Abstract
Accelerating hippocampal sprouting by making unilateral progressive lesions of the entorhinal cortex spared the spatial memory of rats tested for retention of a learned alternation task. Subsequent transection of the sprouted crossed temporodentate pathway (CTD), as well as a simultaneous CTD transection and progressive entorhinal lesion, produced a persistent deficit on the memory task. These results suggest that CTD sprouting, which is homologous to the original perforant path input to the dentate gyrus of the hippocampus, is behaviorally significant and can ameliorate at least some of the memory deficits associated with hippocampal deafferentation.
Keywords: hippocampal formation, learned alternation, neuroplasticity, recovery of function, Y maze
To account for recovery of function observed after human brain trauma, clinicians often invoke concepts such as functional reorganization or functional substitution (1–3). Although the notion that the central nervous system (CNS) may undergo reorganization to compensate for the loss of some behavioral capacity is appealing, the experimental support for such a notion is scant. Numerous investigations over the last two decades, however, have established that neurons surviving an insult to the CNS may undergo extensive terminal proliferation and form functional contacts with cells deprived of their original inputs (4, 5). Given the ubiquitous nature of this “sprouting” response, the possibility exists that the formation of new contacts after brain injury may be a neural substrate mediating functional reorganization. The wealth of studies documenting the neuroplasticity of the CNS notwithstanding, the behavioral significance of these new connections remains as yet unclear.
Lesion-induced sprouting by the crossed temporodentate pathway (CTD) in rats has been implicated in the recovery of spatial memory after deafferentation of the hippocampus, a structure which appears to be crucial for learning and memory (6–10). Following a unilateral lesion of the entorhinal cortex (EC), at least three hippocampal inputs expand their synaptic fields to reinnervate the denervated dentate gyrus (DG) of the hippocampus (4, 5). These are the crossed entorhinal projection (i.e., the CTD), the acetylcholinesterase-containing septodentate pathway, and the commissural/associational (C/A) inputs. The CTD, which projects to the rostral dentate via the dorsal psalterium (DP), is particularly interesting. It originates in the contralateral homologue of the damaged area and, upon sprouting 6 to 10 days postlesion, shares many of the physiological characteristics of the original input, the perforant path. The proliferated CTD input: (i) is capable of discharging the granule cells, the targets of the perforant path (11); (ii) exhibits habituation-like decrements in transmission reminiscent of the normal ipsilateral input (12); and (iii) potentiates population excitatory postsynaptic potentials of the granule cells as does the perforant path (13).
If sprouting by the crossed entorhinal pathway contributes to recovery of function, procedures that accelerate this sprouting should concomitantly accelerate behavioral recovery. Scheff et al. (14, 15) have demonstrated that progressive entorhinal lesions (i.e., two-stage lesions in the same hemisphere) accelerate septodentate and C/A sprouting to the DG, but their effect on recovery from memory deficits is unknown. Also unclear is the effect of progressive lesions on CTD sprouting. This study had three goals: (i) to determine whether performing EC lesions in two stages would accelerate recovery from a deficit in spatial memory, as would be expected if sprouting supports behavioral recovery; (ii) to determine, using quantitative autoradiography, if CTD sprouting is accelerated in parallel with that of the other pathways; and (iii) to determine if CTD sprouting is essential to behavioral recovery by transecting the pathway either after recovery or at the time of the final entorhinal lesion.
METHODS
For the behavioral study, male Sprague–Dawley rats (350– 400 g) were randomly assigned to one of four treatment conditions: (i) priming lesion of the lateral EC followed by secondary lesion of remaining EC (Prog; n = 12); (ii) priming lesion of the lateral EC followed by secondary lesion of remaining EC and simultaneous transection of the DP to cut the CTD (Prog/Tran; n = 7); (iii) sham operation followed by one-stage EC lesion (One-stage; n = 15); (iv) sham operation followed by sham operation (Sham; n = 22). Since Scheff et al. (15) demonstrated that a 4- to 10-day interoperation interval (IOI) accelerated the rate of septodentate and C/A sprouting in the DG most effectively, an IOI of 6 days was used in this study.
