Abstract
Lignocellulosic feedstocks are thought to have great economic and environmental significance for future biotechnological production processes. For cost-effective and efficient industrial processes, complete and fast conversion of all sugars derived from these feedstocks is required. Hence, simultaneous or fast sequential fermentation of sugars would greatly contribute to the efficiency of production processes. One of the main challenges emerging from the use of lignocellulosics for the production of ethanol by the yeast Saccharomyces cerevisiae is efficient fermentation of d-xylose and l-arabinose, as these sugars cannot be used by natural S. cerevisiae strains. In this study, we describe the first engineered S. cerevisiae strain (strain IMS0003) capable of fermenting mixtures of glucose, xylose, and arabinose with a high ethanol yield (0.43 g g−1 of total sugar) without formation of the side products xylitol and arabinitol. The kinetics of anaerobic fermentation of glucose-xylose-arabinose mixtures were greatly improved by using a novel evolutionary engineering strategy. This strategy included a regimen consisting of repeated batch cultivation with repeated cycles of consecutive growth in three media with different compositions (glucose, xylose, and arabinose; xylose and arabinose; and only arabinose) and allowed rapid selection of an evolved strain (IMS0010) exhibiting improved specific rates of consumption of xylose and arabinose. This evolution strategy resulted in a 40% reduction in the time required to completely ferment a mixture containing 30 g liter−1 glucose, 15 g liter−1 xylose, and 15 g liter−1 arabinose.
In recent years, the need for biotechnological manufacturing based on lignocellulosic feedstocks has become evident (6, 10). In contrast to the readily fermentable, mainly starch- or sucrose-containing feedstocks used in current biotechnological production processes, lignocellulosic biomass requires intensive pretreatment and hydrolysis, which yield complex mixtures of sugars (3, 7, 14, 27). For cost-effective and efficient industrial processes, complete and fast conversion of all sugars present in lignocellulosic hydrolysates is a prerequisite. The major hurdles encountered in implementing these production processes are the conversion of substrates that cannot be utilized by the organism of choice and, even more importantly, the subsequent improvement of sugar conversion rates and product yields.
The use of evolutionary engineering has proven to be very valuable for obtaining phenotypes of (industrial) microorganisms with improved properties, such as an expanded substrate range, increased stress tolerance, and efficient substrate utilization (16, 17). Also, for the yeast Saccharomyces cerevisiae, the preferred organism for large-scale ethanol production for the past few decades, evolutionary engineering has been extensively used to select for industrially relevant phenotypes. For ethanol production from lignocellulose by S. cerevisiae, one of the main challenges is efficient conversion of the pentoses d-xylose and l-arabinose to ethanol. To deal with this challenge, S. cerevisiae strains have been metabolically engineered since the early 1990s for the conversion of xylose into ethanol by the introduction of heterologous xylose utilization pathways (for recent reviews, see references 9 and 20). Arabinose utilization, however, has been addressed only quite recently. The most successful approach for obtaining arabinose consumption in S. cerevisiae has been the introduction of a bacterial arabinose utilization pathway (5, 26). In addition to metabolic engineering, extensive evolutionary engineering (by prolonged cultivation of recombinant S. cerevisiae strains in either anaerobic chemostat or repeated anaerobic batch cultures) was required to obtain S. cerevisiae strains that ferment either xylose (13, 19) or arabinose (5, 26) fast or to improve fermentation performance with mixtures containing glucose and xylose (12). In contrast, (evolutionary) engineering has still not resulted in fast and efficient fermentation of both xylose and arabinose to ethanol by a single recombinant S. cerevisiae strain. At best, simultaneous utilization of xylose and arabinose yielded large amounts of the undesirable side products xylitol and arabinitol (11). Hence, a major remaining challenge is the conversion of both xylose and arabinose with high ethanol production rates and yields.
In a previous study, an S. cerevisiae strain was metabolically engineered to obtain both xylose and arabinose utilization. For this, the Piromyces XylA, S. cerevisiae XKS1, and Lactobacillus plantarum araA, araB, and araD genes, as well as the endogenous genes of the pentose phosphate pathway (RPE1, RKI1, TKL1, and TAL1), were overexpressed. Selection by sequential batch cultivation under conditions with arabinose as the sole carbon source resulted in strain IMS0002, which is capable of fermenting arabinose to ethanol under anaerobic conditions (26). Unfortunately, the ability to ferment xylose to ethanol was largely lost during long-term selection for improved l-arabinose fermentation. During anaerobic batch cultivation of strain IMS0002 in a glucose-xylose-arabinose mixture, xylose was not consumed completely and was converted to almost equimolar amounts of xylitol. This loss of xylose metabolism illustrates the limitations of selection in media supplemented with a single carbon and energy source.
The goal of the present study was to evaluate and optimize selection strategies for evolutionary optimization of the utilization of substrate mixtures. Fermentation of glucose, xylose, and arabinose mixtures by engineered S. cerevisiae strains was used as the model.
MATERIALS AND METHODS
Strains and maintenance.
The S. cerevisiae strains used in this study are listed in Table 1. Culture samples from either shake flasks, chemostats, or (sequential) batch cultivation were prepared by addition of 30% (vol/vol) glycerol and were stored as 2-ml aliquots at −80°C.
