Abstract
Pyruvate dehydrogenase complex-deficient strains of Corynebacterium glutamicum produce l-valine from glucose only after depletion of the acetate required for growth. Here we show that inactivation of the DeoR-type transcriptional regulator SugR or replacement of acetate by ethanol already in course of the growth phase results in efficient l-valine production.
Corynebacterium glutamicum is a gram-positive organism that grows on a variety of sugars, organic acids, and alcohols and is used for the production of amino acids, such as l-glutamate, l-lysine, and on a small scale also l-valine (6, 11, 12, 14, 16). l-Valine is essential for vertebrates and is used in infusion solutions and cosmetics and as a precursor for the chemical synthesis of herbicides (6, 10, 13). Recently, we engineered C. glutamicum for the production of l-valine by deletion of the gene aceE encoding the E1p enzyme of the pyruvate dehydrogenase complex (PDHC) (3). C. glutamicum ΔaceE produced significant amounts of pyruvate, l-alanine, and l-valine from glucose. Additional plasmid-bound overexpression of the l-valine biosynthetic genes ilvBNCE in C. glutamicum ΔaceE shifted the product spectrum from pyruvate and l-alanine toward l-valine. Deletion of the pyruvate:quinone oxidoreductase, phosphoglucose isomerase, and pyruvate carboxylase genes (pqo, pgi, and pyc, respectively) in C. glutamicum ΔaceE(pJC4ilvBNCE) further improved l-valine production (5). However, a common feature of the PDHC-deficient l-valine-producing strains was that l-valine production was decoupled from growth, i.e., took place only after the depletion of acetate, which is required for growth of all PDHC-deficient C. glutamicum strains. In this study, we succeeded in overcoming the nonproducing phenotype in the growth phase and present PDHC-deficient C. glutamicum strains producing l-valine in the presence of cellular growth.
The strains used in this study were C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) (5) and C. glutamicum ΔaceE Δpqo ΔsugR(pJC4ilvBNCE). Deletion of sugR in C. glutamicum ΔaceE Δpqo was performed as described previously for C. glutamicum ΔsugR (7), except that the selection agar plates additionally contained 85 mM potassium acetate. Deletion of sugR was verified by PCR using the primers sugRfow (5′-GTTCGTCGCGGCAATGATTGACG-3′) and sugRrev (5′-CTCACCACATCCACAAACCACGC-3′). DNA preparation, transformation, determination of amino acids, and preparation of media were performed as described previously (3). Shake flask fermentations were done aerobically at 30°C with 50-ml cultures in 500-ml baffled Erlenmeyer flasks on a rotary shaker (diameter, 5 cm) at 120 rpm. The fed-batch fermentations were performed at 30°C as 200-ml cultures in a fed-batch-pro fermentation system from Dasgip. The pH was maintained at 7.0 by online measurement using a standard pH electrode (Mettler Toledo) and the addition of 2 M KOH and 2 M H2SO4. The dissolved oxygen was measured online by use of a polarimetric oxygen electrode (Mettler Toledo), which was adjusted to 30% saturation in a cascade by stirring at 100 to 1,200 rpm and aerating with 0.2 to 2 liters of air per minute. Foam development was prohibited by manual injection of small amounts of silicon antifoam (Roth). The fermentations were started with 4% (wt/vol) glucose, 0.5% (wt/vol) brain heart infusion (BHI) powder (Merck) plus 1% (wt/vol) acetate or plus 1% (vol/vol) ethanol. During the fed-batch processes, adequate amounts of 50% (wt/vol) glucose, 50% (wt/vol) acetate, or pure ethanol were injected. Glucose, acetate, lactate, and ethanol concentrations were determined by enzymatic tests from Roche Diagnostics.