After random assignment to experimental and control conditions, rats were pretrained and tested for retention of a learned alternation task on a Y maze according to our standard procedures (16). Alternation was reinforced with three 45-mg Noyes pellets, and the intertrial interval was 40 sec. All the animals were tested daily, received surgery approximately 24 hr after attaining criterion performance (3 consecutive days of at least 80% alternation), and were given 2 days to recover from the surgery. After the first operation, the rats were tested for retention of the alternation task during the last 3 days of the IOI to assess the behavioral effects of the surgery. Subsequently, the animals were subjected to a second operation and were retested; the Prog/Tran group was tested for a maximum of 20 days after surgery. Upon reaching criterion performance again, a random sample of the animals were given DP transections and were tested for a 20-day maximum (Sham n = 16; Prog n = 5; One-stage n = 7).
Immediately prior to surgery, the rats were injected intraperitoneally with 0.1 ml of 0.54 mg/ml atropine sulfate followed by sodium pentobarbital (Nembutal, 50 mg/kg) anesthetic. In surgery, the animals sustained sham operations, one-stage EC lesions, progressive EC lesions, and/or DP transections. Stereotaxic coordinates for the electrolytic unilateral EC lesion were 1.5 mm anterior to the transverse sinus; 3, 4, and 5 mm lateral to the sagittal sinus; and 2, 4, and 6 mm ventral from dura (6). The priming EC lesion was made at the lateral-most set of coordinates (i.e., at 5 mm). To transect the DP in the hemisphere contralateral to the EC lesion, a Beaver Eye blade (Beaver Surgical Products, Waltham, MA) was dropped 4.5 mm deep from dura at bregma and 0.5 mm lateral to the sagittal sinus. The blade was drawn back to 1 mm anterior to lambda and subsequently withdrawn (6). Sham operations consisted of craniotomies of the skull overlying the entorhinal area.
At the termination of testing, the rats were overdosed with Nembutal and were perfused transcardially with physiological saline (0.9%) followed by 10% buffered formalin. The tissue was frozen-sectioned in 30-μm-thick sections, stained with cresyl violet acetate, and coverslipped to determine the extent of the lesions.
For the anatomical study, we examined the time course of the sprouting response at 4, 6, or 8 days postlesion. Male Sprague–Dawley rats (350–400 g) were randomly assigned to one of four treatment conditions wherein unilateral entorhinal lesions in combination with contralateral [3H]proline injections [10 μCi (370 kBq) in 1 μl of 0.9% saline, 6-day survival] into intact medial EC or unilateral [3H]proline injections alone into intact medial EC were made: (i) a priming lesion (lateral EC) followed by both an ipsilateral secondary lesion (Prog; 4 day, n = 10; 6 day, n = 9; 8 day, n = 7) and a [3H]proline injection; (ii) a one-stage unilateral EC lesion (One-stage; 4 day, n = 10; 6 day, n = 10; 8 day, n = 11) and a [3H]proline injection; (iii) a normal control group (Control; n = 7) with a unilateral [3H]proline injection into EC; (iv) a unilateral priming lesion of lateral EC followed by a [3H]proline injection (Prim; 4 day, n = 9; 6 day, n = 10; 8 day, n = 8). The experimental animals were sacrificed at 4, 6, or 8 days after the IOI. The control animals were killed 6 days after the [3H]proline injection. Group Prim was included to determine whether the priming lesion itself initiates a sprouting response. To make the behavioral and anatomical studies equivalent, we used a 6-day IOI for the anatomical study as well as an identical surgical procedure to ablate the EC. The quantitative autoradiographic procedures used were those of Steward and Loesche (17).
We counted the number of silver grains in the terminal field of the entorhinal inputs to the DG molecular layer both ipsilateral and contralateral to the injection with adjustments made for background. The grain density was expressed as a ratio of contralateral to ipsilateral grain counts (i.e., C/I ratios; cf. ref. 17). We sampled areas of approximately 2.25 × 104 μm2 per leaf depicted in Fig. 1. Images were analyzed with an Optimas Image Analysis System (Optimas, Bothell, WA).
Figure 1.