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Characteristics | Reference |
|---|---|---|
| IMS0002 | MATaura3-52 leu2-112 loxP-PTPI::(−266,−1)TAL1 gre3::hphMX pUGPTPI-TKL1 pUGPTPI-RPE1 loxP-PTPI::(−40,−1)RKI1 {pRW231, pRW243}; strain harboring Piromyces sp. strain E2 XylA and Lactobacillus plantarum araA and araD on 2μ-based plasmid pRW231 and XKS1 and L. plantarum araB on integration plasmid pRW243; selected for anaerobic growth on l-arabinose | 26 |
| IMS0003 | Single-colony isolate of strain IMS0002 cultivated anaerobically on solid MY-X; capable of cofermenting mixtures of glucose, xylose, and arabinose to ethanol | This study |
| IMS0007 | Single-colony isolate obtained after long-term chemostat cultivation in MY-XA | This study |
| IMS0010 | Single-colony isolate obtained after repeated consecutive batch cultivation in MY supplemented with a mixture of glucose, xylose, and arabinose as the carbon and energy sources, in MY supplemented with a mixture of xylose and arabinose as the carbon and energy sources, and in MY supplemented with arabinose as the sole carbon and energy source | This study |
Media and shake flask cultivation.
Shake flask cultivation was performed at 30°C in synthetic medium (MY) containing 5 g liter−1 (NH4)2SO4, 3 g liter−1 KH2PO4, 0.5 g liter−1 MgSO4·7H2O, 0.05 ml liter−1 silicon antifoam, and trace elements (23). For cultivation in shake flasks, the pH of the medium was adjusted to 6.0 with 2 M KOH prior to sterilization. After heat sterilization (121°C, 20 min), a filter-sterilized vitamin solution (23) and an appropriate carbon and energy source were added. Shake flask cultures were prepared by inoculating 100 ml medium containing the appropriate sugar in a 500-ml shake flask with a frozen stock culture and were incubated at 30°C in an orbital shaker (200 rpm).
Solid MY plates containing 20 g liter−1 xylose (MY-X) or 20 g liter−1 arabinose (MY-A) were prepared by adding 1.5% agar to MY. Plates were incubated at 30°C until growth was observed.
Chemostat cultivation.
Anaerobic chemostat cultivation was carried out at 30°C in 2-liter laboratory fermentors (Applikon, Schiedam, The Netherlands) with a working volume of 1 liter. Cultures were grown in MY supplemented with 0.01 g liter−1 ergosterol, 0.42 g liter−1 Tween 80 dissolved in ethanol (1, 2), silicon antifoam, trace elements (23), and the appropriate carbon and energy source and were maintained at pH 5.0 by automatic addition of 2 M KOH. Cultures were stirred at 800 rpm and sparged with 0.5 liters min−1 nitrogen gas (<10 ppm oxygen). To minimize diffusion of oxygen, the fermentors were equipped with Norprene tubing (Cole Palmer Instrument Company, Vernon Hills, IL). In addition, the presence of oxygen was monitored with an oxygen electrode (Applisens, Schiedam, The Netherlands). After inoculation and completion of the batch phase, chemostat cultivation was initiated by addition of MY containing 20 g liter−1 xylose and 20 g liter−1 arabinose (MY-XA) to the fermentor at a fixed dilution rate. The working volume of the culture was kept constant using an effluent pump controlled by an electric level sensor.
Sequential batch cultivation.
For anaerobic sequential batch cultivation (in sequential batch reactors [SBR]) the fermentor setup and MY composition that were used for chemostat cultivation were used. Each fermentor was filled to obtain a working volume of 1 liter using a feed pump controlled by an electric level sensor. When the carbon and energy source was depleted, as indicated by a CO2 level in the exhaust gas that was less than 0.05% after the CO2 production peak, a new cycle of batch cultivation was initiated by either manual or automated replacement of approximately 90% of the culture with fresh synthetic medium containing the appropriate carbon and energy source. For each cycle, the maximum specific growth rate (μmax) was estimated from the CO2 profile in the exponential growth phase.
Preparation of single-colony-isolate cultures.
Culture samples, either from the chemostat or from sequential batch cultures, were diluted and spread on solid MY-A and incubated at 30°C until colonies appeared. Separate colonies were restreaked twice on solid MY-A. Single colonies were cultivated at 30°C in shake flasks containing 100 ml MY-A. Frozen stock cultures were prepared by addition of sterile 30% (vol/vol) glycerol in the stationary growth phase and storage of 2-ml aliquots at −80°C.
Batch cultivation.
To characterize single-colony isolates selected from the long-term chemostat and sequential batch cultures, anaerobic batch cultivation was performed in 1 liter of MY containing 30 g liter−1 glucose, 15 g liter−1 d-xylose, and 15 g liter−1 l-arabinose, using a fermentor setup similar to that used for the chemostat and sequential batch cultivation experiments. Inocula for the anaerobic batch fermentations were prepared by cultivation at 30°C in shake flasks containing MY-A.
Determination of biomass dry weight.
Culture samples (10.0 ml) were filtered with preweighed nitrocellulose filters (pore size, 0.45 μm; Gelman Laboratory, Ann Arbor, MI). After filtration of the broth, the biomass was washed with demineralized water, dried in a microwave oven for 20 min at 360 W, and weighed. Duplicate determinations varied by less than 1%.
Gas analysis.
