Abstract
By using cryo-scanning electron microscopy and quantification with lectin-conjugated probes, we have detected the production of exopolysaccharides (EPS) in Bifidobacterium animalis subsp. lactis in the presence of bile. In addition, the expression of gtf01207, which codifies a putative priming glycosyltransferase involved in EPS synthesis, was induced by bile.
The genus Bifidobacterium is a common taxon found in the human gastrointestinal tract which has received special attention because its probiotic effects and its long history of safe use in foods (16). Particularly, the species Bifidobacterium animalis subsp. lactis is widely employed in the formulation of functional dairy products (3). The gut environment presents several biological barriers that this microorganism must overcome, such as the presence of bile. Therefore, intestinal microorganisms have developed several strategies to tolerate physiological bile salt concentrations during the passage through the gut (1).
Bile promotes in Bifidobacterium a very complex response, involving several cellular mechanisms. Study of the bile-adapted strain B. animalis subsp. lactis 4549dOx and its parental strain, IPLA4549, has previously shown that bile response is related to changes in enzymatic profiles, protein expression patterns, and surface properties. Physiological data demonstrated that the acquisition of bile resistance produced a shift in the catabolism of carbohydrates (12, 18). Besides, transmission electron microscopy (TEM) analysis showed that bile promotes the occurrence of surface-displayed vesicle-like structures which could act as a mechanism for detoxification (15).
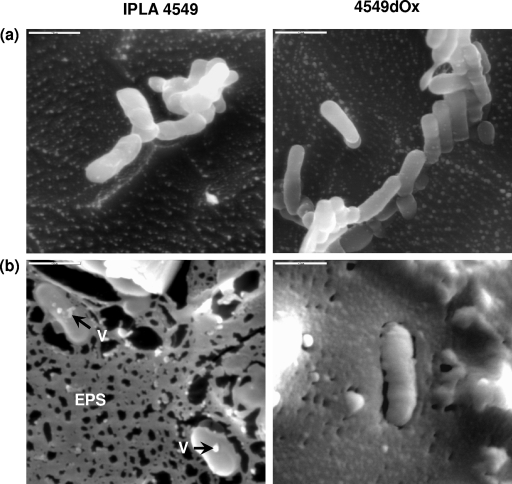
In the present work, we try to gain insight into the physiological changes produced in B. animalis subsp. lactis in the presence of bile. The strains IPLA4549 and 4549dOx were grown in MRSc (MRS broth plus 0.05% [wt/vol] l-cysteine) as previously described (15). Cultures were employed to inoculate (2% [vol/vol]) 100 ml of fresh MRSc broth containing 0, 0.3, and 0.6% (wt/vol) bovine bile (Sigma Chemical Co., St. Louis, MO), which was filtered (0.45-μm pore size) and added to the sterilized media. Strains were cultured for 24 h, and in the absence of bile, both strains showed similar growth rates, whereas, as previously reported (17), in the presence of bile the bile-adapted 4549dOx strain grew faster than the parental strain, IPLA4549. For microscopy, strains were grown until an optical density at 600 nm (OD600) of 0.4 ± 0.05 was reached, which corresponded to the beginning of the exponential growth phase for all strains/conditions tested. The counts obtained at this OD for the parental and bile-adapted strains, respectively, were as follows: in MRSc, 8.31 ± 0.12 and 8.08 ± 0.21 log CFU/ml; in MRSc plus 0.3% bile, 7.32 ± 0.26 and 8.07 ± 0.01 log CFU/ml; and in MRSc plus 0.6% bile, 6.46 ± 0.54 and 7.54 ± 0.04 log CFU/ml. After sample collection, cultures were centrifuged (10,000 × g for 20 min), washed once with Ringer solution, concentrated 10 times, frozen in liquid N2, and stored at −80°C before cryo-scanning electron microscopy (cryo-SEM) analysis (Fig. 1). The most noticeable feature observed in the micrographs was the presence of a matrix surrounding the bacteria which was only evident in cultures grown in the presence of bile salts (data for MRSc plus 0.3% bile not shown). This matrix resembles that of exopolysaccharides (EPS) visualized by cryo-SEM in milk samples fermented with EPS-producing lactic acid bacteria (6). In addition, some vesicle-like structures became evident in the surface of the bacteria, as we had previously noticed through TEM (15).
FIG. 1.