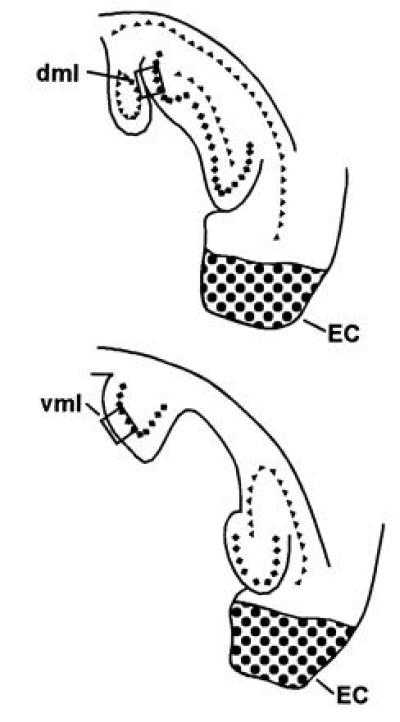
The hippocampus reconstructed in the horizontal plane, wherein silver grains were counted to assess the CTD. All counts were made from horizontal sections in the rostral DG molecular layer of both dorsal (dml) and ventral leaves (vml) in bright field with a 100× oil-immersion lens (cf. ref. 17). The stippled area illustrates representative EC lesions. ♦, Granule cell layer; ▴, pyramidal cell layer.
RESULTS
As Fig. 2A illustrates, one-way analyses of variance (ANOVA) and a priori Dunnett comparisons (Systat, Evanston, IL) of the number of errors committed and the number of days required to attain criterion after the second operation indicate that the Prog group’s alternation performance was spared relative to the One-stage group. Whereas the One-stage group committed significantly more errors (mean ± SEM = 29.1 ± 4.0; omnibus F = 158.05; df = 3, 52; P < 0.001) and required significantly more days (mean ± SEM = 9.5 ± 0.6; omnibus F = 15.78; df = 2, 46; P < 0.001) to attain criterion performance than the Sham group (mean errors ± SEM = 7.2 ± 1.8; mean days ± SEM = 4.3 ± 0.4; Dunnett test for two-tailed comparisons: Ps < 0.001), the Prog group was not statistically different from the Sham operates (mean errors ± SEM = 6.3 ± 0.8; mean days ± SEM = 4.1 ± 0.6; Ps > 0.5). In contrast to the Prog group, the rats that sustained a progressive lesion and simultaneous DP transection (Prog/Tran) committed more errors than the control animals (mean errors ± SEM = 109.9 ± 6.6; P < 0.001) and none reattained criterion performance. Fisher’s exact tests for two-tailed comparisons, performed to determine whether the Prog/Tran group’s failure to attain criterion was significant, indicated that the Prog/Tran group was indeed substantially more impaired than the other groups (Ps < 0.001). A one-way ANOVA with Scheffé post hoc contrasts on the first day’s performance after secondary surgery (omnibus F = 29.66; df = 3, 52; P < 0.001) indicated that the Prog/Tran and the One-stage groups were not different from one another (P > 0.06), but both groups committed more errors than the Prog or the Sham operates (Ps < 0.001). By the second test day after secondary surgery, the Prog/Tran group performed more poorly than all the other groups and the One-stage group was more impaired than the Prog or Sham groups (omnibus F = 30.30; df = 3, 52; P < 0.001; Scheffé test: Ps < 0.009). A two-way ANOVA with repeated measures on days after the first operation indicated the priming lesion did not impair the Prog or the Prog/Tran groups relative to the Sham group or the sham operations of the One-stage group (between subjects: F = 2.57; df = 3, 52; P > 0.06; group × days: F = 0.87; df = 6, 104; P > 0.50), though there was an improvement during the course of the IOI testing (days: F = 3.47; df = 2, 104; P < 0.04).
Figure 2.
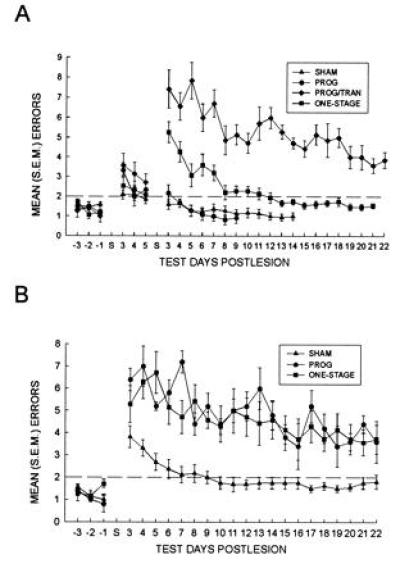
Alternation performance following sham operations, EC ablation, and/or DP transections. Whereas the One-stage group recovered to criterion levels of performance 8–12 days postlesion, the Prog group alternated at criterion levels as early as the fourth day after surgery (A). Simultaneously transecting the DP and ablating the remaining EC in a secondary operation (Prog/Tran) impaired alternation performance for 20 days of postoperative testing (A). DP transections made upon recovery from the secondary operation impaired control animals only transiently (7–9 days after surgery); the Prog and One-stage groups were impaired for the 20 days of postoperative testing (B). S on the x-axis represents the day of surgery; bars indicate ±SEM.