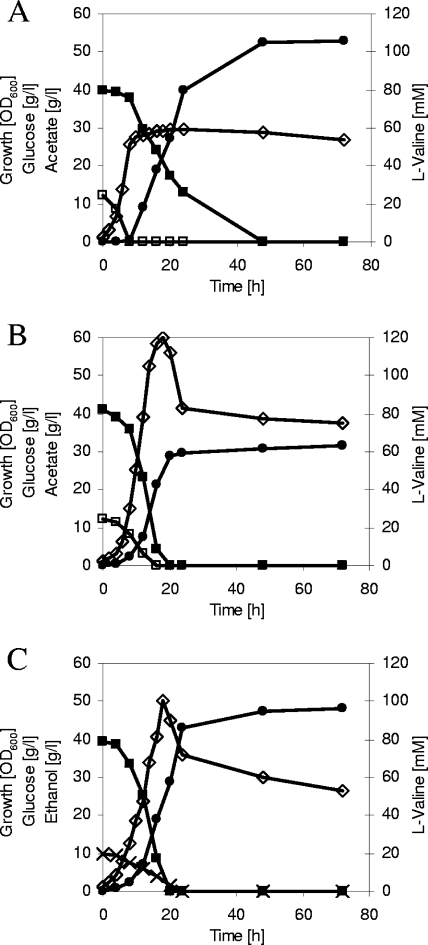
Figure 1A shows the course of a shake flask fermentation of C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE), representative of PDHC-deficient l-valine-overproducing C. glutamicum strains in minimal medium with glucose and acetate. Within 8 h, C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) grew to an optical density at 600 nm (OD600) of about 26, consuming acetate completely and consuming a minor part of glucose. After depletion of acetate, the cells stopped growing, further consumed glucose, and produced more than 100 mM l-valine within 40 h. The cells secreted neither l-alanine nor l-lactate into the medium; however, they formed about 13 mM pyruvate. The growth and fermentation parameters for C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) are summarized in Table 1.
FIG. 1.
l-Valine accumulation during representative shake flask batch cultivations of C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) (A), of C. glutamicum ΔaceE Δpqo ΔsugR(pJC4ilvBNCE) in minimal medium initially containing glucose (4%), potassium acetate (1%), and BHI powder (0.5%) (B), and of C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) in minimal medium initially containing glucose (4%), ethanol (1%), and BHI powder (0.5%) (C). ⋄, growth; ▪, glucose; □, acetate; ×, ethanol; •, l-valine. Three independent fermentations were performed, and all three showed comparable results.
TABLE 1.
Growth rates, substrate consumption rates, YX/S, final l-valine concentrations, and by-product concentrations in shake flask fermentations of C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) and C. glutamicum ΔaceE Δpqo ΔsugR(pJC4ilvBNCE)a
| Strain | Substrates | Growth rate (h−1) | Substrate consumption rate [mmol/(g CDW × h)]b
|
YX/S (g CDW/mol C)c | Final l-valine concn (mM) | By-product (mM)
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Acetate or ethanol | Glucose | l-Alanine | Pyruvate | l-Lactate | |||||
| C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) | Glucose + acetate | 0.38 ± 0.02 | 3.30 ± 0.30 | 0.18 ± 0.04 | 19 ± 3 | 106 ± 9 | <1 | 13 ± 5 | <1 |
| C. glutamicum ΔaceE Δpqo ΔsugR(pJC4ilvBNCE) | Glucose + acetate | 0.31 ± 0.02 | 1.90 ± 0.10 | 0.80 ± 0.05 | 38 ± 7 | 63 ± 4 | 5 ± 2 | 14 ± 6 | 47 ± 10 |
| C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) | Glucose + ethanol | 0.31 ± 0.02 | 1.80 ± 0.12 | 1.05 ± 0.08 | 36 ± 7 | 93 ± 6 | 5 ± 2 | 3 ± 1 | <1 |
Values are means ± standard deviations.
Cell dry weight (CDW) was calculated from the OD600 (after 8 h) using a ratio of 0.3 g CDW liters−1 per OD600 (4). Substrate consumption rates were calculated for acetate, ethanol, and glucose for the first 8 h of fermentation.
