complimentary" polypeptide fragments of tetanus neurotoxin. Infect Immun. 1975 Nov;12(5):1147–1153. doi: 10.1128/iai.12.5.1147-1153.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel J., Cohen H. Studies on tetanus antitoxins. II. Demonstration of at least four antitoxins of different specificity in antitoxic sera. J Immunol. 1973 May;110(5):1388–1395. [PubMed] [Google Scholar]
- Remmers E. F., Colwell R. R., Goldsby R. A. Production and characterization of monoclonal antibodies to cholera toxin. Infect Immun. 1982 Jul;37(1):70–76. doi: 10.1128/iai.37.1.70-76.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb M., Nichols J. C., Whoriskey S. K., Murphy J. R. Isolation of hybridoma cell lines and characterization of monoclonal antibodies against cholera enterotoxin and its subunits. Infect Immun. 1982 Oct;38(1):267–272. doi: 10.1128/iai.38.1.267-272.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wellhöner N. H. Tetanus neurotoxin. Rev Physiol Biochem Pharmacol. 1982;93:1–68. doi: 10.1007/BFb0032668. [DOI] [PubMed] [Google Scholar]
- Yount W. J., Dorner M. M., Kunkel H. G., Kabat E. A. Studies on human antibodies. VI. Selective variations in subgroup composition and genetic markers. J Exp Med. 1968 Mar 1;127(3):633–646. doi: 10.1084/jem.127.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heyningen S. Binding of ganglioside by the chains of tetanus toxin. FEBS Lett. 1976 Sep 15;68(1):5–7. doi: 10.1016/0014-5793(76)80391-2. [DOI] [PubMed] [Google Scholar]