One-way ANOVAs and a priori Dunnett comparisons revealed that DP transections sustained in a third operation produced alternation deficits which persisted for at least 20 days in the Prog and the One-stage groups (Fig. 2B). As determined by the number of errors committed, the Prog (mean errors ± SEM = 98.2 ± 6.2) and the One-stage (mean errors ± SEM = 87.6 ± 11.5) groups suffered profound impairments after the DP transection. Both groups committed more errors than the Sham operates with DP transections (mean errors ± SEM = 21.5 ± 5.2; omnibus F = 33.33; df = 2, 25; P < 0.001; Dunnett test for two-tailed comparisons: Ps < 0.001).
It is of interest that the extent of the alternation impairment of the Prog/Tran group was similar to that of the Prog and the One-stage groups after DP transection. A two-way ANOVA with days as a repeated measure indicated that these groups were statistically indistinguishable from one another (between subjects: F = 0.92; df = 2, 16; P > 0.40; group × days interaction: F = 0.89; df = 38, 304; P > 0.50), although there was a significant days effect (F = 7.46; df = 19, 304; P < 0.001), indicating the groups’ performance improved during testing. While none of the Prog or Prog/Tran rats recovered after the CTD transection, two from the One-stage group reattained criterion performance. Although one Sham rat failed to reattain criterion performance after the transection, all others from the Sham group succeeded.
Analyses of the autoradiographic material indicated that whereas neither the priming lesion alone nor the one-stage unilateral EC lesion resulted in a significant CTD sprouting response either 4 or 6 days postlesion, progressive lesions increased the C/I ratio by approximately 83% (dorsal leaf) to 99% (ventral leaf) relative to the Control group as early as 4 days postlesion. Statistical analyses with one-way ANOVAs (omnibus F tests) and a priori Dunnett comparisons for both dorsal and ventral leaves at the 4- and 6-day time points indicated that the progressive lesion group was the only group to differ from the control group (dorsal leaf: 4-day, F = 4.54; df = 3, 32; P < 0.01; 6-day, F = 4.55; df = 3, 32; P < 0.01; ventral leaf: 4-day, F = 5.46; df = 3, 32; P < 0.005; 6-day, F = 6.63; df = 3, 32; P < 0.002; Dunnett test for two-tailed comparisons indicated Ps < 0.02; Figs. 3 and 4). Comparisons at the 8-day time point revealed that both the Prog group and the One-stage group were the only groups to differ from the Control group (dorsal leaf: F = 6.64; df = 3, 29; P < 0.002; ventral leaf: F = 4.65; df = 3, 29; P < 0.01; Dunnett test for two-tailed comparisons indicated Ps < 0.03; Fig. 4). It is important to note that although the rats in the priming lesion group (Prim) were given a total of 10, 12, or 14 days survival after the priming lesion (depending upon experimental condition), the priming lesions did not produce a significant increase in the C/I ratio.
Figure 3.
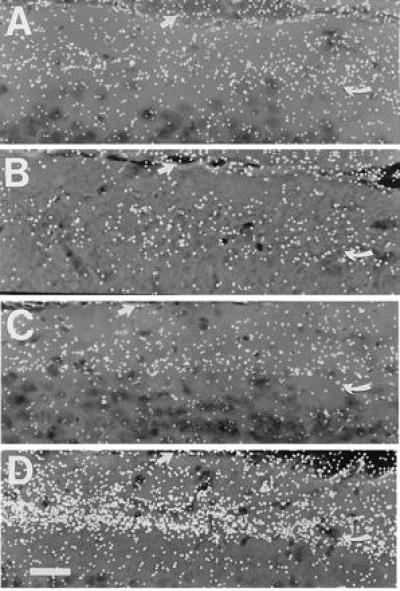
The pattern of labeling in the ventral leaf of the DG molecular layer. The figure illustrates the silver grain density observed in dark-field autoradiograms of the molecular layer from a control rat (A), a rat with a priming lesion only (B), a rat with a one-stage lesion (C), and a rat with a progressive lesion (D). Note the increased grain density in D relative to C. These dark-field photomicrographs of the ventral leaf (area vml of Fig. 1) from rats at the 6-day time point were taken with a 20× objective and an oil-immersion dark-field condenser. The curved arrow indicates the denervated/reinnervated zone and the large arrow indicates the outer limit of the molecular layer. (Bar = 40 μm.)