YX/S is given as g CDW per mol C from either acetate or ethanol.
Wendisch et al. (17) previously showed that glucose consumption of wild-type C. glutamicum (ATCC 13032) was reduced during growth on glucose plus acetate compared to growth on glucose as the sole carbon source. In accordance, several authors (7, 8, 15) recently identified the DeoR-type regulator SugR to be responsible for acetate-mediated repression of ptsG, ptsI, and ptsH, encoding the glucose-specific enzyme II and the general components enzyme I and Hpr of the phosphoenolpyruvate:sugar phosphotransferase system (PTS). To test whether the repressive effect of SugR on the PTS genes and, thus, a decreased glucose consumption in the presence of acetate are the reasons for the nonproducing phenotype of C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) in the growth phase, we deleted the sugR gene in C. glutamicum ΔaceE Δpqo, transformed the resulting strain with plasmid pJC4ilvBNCE, and performed shake flask fermentations. In minimal medium with glucose and acetate, C. glutamicum ΔaceE Δpqo ΔsugR(pJC4ilvBNCE) grew within 18 h to an OD600 of about 60 and in fact produced about 60 mM l-valine in the growth phase (t = 0 to 20 h) (Fig. 1B). After depletion of both substrates, the OD600 dropped to about 40. In addition to l-valine, the strain secreted small amounts of l-alanine and pyruvate and about 50 mM l-lactate (Table 1). The glucose consumption rate of C. glutamicum ΔaceE Δpqo ΔsugR(pJC4ilvBNCE) in the first 8 h of fermentation was about five times higher than that of C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE), whereas the acetate consumption rate was significantly lower (Table 1). These results indicate an intracellular surplus of the precursor pyruvate in the presence of acetate in the SugR-deficient mutant. In addition, these findings suggest that the repressive effect of SugR on the PTS genes and, in consequence, the reduced glucose consumption rate in the presence of acetate might be responsible for the nonproducing phenotype of C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) during growth.
Although C. glutamicum ΔaceE Δpqo ΔsugR(pJC4ilvBNCE) showed l-valine production in the growth phase, the overall l-valine formation was about 40% lower than that of C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE). Therefore, we tested for an alternative possibility to overcome the nonproduction phenotype of this strain during growth. Since C. glutamicum possesses ethanol and acetaldehyde dehydrogenase activities (1, 2, 9) and since glucose consumption seemed not to be affected by the presence of ethanol in the growth medium (1), we characterized the growth and (by-)product formation of C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) in minimal medium with glucose plus ethanol instead of glucose plus acetate. The cells grew within 18 h to an OD600 of about 50, and within 24 h they produced about 90 mM l-valine and small amounts of l-alanine and pyruvate (Fig. 1C) (Table 1). The glucose consumption rate of C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) in medium containing glucose plus ethanol was about sixfold higher than that in medium containing glucose plus acetate, and the ethanol consumption rate was lower than that of acetate (Table 1). Replacement of acetate in the fermentation medium by ethanol thus represents an elegant way to achieve l-valine production in the growth phase of PDHC-deficient C. glutamicum strains.
Although C. glutamicum ΔaceE Δpqo ΔsugR(pJC4ilvBNCE) on glucose plus acetate and C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) on glucose plus ethanol produced l-valine already in the course of growth, the overall l-valine formation was lower than that with C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) on glucose plus acetate as substrates (Fig. 1A, B, and C). Comparing the data shown in the figures, we speculated that the lower overall l-valine production is due to the higher biomass formation. To further study l-valine production, we carried out comparative fed-batch fermentations with both strains and determined l-valine accumulation and other fermentation parameters (Table 2). As in the shake flask fermentations, we observed a strict separation into growth and production phases with C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) on glucose plus acetate. In contrast, C. glutamicum ΔaceE Δpqo ΔsugR(pJC4ilvBNCE) on glucose plus acetate and C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) on glucose plus ethanol formed l-valine throughout the cultivations (data not shown). The biomass-specific yield (YX/S) of C. glutamicum ΔaceE Δpqo ΔsugR(pJC4ilvBNCE) was higher, and l-valine yield (YP/S) and productivity were about the same; however, the final l-valine concentration was significantly lower than that of C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) (Table 2). In addition, the cells formed significant amounts of l-alanine, pyruvate, and l-lactate (Table 2). When C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) was cultivated in the presence of ethanol instead of acetate, the overall l-valine accumulation and also YX/S were higher, whereas the YP/S and productivity were about the same under both conditions (Table 2). However, the cells cultivated in the presence of ethanol secreted significant amounts of l-alanine and pyruvate (Table 2), indicating an intracellular accumulation of the l-valine precursor pyruvate under these conditions and thus suggesting a further potential to increase l-valine production with C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE).