Exhaust gas from the anaerobic fermentor cultures was cooled in a condenser (2°C) and dried with a Permapure dryer (type MD-110-48P-4; Permapure, Toms River, NJ). Oxygen and carbon dioxide concentrations were determined with an NGA 2000 analyzer (Rosemount Analytical, Orrville, OH). The exhaust gas flow rate and specific carbon dioxide production rates were determined as described previously (21, 24). When the cumulative carbon dioxide production was calculated, volume changes caused by withdrawing culture samples were taken into account.
Metabolite analysis.
Glucose, xylose, arabinose, xylitol, arabinitol, organic acids, glycerol, and ethanol were analyzed by high-performance liquid chromatography using a Waters Alliance 2690 high-performance liquid chromatograph (Waters, Milford, MA) equipped with a Bio-Rad HPX 87H column (Bio-Rad, Hercules, CA), a Waters 2410 refractive index detector, and a Waters 2487 UV detector. The column was eluted at 60°C with 0.5 g liter−1 sulfuric acid at a flow rate of 0.6 ml min−1.
Carbon recovery.
Carbon recovery was calculated by dividing the amount of carbon in the products formed by the total amount of sugar carbon consumed and was based on a biomass carbon content of 48%. To correct for ethanol evaporation during fermentation, the amount of ethanol produced was assumed to be equal to the measured cumulative production of CO2 minus the CO2 production that occurred due to biomass synthesis (5.85 mmol CO2 per g biomass [22]) and acetate formation.
Rate calculation.
For calculation of the specific rates of glucose, arabinose and xylose consumption, the time-dependent sugar concentrations [y(t)] were fitted with the following sigmoidal equation:
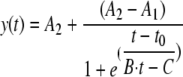 |
where A1 is the initial value (left horizontal asymptote), A2 is the final value (right horizontal asymptote), t0 is the center (point of inflection), and B·t − C is the time-dependent width τ, which was defined as the change in t corresponding to the most significant change in y.
For each time point, the specific sugar consumption rates were calculated by dividing the derivative of the fitted curves by the dry weight.
RESULTS
Cofermentation of glucose, xylose, and arabinose by S. cerevisiae IMS0003.
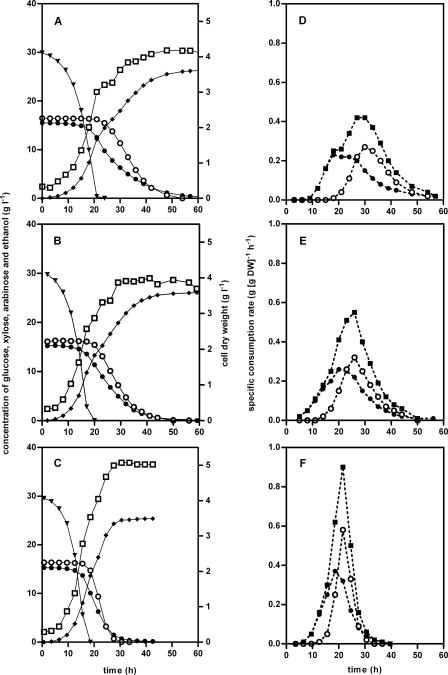
In a previous study, we described construction and evolutionary optimization of S. cerevisiae strain IMS0002, which is capable of fermenting arabinose to ethanol under anaerobic conditions (26). Although this strain was engineered for both xylose and arabinose utilization, the ability to ferment xylose to ethanol was lost during the long-term selection for anaerobic l-arabinose fermentation. The ability to ferment xylose was recovered by anaerobic cultivation of single colonies of strain IMS0002 on solid MY-X at 30°C. Small colonies appeared after 10 days, and after 30 days of incubation, colonies were transferred to solid MY-A again to confirm that the organism could grow on arabinose. A single colony was restreaked twice on solid MY-A, cultivated in a shake flask containing MY-A, and designated strain IMS0003. To verify that there was anaerobic utilization of both xylose and arabinose, strain IMS0003 was cultivated anaerobically in MY supplemented with a mixture containing 30 g liter−1 glucose, 15 g liter−1 d-xylose, and 15 g liter−1 l-arabinose. All sugars present were consumed within approximately 60 h, as shown by the sugar consumption profiles (Fig. 1A). The xylose and arabinose profiles had a long “tail,” indicating that further improvement of the affinity for these sugars is possible.
FIG. 1.
Product formation and sugar consumption (A, B, and C) and specific consumption rates (D, E, and F) during anaerobic batch cultivation of strains IMS0003 (A and D), IMS0007 (B and E), and IMS0010 (C and F) in MY containing a mixture of 30 g liter−1 glucose, 15 g liter−1 d-xylose, and 15 g liter−1 l-arabinose. The data are data from single batch cultivations and are representative of duplicate experiments. To correct for small differences in the initial biomass, the profiles were aligned using the beginning of the glucose consumption peak. Ethanol concentrations were derived from the cumulative CO2 production. (A, B, and C) Symbols: ▾, concentration of glucose; •, concentration of xylose; ○, concentration of arabinose; ⧫, concentration of ethanol; □, dry weight of cells. (D, E, and F) Symbols: •, specific consumption rate for xylose; ○, specific consumption rate for arabinose; ▪, sum of the specific consumption rates for the two pentoses. DW, dry weight of cells.
Minor improvement of the fermentation kinetics by prolonged chemostat cultivation on xylose-arabinose mixtures.