Cryo-SEM micrographs of B. animalis subsp. lactis parent strain IPLA4549 and its bile-adapted 4549dOx mutant growing in MRSc (a) and MRSc plus 0.6% bile (b). Cryo-SEM visualization was done at the Electron Microscopy Unit of Centro de Ciencias Medioambientales (CCMA-CSIC, Madrid, Spain) using a low-temperature scanning electron microscope coupled with an Oxford CT-1500 HF cryo-preparation system (Oxford Instruments, Cambridge, United Kingdom). Samples were prepared by being poured on top of copper holders and then immersed in liquid N2 under vacuum at −200°C. Frozen samples were introduced into the 1500 HF cryo-preparation system, where they were fractured. The samples were etched for 2 min (from −178°C to −90°C) and coated with gold. They were transferred into the observation chamber maintained at −150°C for visualization. Bars, 2 μm. Arrows indicate vesicle-like structures (V).
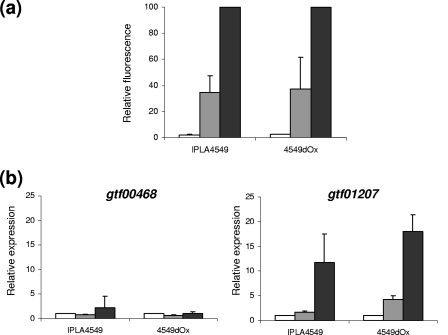
The centrifugation step for cryo-SEM analysis removed part of the loosely attached EPS. Then other methods were employed in order to demonstrate the EPS production in the presence of bile salts. Cultures (100 ml) were obtained under the same conditions previously indicated. From each culture, a sample (500 μl) was mixed with 1 ml of RNAprotect bacterial reagent (Qiagen GmbH, Hilden, Germany) and kept at −20°C until use. The remaining culture was washed, concentrated 10 times, and employed to obtain Ringer solution-standardized (OD600 of 0.2 ± 0.02) cellular suspensions which were dyed with a fluorescence lectin-conjugated probe that selectively binds to α-mannopyranosyl and α-glucopyranosyl residues. Each cellular suspension was mixed to a final concentration of 50 μl/ml (vol/vol) of concanavalin A-Alexa Fluor 488 conjugate (Molecular Probes Invitrogen, Merck, Darmstadt, Germany) dissolved in 0.1 M of sodium bicarbonate. The mixture was kept for 1 h at 4°C in darkness and centrifuged (4,000 × g for 10 min) to eliminate the unattached probe, and the dyed cells were resuspended in the same volume of Ringer solution. Fluorescence emitted (519 nm) by cells after sample excitation (495 nm) was measured in a Cary eclipse fluorescence spectrophotometer (Varian Ibérica S.A., Madrid, Spain). The total protein content of the standardized cellular suspensions was determined using the Lowry method (10). The amount of fluorescence emitted was corrected by the protein concentration of each sample. Finally, the relative fluorescence was referred to the maximum fluorescence value obtained. Experiments were carried out in triplicate, each measured three times. Figure 2a shows a positive correlation between the fluorescence emitted, which corresponds to EPS dyed, and the amount of bile initially present in the culture media. Thus, the presence of bile seems to enhance the production of EPS in both parental and bile-adapted strains.
FIG. 2.
(a) Fluorescence emitted after EPS staining with concanavalin A-Alexa Fluor 488 conjugate by B. animalis subsp. lactis parent strain IPLA4549 and its bile-adapted 4549dOx mutant. Results were referred to the total protein content of the standardized (OD600 of 0.2 ± 0.02) cellular suspension, and the relative fluorescence was calculated with respect to the maximum fluorescence emitted (presence of 0.6% bile). (b) Relative expression of gtf00448 and gtf01207 encoding putative glycosyltransferase from both strains as determined by quantitative reverse transcription-PCR. The expression levels in the presence of different concentrations of bile were referred to those obtained for the control culture (absence of bile). Bars represent bacteria growing in MRSc (white bars), MRSc plus 0.3% bile (gray bars), and MRSc plus 0.6% bile (black bars).