Figure 4.
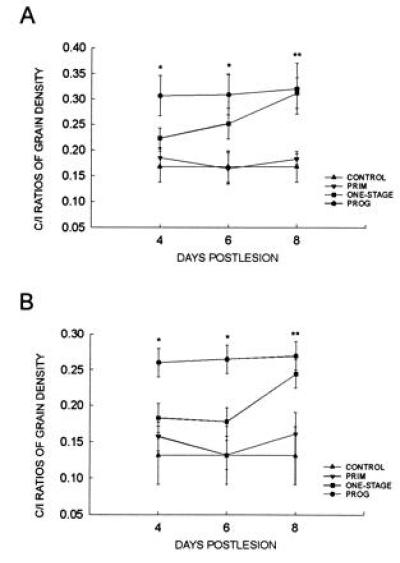
C/I ratios in the DG. Only the Prog C/I ratios were significantly greater (*, Ps < 0.02) than the Control group values in the dorsal (A) and ventral (B) leaves at the 4- and 6-day time points. By 8 days postlesion, both the Prog and the One-stage groups were significantly greater than the Control group (**, Ps < 0.03). Bars indicate ±SEM.
Histological analyses indicated that the entorhinal area, particularly layers II and III (18), was extensively injured, and the pre- and parasubiculum were injured to some extent, in all entorhinectomized rats in these experiments (Fig. 1). The ventral subiculum and the ventral DG granule cell layer were moderately injured in 4 rats from the behavioral study and 5 rats from the anatomical study, but the hippocampus and DG were spared dorsally. The DP of all transected rats was completely injured. The transections invariably impinged on the dorsal-most fornix, and they injured the corpus callosum extending to the splenium, as well as the overlying cingulate and neocortex. The dorsal septum, fimbria, and ventral psalterium were moderately injured in 11 transected rats. The alternation performances or grain densities of the rats with these more extensive injuries were similar to those of their cohorts with well-localized entorhinal or DP damage.
DISCUSSION
Progressive entorhinal lesions accelerated CTD sprouting and concomitantly spared the animals from the memory deficits typically associated with hippocampal deafferentation. The subsequent transection of the sprouted pathway in rats sustaining progressive lesions disrupted alternation performance as severely as that manifested by the one-stage animals. This observation is particularly interesting because rats with progressive lesions had not evidenced memory deficits after the prior entorhinal injury. Finally, whereas the progressive lesion spared alternation performance, the simultaneous transection of the crossed entorhinal pathway and the second stage of the progressive lesion produced a persistent alternation deficit. Taken together, these results are consistent with the hypothesis that lesion-induced CTD sprouting is functionally significant. More importantly, our results suggest that accelerating CTD sprouting spares spatial memory after progressive entorhinal injury.
Our findings corroborate and extend reports that one-stage unilateral EC lesions impair learned alternation performance from which rats recover 8 to 12 days postlesion and that transection of the sprouted CTD reinstates memory deficits from which the animals previously recovered (6). Loesche and Steward (6) have also demonstrated that the recovery is time dependent—i.e., delaying the onset of testing after surgery does not alter the time at which recovery occurs. Thus, in their experiments and in ours, the time course of the recovered alternation behavior parallels the DG reinnervation (4, 5).