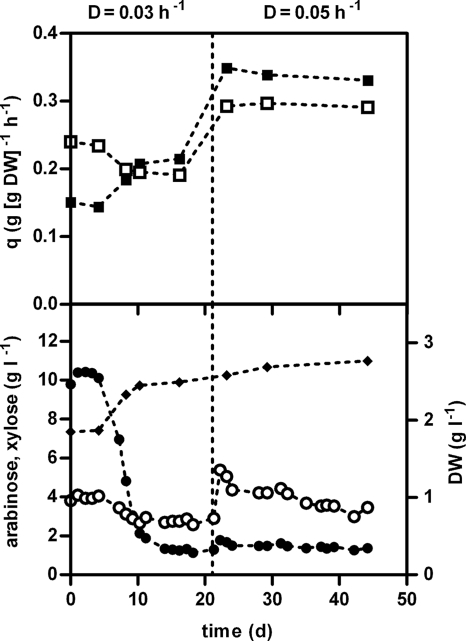
To select for spontaneous mutants with an improved affinity for xylose and arabinose, strain IMS0003 was cultivated in an anaerobic chemostat containing MY-XA at a dilution rate of 0.03 h−1. For the first 6 days, the steady-state xylose and arabinose concentrations were stable at 10.4 and 4.0 g liter−1, respectively (Fig. 2). After this, a decrease in the residual xylose concentration from 10 g liter −1 to approximately 1.3 g liter −1 was observed, while the dry weight of cells increased from 1.9 to 2.5 g liter −1. This resulted in an increase in the specific xylose consumption rate from 0.15 to 0.21 g g (dry weight)−1 h−1. The specific arabinose consumption rate, however, decreased from 0.24 to 0.19 g g (dry weight)−1 h−1 as a result of only a minor decrease in the residual arabinose concentration and the increased dry weight of cells. As expected for chemostat cultivation without drastic shifts in the biomass yield, the sum of the specific consumption rates of the two pentoses remained constant at approximately 0.4 g g (dry weight)−1 h−1 throughout the first 21 days of cultivation.
FIG. 2.
Residual sugar concentrations, dry weights of cells, and specific consumption rates during selective anaerobic chemostat cultivation of S. cerevisiae strain IMS0003 in MY with medium reservoir concentrations of xylose and arabinose of 20 and 20 g liter−1, respectively. The dotted vertical line at 21 days indicates the shift of the dilution rate from 0.03 to 0.05 h−1. Symbols: •, concentration of xylose; ○, concentration of arabinose; ⧫, dry weight of cells; ▪, specific consumption rate for xylose; □, specific consumption rate for arabinose. D, dilution rate; DW, dry weight of cells; q, specific consumption rate.
After 21 days, the dilution rate was increased to 0.05 h−1. After the initial increase in the residual arabinose concentration due to the increased dilution rate, the only significant change during this phase was a decrease in the residual arabinose concentration. After approximately 40 days of selective chemostat cultivation, a sample was removed, and multiple single colonies were isolated. The behavior of cultures resulting from evolutionary engineering can be due to either one dominant strain or a mixture of strains with different phenotypes. To exclude the latter possibility, where none or very few of the individual strains had the phenotype of the combined culture, two randomly selected single-colony isolates were compared to the heterogeneous sample from the chemostat by recording the CO2 production profile during anaerobic batch cultivation in MY supplemented with 30 g liter−1 glucose, 15 g liter−1 d-xylose, and 15 g liter−1 l-arabinose. Since these two single-colony isolates appeared to be representative of the phenotype of the heterogeneous sample, no additional isolates were characterized. The sugar consumption profile of single-colony isolate IMS0007 (Fig. 1B) showed that the total time necessary to consume the three sugars was only approximately 5 h less than the time necessary for strain IMS0003 to consume the three sugars (Fig. 1A).
Fermentation kinetics deteriorated after selective repeated batch cultivation with a xylose-arabinose mixture.
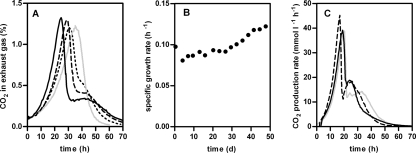
As described above, prolonged cultivation in the chemostat resulted in only a minor improvement in the fermentation performance in medium containing a mixture of glucose, xylose, and arabinose (Fig. 1). To obtain an S. cerevisiae strain with either improved xylose and arabinose coconsumption or faster sequential consumption of both pentose sugars, further selection was performed using automated SBRs. To do this, anaerobic SBR cultivation in MY-XA was performed. The selective SBR cultivation was started by inoculation with a sample from the previous chemostat selection, after which the repeated anaerobic batch cultivation regimen was performed by automated emptying and filling of the fermentor with MY-XA at the end of each batch after both pentoses were consumed. When the CO2 production profiles of the individual sequential batches were compared, a clear increase in the maximum CO2 production rate and a progressively faster increase in CO2 production were observed (Fig. 3A), which were confirmed by the increase in the μmax from 0.08 to 0.13 h−1 (Fig. 3B). Interestingly, the increase in the μmax was accompanied by a gradual shift from a single CO2 production peak in the first cycle of the SBR to a typical diauxic CO2 production profile in cycle 16 (Fig. 3A). Analyses of sugars in supernatant samples from the SBR cultivation showed that the increase in the μmax was a result of increasingly higher xylose consumption rates. The arabinose consumption rates, however, decreased during SBR selection. Clearly, the consumption phases for xylose and arabinose were separated more over time, which eventually resulted in an increase in the total time necessary to completely consume xylose and arabinose from 55 h to more than 70 h (Fig. 3A). The deteriorating arabinose consumption was confirmed by anaerobic batch cultivation of a 100-ml sample from the SBR culture (batch 13) in MY supplemented with 30 g liter−1 glucose, 15 g liter−1 d-xylose, and 15 g liter−1 l-arabinose. Sugar analysis of supernatant samples revealed that the arabinose consumption phase was delayed and longer than that of chemostat isolate IMS0007, which was confirmed by the long “tail” of the CO2 production profile (Fig. 3C).