TABLE 2.
YX/S, final l-valine concentrations, overall YP/S, productivity, and by-product concentrations in fed-batch fermentations of C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) and C. glutamicum ΔaceE Δpqo ΔsugR(pJC4ilvBNCE)a
| Strain | Substrates | Total C consumed (mol C) | Fermentation time (h) | YX/S [g CDW/mol C]b | l-Valine concn (mM) | YP/S (mol-C/mol-C)c | Productivity [mmol/ (g CDW × h)] | By-product (mM)
|
||
|---|---|---|---|---|---|---|---|---|---|---|
| l-Alanine | Pyruvate | l-Lactate | ||||||||
| C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) | Glucose + acetate | 4.57 ± 0.24 | 47 ± 4 | 11 ± 5 | 210 ± 22 | 0.23 ± 0.02 | 0.25 ± 0.03 | <1 | 7 ± 3 | <1 |
| C. glutamicum ΔaceE Δpqo ΔsugR(pJC4ilvBNCE) | Glucose + acetate | 4.70 ± 0.34 | 41 ± 4 | 24 ± 7 | 160 ± 11 | 0.17 ± 0.05 | 0.23 ± 0.01 | 17 ± 3 | 14 ± 6 | 25 ± 16 |
| C. glutamicum ΔaceE Δpqo(pJC4ilvBNCE) | Glucose + ethanol | 7.53 ± 0.41 | 66 ± 5 | 19 ± 5 | 301 ± 27 | 0.20 ± 0.02 | 0.30 ± 0.05 | 26 ± 4 | 20 ± 3 | <1 |
Values are means ± standard deviations.
Cell dry weight (CDW) was calculated from the OD600 (after 8 h) using a ratio of 0.3 g CDW liters−1 per OD600 (4). YX/S is given as g CDW per mol C from either acetate or ethanol.
The overall YP/S were calculated with the total carbon from either acetate plus glucose or ethanol plus glucose.
Taken together, our data show that the nonproduction phenotype in the growth phase of PDHC-deficient C. glutamicum l-valine producers can be overcome either by inactivating of SugR or by replacing acetate by ethanol in the growth medium. However, further studies are necessary to improve the overall l-valine yields and productivity.
Acknowledgments
We thank V. F. Wendisch and V. Engels for providing plasmid pK19mobsacB-ΔsugR.
The support of the Fachagentur Nachwachsende Rohstoffe of the BMVEL (grant no. 04NR004/22000404) is gratefully acknowledged.
Footnotes
Published ahead of print on 16 December 2008.