Another approach to assess the effect of bile on EPS production was the measurement of the expression of genes potentially involved in the EPS synthesis. For this purpose the priming glycosyltransferase (p-GTF), that catalyzes the transfer of a sugar-1-phosphate to a lipophilic carrier molecule anchored in the cellular membrane, was chosen. This is the first step in the assembly of the repeating unit that built the polymer (14). Two putative p-GTF genes (gtf00468 and gtf01207 [GenBank accession no. EU651841 and EU651842, respectively]) were identified in the course of a random genome sequencing of B. animalis subsp. lactis NCC2818 (Nestlé Research Centre, Lausanne, Switzerland). Specific primers were designed for gtf00468 (forward, 5′-TGACGACTCGTTTGCAACTGA-3′; and reverse, 5′-GCGCAGGCAGCGGAATAC-3′) and gtf01207 (forward, 5′-CGTGCTGAGTCGAAAGAATCG-3′; and reverse, 5′-TTGTAGAACGTGATCGGCTCA-3′) by using the corresponding sequences. The designed primers were tested for specificity by qualitative PCR using DNA extracts from B. animalis subsp. lactis IPLA4549. The 16S rRNA gene was employed as an endogenous control by using Bifidobacterium-specific primers (5). The RNA was extracted with the RNeasy minikit (Qiagen) following the manufacturer's instructions with minor modifications: the lysis buffer was supplemented with 30 μg/ml lysozyme and 100 U/ml mutanolysin, and the samples were incubated for 30 min with mild stirring. Contaminant DNA was removed by column digestion using the RNase-free DNase set (Qiagen). Then, 3 μg of RNA was reverse transcribed into cDNA and quantitative reverse transcription-PCR was performed as previously described (4). The expression levels in the presence of bile were referred to those obtained for the control culture (absence of bile). Experiments were carried out in triplicate, each measured in duplicate. Figure 2b depicts the relative expression of gtf00468 and gtf01207 in the presence of 0, 0.3, and 0.6% bile. The expression level for gtf00468 was not modified in the presence of bile, but bile induced the expression of gtf01207 in both parental and bile-adapted strains, mainly at the highest concentration of bile tested (0.6%).
Our results indicate that bile promotes the synthesis of EPS in B. animalis subsp. lactis, probably as a mechanism of protection against a toxic compound. Similar findings have been reported for some gram-negative bacteria. The incubation of Vibrio parahaemolyticus in medium supplemented with bile increased the levels of capsular EPS and its adherence (7). In Vibrio cholerae, bile induces the synthesis of biofilm, which was found to be dependent on vps (Vibrio polysaccharide synthesis) genes and the transcriptional activator vpsR (8). This seems to be a mechanism of resistance that allows Vibrio to evade the adverse gut conditions and establish the infection. Recently, it has been demonstrated that genes of the O-antigen capsule-encoding operon are bile induced in Salmonella and that the capsule produced by the enzymes of this operon is specifically required for biofilm formation on cholesterol gallstones (2). Bile salt treatment of Bacteroides fragilis significantly increased coaggregation, adhesion to intestinal epithelial cells, and biofilm formation (11). It has also been demonstrated that bile stimulates biofilm formation in the gram-positive strain Lactobacillus rhamnosus GG (9). In this way, we have shown that EPS produced by probiotics interfere with bacterial adhesion to human intestinal mucus (13). All of these studies indicate that EPS may play a role in the gut that is mainly related to the colonization of this environment. Our study is the first to report the induction of EPS synthesis by bile in the genus Bifidobacterium.
Acknowledgments
This work was financed by FEDER funds (European Union) and the Spanish Plan Nacional de I+D+i through projects AGL2004-06088-CO2-01/ALI, AGL2006-03336, and AGL2007-61805/ALI. M. Gueimonde was funded by a Ramon & Cajal postdoctoral contract from the Spanish Ministry of Education and Science.
F. Pinto from CCMA-CSIC (Madrid) is acknowledged for his excellent technical assistance with the cryo-SEM analysis.
Footnotes
Published ahead of print on 16 December 2008.