FIG. 3.
(A and B) CO2 production profiles (A) and estimated μmax derived from CO2 production (B) during repeated anaerobic batch cultivation in MY-XA. Gray line, batch 2; dotted line, batch 7; dashed line, batch 12; solid black line, batch 16. (C) CO2 production profiles during anaerobic batch cultivation in MY containing 30 g liter−1 glucose, 15 g liter−1 d-xylose, and 15 g liter−1 l-arabinose inoculated with a 100-ml sample taken from the repeated batch cultivation (solid black line) compared to strain IMS0003 (solid gray line) and single-colony isolate IMS0007 (dashed line). To correct for small differences in the initial biomass, the profiles were aligned using the beginning of the glucose consumption peak.
Substantially improved fermentation kinetics due to repeated consecutive anaerobic batch cultivation in media with alternating glucose, xylose, and arabinose compositions.
Repeated batch cultivation in medium with a fixed sugar composition (MY-XA) resulted in a shift from coconsumption of xylose and arabinose to more sequential utilization of the two sugars. One explanation of this is that the starting culture started consuming xylose before it started consuming arabinose. As a consequence, the number of generations of growth on xylose was higher than the number of generations of growth on arabinose, resulting in an undesired increase in the selection pressure for faster growth on xylose alone and a reduction in the selection pressure for improved growth on arabinose. To select for cells that exhibited improved rates of consumption of both xylose and arabinose, the selection pressure for utilization of arabinose was enhanced by increasing the number of generations of cells growing on arabinose without compromising the selection pressure for xylose. To accomplish this, a new selective anaerobic SBR cultivation strategy was designed, consisting of three consecutive phases of cultivation in (i) MY containing 20 g liter−1 glucose, 20 g liter−1 xylose, and 20 g liter−1 arabinose (MY-GXA), (ii) MY-XA, and (iii) MY-A.
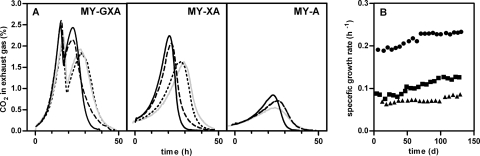
The new SBR selection regimen was started by inoculation with a (whole-broth) sample taken from the selective chemostat cultivation. Consecutive cultivation in MY-GXA, MY-XA, and MY-A resulted in typical CO2 production profiles, as shown by the profiles for cycle 1 in Fig. 4A. During cultivation in MY-GXA, glucose was consumed first, followed by xylose and arabinose, and this resulted in a typical diauxic CO2 production pattern. Xylose and arabinose were consumed simultaneously during batch cultivation in MY-XA, which was illustrated by the single CO2 production peak. When the complete three-phase cycle was repeated 20 times, progressively increasing CO2 production rates were observed for the three medium compositions, which was shown by the gradual increase in the μmax. Within 20 cycles of batch cultivation, the μmax (as determined by CO2 production) increased from 0.19 h−1 to approximately 0.23 h−1 in MY-GXA and from 0.08 to 0.12 h−1 in MY-XA (Fig. 4B). Only a minor increase in the growth rate was observed for batch cultivation with arabinose as the sole carbon source. In addition to the increased μmax values, the total time necessary to consume all sugars present in the medium was substantially reduced from 50 h to approximately 35 h for MY-GXA and from 50 to 30 h for MY-XA (Fig. 4A). Furthermore, the single CO2 production peak for the batch cultivation in MY-XA indicates that the ability to utilize xylose and arabinose simultaneously was preserved during the repeated batch cultivation; this is in contrast to the findings obtained previously with SBR using medium with a fixed sugar composition. In addition to improved coconsumption of xylose and arabinose, the batch cultivation times in medium with only arabinose were shortened from approximately 55 to 40 h.
FIG. 4.
CO2 production profiles (A) and estimated μmax derived from the CO2 production profiles (B) during anaerobic SBR cultivation with repeated cycles consisting of consecutive cultivation in MY-GXA, MY-XA, and MY-A. (A) Gray line, cycle 1; dotted line, cycle 7; dashed line, cycle 13; solid black line, cycle 20. (B) Symbols: •, MY-GXA; ▪, MY-XA; ▴, MY-A.
After 20 cycles of repeated batch cultivation, a sample was removed, and single colonies were isolated by plating on solid MY-A. Two single-colony isolates were characterized to determine their CO2 production profiles with a mixture containing 30 g liter−1 glucose, 15 g liter−1 xylose, and 15 g liter−1 arabinose, and they were found to be representative of the sample obtained directly from the SBR. One of the single-cell isolates, designated strain IMS0010, was used for further characterization. Comparison of the sugar consumption and ethanol production profiles of strains IMS0007 and IMS0010 (Fig. 1B and 1C) revealed that the total time necessary to ferment the glucose-xylose-arabinose mixture was reduced from 55 h to approximately 35 h.