REFERENCES
- 1.Arndt, A., M. Auchter, T. Ishige, V. F. Wendisch, and B. J. Eikmanns. 2008. Ethanol catabolism in Corynebacterium glutamicum. J. Mol. Microbiol. Biotechnol. 15:222-233. [DOI] [PubMed] [Google Scholar]
- 2.Arndt, A., and B. J. Eikmanns. 2007. The alcohol dehydrogenase gene adhA in Corynebacterium glutamicum is subject to carbon catabolite repression. J. Bacteriol. 189:7408-7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blombach, B., M. E. Schreiner, J. Holátko, T. Bartek, M. Oldiges, and B. J. Eikmanns. 2007. l-Valine production with pyruvate dehydrogenase complex-deficient Corynebacterium glutamicum. Appl. Environ. Microbiol. 73:2079-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blombach, B., M. E. Schreiner, M. Moch, M. Oldiges, and B. J. Eikmanns. 2007. Effect of pyruvate dehydrogenase complex deficiency on l-lysine production with Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 76:615-623. [DOI] [PubMed] [Google Scholar]
- 5.Blombach, B., M. E. Schreiner, T. Bartek, M. Oldiges, and B. J. Eikmanns. 2008. Corynebacterium glutamicum tailored for high-yield l-valine production. Appl. Microbiol. Biotechnol. 79:471-479. [DOI] [PubMed] [Google Scholar]
- 6.Eggeling, L., W. Pfefferle, and H. Sahm. 2001. Amino acids, p. 281-303. In C. Ratledge and B. Kristiansen (ed.), Basic bio/technology. Cambridge University Press, London, United Kingdom.
- 7.Engels, V., and V. F. Wendisch. 2007. The DeoR-type regulator SugR represses expression of ptsG in Corynebacterium glutamicum. J. Bacteriol. 189:2955-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaigalat, L., J. P. Schlüter, M. Hartmann, S. Mormann, A. Tauch, A. Pühler, and J. Kalinowski. 2007. The DeoR-type transcriptional regulator SugR acts as a repressor for genes encoding the phosphoenolpyruvate:sugar phosphotransferase system (PTS) in Corynebacterium glutamicum. BMC Mol. Biol. 8:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotrbova-Kozak, A., P. Kotrba, M. Inui, J. Sajdok, and H. Yukawa. 2007. Transcriptionally regulated adhA gene encodes alcohol dehydrogenase required for ethanol and n-propanol utilization in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 76:1347-1356. [DOI] [PubMed] [Google Scholar]
- 10.Leuchtenberger, W. 1996. Amino acids—technical production and use, p. 465-502. In H.-J. Rehm, G. Reed, A. Pühler, P. Stadler, and M. Roeh (ed.), Biotechnology, vol. 6. VCH, Weinheim, Germany. [Google Scholar]
- 11.Leuchtenberger, W., K. Huthmacher, and K. Drauz. 2005. Biotechnological production of amino acids and derivates: current status and prospects. Appl. Microbiol. Biotechnol. 69:1-8. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama, K., S. Kitada, and S. Kinoshita. 1961. l-Valine production using microbial auxotroph. J. Gen. Appl. Microbiol. (Tokyo) 7:52-69. [Google Scholar]
- 13.Park, J. H., K. H. Lee, T. Y. Kim, and S. Y. Lee. 2007. Metabolic engineering of Escherichia coli for the production of l-valine based on transcriptome analysis and in silico knockout simulation. Proc. Natl. Acad. Sci. USA 104:7797-7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takors, R., B. Bathe, M. Rieping, S. Hans, R. Kelle, and K. Huthmacher. 2007. Systems biology for industrial strains and fermentation processes—example: amino acids. J. Biotechnol. 129:181-190. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka, Y., H. Teramoto, M. Inui, and H. Yukawa. 2008. Regulation of expression of general components of the phosphoenolpyruvate:carbohydrate phosphotransferase system (PTS) by the global regulator SugR in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 78:309-318. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchida, T., F. Yoshinaga, and K. Kubota. 1975. Production of l-valine by 2-thiazolealanine resistant mutants derived from glutamic acid bacteria. Agric. Biol. Chem. (Tokyo) 39:1319-1322. [Google Scholar]
- 17.Wendisch, V. F., A. A. de Graaf, H. Sahm, and B. J. Eikmanns. 2000. Quantitative determination of metabolic fluxes during coutilization of two carbon sources: comparative analyses with Corynebacterium glutamicum during growth on acetate and/or glucose. J. Bacteriol. 182:3088-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]