REFERENCES
- 1.Begley, M., C. G. M. Gahan, and C. Hill. 2005. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29:625-651. [DOI] [PubMed] [Google Scholar]
- 2.Crawford, R. W., D. L. Gibson, W. W. Kay, and J. S. Gunn. 2008. Identification of bile-induced exopolysaccharide required for Salmonella biofilm formation on gallstone surfaces. Infect. Immun. 76:5341-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gueimonde, M., S. Delgado, B. Mayo, P. Ruas-Madiedo, A. Margolles and C. G. de los Reyes-Gavilán. 2004. Viability and diversity of probiotic Lactobacillus and Bifidobacterium populations included in commercial fermented milks. Food Res. Int. 37:839-850. [Google Scholar]
- 4.Gueimonde, M., L. Noriega, A. Margolles, and C. G. de los Reyes-Gavilán. 2007. Induction of α-l-arabinofuranosidase activity by monomeric carbohydrates in Bifidobacterium longum and ubiquity of encoding genes. Arch. Microbiol. 187:145-153. [DOI] [PubMed] [Google Scholar]
- 5.Gueimonde, M., S. Tölkkö, T. Korpimäki, and S. Salminen. 2004. New real-time quantitative PCR procedure for quantification of bifidobacteria in human fecal samples. Appl. Environ. Microbiol. 70:4165-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassan, A. N., J. F. Frank, and M. Elsoda. 2003. Observation of bacterial exopolysaccharide in dairy products using cryo-scanning electron microscopy. Int. Dairy J. 13:755-762. [Google Scholar]
- 7.Hsieh, Y.-C., S.-M. Liang, W.-L. Tsai, Y.-H. Chen, T.-Y. Liu, and C.-M. Liang. 2003. Study of capsular polysaccharide from Vibrio parahaemolyticus. Infect. Immun. 71:3329-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung, D. T., J. Zhu, D. Sturtevant, and J. J. Mekalanos. 2006. Bile acids stimulate biofilm formation in Vibrio cholerae. Mol. Microbiol. 59:193-201. [DOI] [PubMed] [Google Scholar]
- 9.Lebeer, S., T. L. A. Verhoeven, M. Perea Vélez, J. Vanderleyden, and S. C. J. De Keersmaecker. 2007. Impact of environmental and genetic factors on biofilm formation by the probiotic strain Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 73:6768-6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 11.Pumbwe, L., C. A. Skilbeck, V. Nakano, M. J. Avila-Campos, R. M. F. Piazza, and H. M. Wexler. 2007. Bile salts enhance bacterial co-aggregation, bacterial-intestinal epithelial cell adhesion, biofilm formation and antimicrobial resistance of Bacteroides fragilis. Microb. Pathog. 43:78-87. [DOI] [PubMed] [Google Scholar]
- 12.Ruas-Madiedo, P., A. Hernández-Barranco, A. Margolles, and C. G. de los Reyes-Gavilán. 2005. A bile salt-resistant derivative of Bifidobacterium animalis has an altered fermentation pattern when grown on glucose and maltose. Appl. Environ. Microbiol. 71:6564-6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruas-Madiedo, P., M. Gueimonde, A. Margolles, C. G de los Reyes-Gavilán, and S. Salminen. 2006. Exopolysaccharides produced by probiotic strains modify the adhesion of probiotics and enteropathogens to human intestinal mucus. J. Food Prot. 69:2011-2015. [DOI] [PubMed] [Google Scholar]
- 14.Ruas-Madiedo, P., J. A. Moreno, N. Salazar, S. Delgado, B. Mayo, A. Margolles, and C. G. de los Reyes-Gavilán. 2007. Screening of exopolysaccharide-producing Lactobacillus and Bifidobacterium strains isolated from the human intestinal microbiota. Appl. Environ. Microbiol. 73:4385-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruiz, L., B. Sánchez, P. Ruas-Madiedo, C. G. de los Reyes-Gavilán, and A. Margolles. 2007. Cell envelope changes in Bifidobacterium animalis ssp. lactis as a response to bile. FEMS Microbiol. Lett. 274:316-322. [DOI] [PubMed] [Google Scholar]
- 16.Salminen, S. J., M. Gueimonde, and E. Isolauri. 2005. Probiotics that modify disease risk. J. Nutr. 135:1294-1298. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez, B., C. G. de los Reyes-Gavilán, and A. Margolles. 2006. The F1F0-ATPase of Bifidobacterium animalis is involved in bile tolerance. Environ. Microbiol. 8:1825-1833. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez, B., M.-C. Champomier-Vergès, B. Stuer-Lauridsen, P. Ruas-Madiedo, P. Anglade, F. Baraige, C. G. de los Reyes-Gavilán, E. Johansen, M. Zagorec, and A. Margolles. 2007. Adaptation and response of Bifidobacterium animalis subsp. lactis to bile: a proteomic and physiological approach. Appl. Environ. Microbiol. 73:6757-6767. [DOI] [PMC free article] [PubMed] [Google Scholar]