Fermentation characteristics and kinetics of strains selected for improved mixed-sugar fermentation.
To compare physiological characteristics of the S. cerevisiae strains obtained by the different selection methods described in this paper, anaerobic batch cultivation of strains IMS0003, IMS0007, and IMS0010 in mixtures containing 30 g liter−1 glucose, 15 g liter−1 xylose, and 15 g liter−1 arabinose was performed (Fig. 1). Strain IMS0003 was able to consume the sugar mixture within approximately 65 h (Fig. 1A), and the maximum specific consumption rates (qmax) were 0.27 and 0.23 g g (dry weight)−1 h−1 for xylose and arabinose, respectively (Fig. 1D and Table 2). As expected from the marginal reduction in total fermentation time after chemostat selection, no drastic changes in sugar consumption occurred when IMS0003 and IMS0007 were compared. While the qmax for xylose increased from 0.21 to 0.31 g g (dry weight)−1 h−1, the qmax for arabinose did not change significantly (Table 2). The slightly improved coconsumption of both pentoses observed for IMS0007 is illustrated by the increased sum of the two pentose-specific consumption rates (Fig. 1E) compared to that of IMS0003.
TABLE 2.
Products yields, levels of carbon recovery, and specific consumption rates determined for anaerobic batch cultivation of strains IMS0003, IMS0007, and IMS0010 in MY supplemented with 30 g liter−1 glucose, 15 g liter−1 xylose, and 15 g liter−1 arabinosea
| Strain | Yield (g g−1 of total sugar)
|
Carbon recovery (%) | Observed qmax (g g [dry wt]−1 h−1)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomass | CO2 | Ethanolb | Glycerol | Acetate | Lactate | Pyruvate | Succinate | Xylose | Arabinose | ||
| IMS0003 | 0.060 ± 0.003 | 0.44 ± 0.01 | 0.44 ± 0.01 | 0.071 ± 0.002 | 0.007 ± 0.000 | 0.006 ± 0.000 | 0.001 ± 0.000 | 0.004 ± 0.000 | 103 ± 3 | 0.21 ± 0.03 | 0.23 ± 0.06 |
| IMS0007 | 0.056 ± 0.001 | 0.44 ± 0.00 | 0.44 ± 0.00 | 0.078 ± 0.002 | 0.011 ± 0.001 | 0.006 ± 0.000 | 0.001 ± 0.000 | 0.005 ± 0.001 | 103 ± 2 | 0.31 ± 0.08 | 0.28 ± 0.07 |
| IMS0010 | 0.075 ± 0.002 | 0.43 ± 0.01 | 0.43 ± 0.01 | 0.078 ± 0.000 | 0.003 ± 0.000 | 0.007 ± 0.000 | 0.001 ± 0.000 | 0.003 ± 0.000 | 102 ± 2 | 0.35 ± 0.02 | 0.53 ± 0.05 |
The values are the averages ± the standard deviations for two (IMS0003 and IMS0007) or three (IMS0010) independent anaerobic batch fermentations.
The amounts ethanol were derived from the cumulative CO2 production.
The strain resulting from repeated consecutive batch cultivation with sugar mixtures having alternating compositions (IMS0010) exhibited clearly improved fermentation performance. While the qmax for xylose, 0.35 g g (dry weight)−1 h−1, was comparable to that of strain IMS0007, arabinose was consumed with a qmax of 0.53 g g (dry weight)−1 h−1, which corresponded to a twofold increase compared to strain IMS0007 (Fig. 1F and Table 2). As a result, the sum of the specific consumption rates of the two pentoses increased 1.5-fold (Fig. 1F), which contributed to a great extent to the reduction in the total fermentation time from 55 h to approximately 35 h (Fig. 1C).
All three xylose- and arabinose-consuming strains described in this study exhibited high ethanol yields (0.43 to 0.44 g g−1 of total sugar) (Table 2). In line with these yields, the levels of xylitol and arabinitol remained below the detection level. This indicated that the ethanol yields were not (negatively) affected either by the chemostat selection or the selective sequential batch cultivation. The biomass yield of strain IMS0010, however, was significantly higher than that of strains IMS0003 and IMS0007. The increased biomass yield of strain IMS0010 can be explained as an effect of a decreased cumulative energy requirement for maintenance as a result of the decreased fermentation times.
DISCUSSION
The use of evolutionary engineering has proven to be a very successful tool for selecting for recombinant S. cerevisiae strains capable of anaerobically utilizing sugars such as xylose and arabinose (5, 13, 19, 26). Natural selection even enabled a non-metabolically engineered S. cerevisiae strain to utilize xylose as a sole carbon and energy source, which demonstrates that trace activities of enzymes in the targeted metabolic route can result in growth, provided that strong selection pressure is applied (4). Hitherto, research on evolutionary engineering of pentose utilization by S. cerevisiae has focused primarily on the use of single sugars, and there have been only a few examples of improved utilization of multiple sugars (12). Improvement of the utilization of multiple substrates has specific challenges, as it is rather complicated to select for multiple mutations in different metabolic routes that require different kinds of (potentially conflicting) selective pressure (17). In line with this observation, previous evolutionary engineering for anaerobic utilization of arabinose as the sole carbon source resulted in the loss of the xylose-utilizing capacities of an engineered S. cerevisiae strain (26).
Given the complexity of biomass hydrolysates, evolutionary engineering strategies that enable efficient utilization of mixtures containing three (or more) different sugars are required. The sequencing batch evolution strategy with 20 g liter−1 xylose and 20 g liter−1 arabinose (Fig. 3) clearly demonstrated that a slight preference for xylose over arabinose resulted in increasingly more generations on xylose and thus a shift of the selection pressure to the preferred sugar. Although, as a result, growth on xylose improved dramatically, the deteriorated kinetics of arabinose utilization resulted in overall less favorable fermentation characteristics. If the expression of a native or heterologous pathway for a less-preferred substrate confers even a slight selective disadvantage (for example, via protein burden [18]), repeated cultivation on a fixed substrate mixture may ultimately select for strains that exhibit the well-known phenomenon of diauxic growth (15). To minimize such unequal selection pressure on the utilization of glucose, xylose, and arabinose, a novel selection strategy involving consecutive anaerobic batch cultivation in MY-GXA, MY-XA, and MY-A was used. This selection strategy allows more even distribution of the number of generations grown on each carbon source, even when cells have a preference for one substrate over another.
The regimen involving repetitive consecutive cultivation with the three different sugar mixtures yielded an S. cerevisiae strain (strain IMS0010) that exhibited rapid anaerobic fermentation of mixtures of glucose, xylose, and arabinose to ethanol. The underlying genetic changes involved in the improvement in xylose and arabinose utilization by the evolved strain IMS0010 remain to be investigated. The possible changes include mutations that resulted in improved codon usage of introduced genes, as codon optimization for AraA, AraB, and AraD in engineered S. cerevisiae strains has been shown to result in improved arabinose conversion rates (25). Alternatively, changes in plasmid copy numbers may have played a role in fine-tuning the levels of expression of introduced genes, as described previously for S. cerevisiae strains evolved for lactose utilization (8). Interestingly, the specific combined pentose consumption rate of IMS0010 nearly equals the maximum xylose consumption rate of RWB218 with glucose-xylose mixtures (12), which might indicate that there is a common flux-controlling step during pentose utilization by these strains.
In plant biomass hydrolysates, xylose, and especially arabinose comprise only a small fraction of the total carbon. The use of such hydrolysates for evolutionary engineering would limit the selection pressure for the substrates that are less preferred by the organism of choice and therefore might not result in improved kinetics for utilization of the minor components of the medium. A simple solution would be to add these substrates to the hydrolysates in various stages of the evolutionary engineering process to obtain enough generations on the desired substrates. In this way, rapid consumption of the less predominant substrates, which often are crucial for the overall process economics, in the production environment may be achieved. Equal selection pressures for multiple sugars can be achieved in multiple ways, and the fermentation setup described above is not unique. For example, repeated cultivation with a (fixed) mixture of 5 g liter−1 glucose, 15 g liter−1 xylose, and 45 g liter−1 arabinose would also result in a more even distribution of the selection pressures. However, although the number of generations is expected to be similar, the simultaneous presence of various sugars at high (repressing) concentrations in the strategy described in this paper add selective pressure for rapid subsequent or even partially simultaneous use of the substrates.
To our knowledge, the strategy described here for improving the utilization of mixtures of three or more substrates via consecutive batch cultivation in media with alternating sugar compositions has not been described previously. Moreover, although the strategy has to be tested in practice, its applications do not seem to be limited to the selection of improved S. cerevisiae phenotypes for ethanol production; this strategy might also be applicable to other microorganisms used in (industrial) biotechnological processes based on the conversion of lignocellulosic hydrolysates or other substrate mixtures.
Acknowledgments
This project was financially supported by The Netherlands Ministry of Economic Affairs and the B-Basic partner organizations (www.b-basic.nl) through B-Basic, a public-private NWO-Advanced Chemical Technologies for Sustainability program. The Kluyver Centre for Genomics of Industrial Fermentation is supported by The Netherlands Genomics Initiative.
We thank Eleonora Bellissimi for sharing her expertise on repeated batch cultivation and for assistance during overnight batch cultivation.
Footnotes
Published ahead of print on 12 December 2008.
REFERENCES
- 1.Andreasen, A. A., and T. J. Stier. 1953. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J. Cell Physiol. 41:23-36. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen, A. A., and T. J. Stier. 1954. Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated fatty acid requirement for growth in a defined medium. J. Cell Physiol. 43:271-281. [DOI] [PubMed] [Google Scholar]
- 3.Aristidou, A., and M. Penttilä. 2000. Metabolic engineering applications to renewable resource utilization. Curr. Opin. Biotechnol. 11:187-198. [DOI] [PubMed] [Google Scholar]
- 4.Attfield, P. V., and P. J. L. Bell. 2006. Use of population genetics to derive nonrecombinant Saccharomyces cerevisiae strains that grow using xylose as a sole carbon source. FEMS Yeast Res. 6:862-868. [DOI] [PubMed] [Google Scholar]
- 5.Becker, J., and E. Boles. 2003. A modified Saccharomyces cerevisiae strain that consumes l-arabinose and produces ethanol. Appl. Environ. Microbiol. 69:4144-4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dale, B. E. 2003. ‘Greening’ the chemical industry: research and development priorities for biobased industrial products. J. Chem. Technol. Biotechnol. 78:1093-1103. [Google Scholar]
- 7.Galbe, M., and G. Zacchi. 2002. A review of the production of ethanol from softwood. Appl. Microbiol. Biotechnol. 59:618-628. [DOI] [PubMed] [Google Scholar]
- 8.Guimaraes, P. M. R., J. Francois, J. L. Parrou, J. A. Teixeira, and L. Domingues. 2008. Adaptive evolution of a lactose-consuming Saccharomyces cerevisiae recombinant. Appl. Environ. Microbiol. 74:1748-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahn-Hägerdal, B., K. Karhumaa, C. Fonseca, I. Spencer-Martins, and M. F. Gorwa-Grauslund. 2007. Towards industrial pentose-fermenting yeast strains. Appl. Microbiol. Biotechnol. 74:937-953. [DOI] [PubMed] [Google Scholar]
- 10.Horvath, I. T., and P. T. Anastas. 2007. Innovations and green chemistry. Chem. Rev. 107:2169-2173. [DOI] [PubMed] [Google Scholar]
- 11.Karhumaa, K., B. Wiedemann, B. Hahn-Hägerdal, E. Boles, and M. F. Gorwa-Grauslund. 2006. Co-utilization of l-arabinose and d-xylose by laboratory and industrial Saccharomyces cerevisiae strains. Microb. Cell Fact. 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuyper, M., M. J. Toirkens, J. A. Diderich, A. A. Winkler, J. P. Van Dijken, and J. T. Pronk. 2005. Evolutionary engineering of mixed-sugar utilization by a xylose-fermenting Saccharomyces cerevisiae strain. FEMS Yeast Res. 5:925-934. [DOI] [PubMed] [Google Scholar]
- 13.Kuyper, M., A. A. Winkler, J. P. Van Dijken, and J. T. Pronk. 2004. Minimal metabolic engineering of Saccharomyces cerevisiae for efficient anaerobic xylose fermentation: a proof of principle. FEMS Yeast Res. 4:655-664. [DOI] [PubMed] [Google Scholar]
- 14.Lee, R. L. 1997. Overview and evaluation of fuel ethanol from cellulosic biomass: technology, economics, the environment and policy. Annu. Rev. Energy Environ. 21:403-465. [Google Scholar]
- 15.Monod, J. 1945. Sur la nature du phenomene de diauxie. Ann. Inst. Pasteur Microbiol. 71:37-40. [Google Scholar]
- 16.Patnaik, R. 2008. Engineering complex phenotypes in industrial strains. Biotechnol. Prog. 24:38-47. [DOI] [PubMed] [Google Scholar]
- 17.Sauer, U. 2001. Evolutionary engineering of industrially important microbial phenotypes. Adv. Biochem. Eng. Biotechnol. 73:129-169. [DOI] [PubMed] [Google Scholar]
- 18.Snoep, J. L., L. P. Yomano, H. V. Westerhoff, and L. O. Ingram. 1995. Protein burden in Zymomonas mobilis—negative flux and growth control due to overproduction of glycolytic enzymes. Microbiology 141:2329-2337. [Google Scholar]
- 19.Sonderegger, M., and U. Sauer. 2003. Evolutionary engineering of Saccharomyces cerevisiae for anaerobic growth on xylose. Appl. Environ. Microbiol. 69:1990-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Maris, A. J. A., D. A. Abbott, E. Bellissimi, J. van den Brink, M. Kuyper, M. A. H. Luttik, H. W. Wisselink, W. A. Scheffers, J. P. Van Dijken, and J. T. Pronk. 2006. Alcoholic fermentation of carbon sources in biomass hydrolysates by Saccharomyces cerevisiae: current status. Antonie van Leeuwenhoek 90:391-418. [DOI] [PubMed] [Google Scholar]
- 21.Van Urk, H., P. R. Mak, W. A. Scheffers, and J. P. Van Dijken. 1988. Metabolic responses of Saccharomyces cerevisiae CBS 8066 and Candida utilis CBS 621 upon transition from glucose limitation to glucose excess. Yeast 4:283-291. [DOI] [PubMed] [Google Scholar]
- 22.Verduyn, C., E. Postma, W. A. Scheffers, and J. P. Van Dijken. 1990. Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J. Gen. Microbiol. 136:395-403. [DOI] [PubMed] [Google Scholar]
- 23.Verduyn, C., E. Postma, W. A. Scheffers, and J. P. Van Dijken. 1992. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 8:501-517. [DOI] [PubMed] [Google Scholar]
- 24.Weusthuis, R. A., W. Visser, J. T. Pronk, W. A. Scheffers, and J. P. Van Dijken. 1994. Effects of oxygen limitation on sugar metabolism in yeasts—a continuous-culture study of the Kluyver effect. Microbiology 140:703-715. [DOI] [PubMed] [Google Scholar]
- 25.Wiedemann, B., and E. Boles. 2008. Codon-optimized bacterial genes improve l-arabinose fermentation in recombinant Saccharomyces cerevisiae. Appl. Environ. Microbiol. 74:2043-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wisselink, H. W., M. J. Toirkens, M. R. Franco Berriel, A. A. Winkler, J. P. Van Dijken, J. T. Pronk, and A. J. A. van Maris. 2007. Engineering of Saccharomyces cerevisiae for efficient anaerobic alcoholic fermentation of l-arabinose. Appl. Environ. Microbiol. 73:4881-4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaldivar, J., J. Nielsen, and L. Olsson. 2001. Fuel ethanol production from lignocellulose: a challenge for metabolic engineering and process integration. Appl. Microbiol. Biotechnol. 56:17-34. [DOI] [PubMed] [Google Scholar]