Because septodentate and C/A sprouting are accelerated after progressive lesions, we cannot rule out the possibility that they may also contribute to the observed behavioral sparing by working in conjunction with the CTD. Disruption of any of the three inputs may compromise the ability of the hippocampal formation to process spatial memory. Nevertheless, the persistent impairment resulting from the DP transection suggests that the sprouted crossed entorhinal pathway is indeed important for spatial memory. For example, the simultaneous DP transection and progressive lesion eliminated the CTD before it could sprout, yet presumably permitted sprouting by the septodentate and C/A inputs. Although the Prog/Tran group’s performance improved somewhat, none of the rats in this group reattained criterion performance after 20 days of testing.
The Prog and One-stage groups after DP transection as well as the Prog/Tran group evidenced a behavioral impairment and improvement similar to that reported by Loesche and Steward (6) for DP-transected rats that had previously recovered from one-stage EC lesions. Although the groups do not fully recover to preoperative levels of performance during the testing period, the recovery rates of these three groups are very similar. Conceivably, the gradual improvement may be related to postoperative testing and/or the remaining sprouting afferents. If these afferents were primarily responsible for the recovery, though, the performance level should have been more stable at the outset of testing rather than gradually improving as observed, since substantial septodentate and C/A sprouting have already occurred by the time of the DP transection. In a similar vein, it appears that postoperative training may be more salient than sprouting by the septodentate and C/A pathways for the recovery from the perseverative behavior associated with bilateral entorhinal lesions (19).
One might postulate that a sprouting response during the IOI, elicited by the priming lesion itself, sustained the learned alternation behavior immediately following the secondary lesion. Our neuroanatomical findings argue against this possibility, however, since the priming lesion failed to induce a significant amount of sprouting as measured autoradiographically at all the time points we investigated.
The time course of CTD sprouting observed in the rats that sustained one-stage entorhinal lesions is consistent with earlier reports (17). The grain densities for the One-stage group were not considerably different from the control preparations at either 4 or 6 days postlesion. But by 8 days postlesion, there was a significant increase in the C/I ratio. The time course of the increasing C/I ratios also corresponds to the time course of the increasing synaptic efficacy in the CTD after unilateral entorhinal lesions (7). Although it is possible that increases in synaptic efficacy may reflect alterations in receptor activity such as denervation supersensitvity, the fact that the anatomical and electrophysiological time courses are synchronous strengthens the interpretation that the two methods indeed reveal CTD sprouting (17).
In contrast to the One-stage group, the time course of CTD sprouting in rats with progressive lesions evidences an accelerated response. As early as 4 days after the secondary lesion, the Prog rats exhibited a much greater C/I ratio, which remained elevated throughout the time periods examined. It should be noted that Scheff et al. (15) reported a similar observation for the sprouting response of the septodentate and C/A inputs to the dentate after progressive EC lesions. By 8 days postlesion, the sprouting responses of the One-stage and Prog groups converged such that the C/I ratios were no longer substantially different. The latter finding is noteworthy because the performance of the One-stage group on the spatial memory task is relatively unimpaired by 8 days postlesion and approaches the performance level of the Prog group (see Fig. 2A).
The mechanisms responsible for enhancing the rate of reinnervation after progressive lesions are as yet unknown. Several phenomena associated with axonal sprouting are potential candidates for initiating the events involved in terminal proliferation and synaptogenesis. These include clearing of terminal degeneration by macrophages (20, 21), microglial induction of astrocytic hypertrophy or hyperplasia (22), and the synthesis of neurotrophic factors in the target (23–25). Previously Scheff et al. (15) had demonstrated that the catalytic effect of the priming EC lesion was limited to a window of 4–13 days between the primary and secondary lesions. It is unknown whether this window reflects alterations in the microenvironment necessary to elicit a sprouting response, in the neuron undergoing terminal proliferation, or in the postsynaptic neuron undergoing synaptogenesis.
One feature of our anatomical findings suggests that the factors responsible for the accelerated CTD sprouting may act at a distance from the site of denervation. The field of termination of the lateral and medial entorhinal inputs to the molecular layer are adjacent but nonoverlapping. The lateral EC projects to the outer third of the molecular layer, whereas the medial EC projects to the middle third (26, 27). Yet the priming lateral entorhinal lesions used in our study were capable of conditioning the medial inputs to sprout more rapidly. There are at least three possible ways to account for this finding: (i) the factor responsible for eliciting a sprouting response is a diffusible substance and consequently affected the medial inputs by diffusing into their terminal field; (ii) the proximity of the macrophages to the middle molecular layer facilitated their subsequent invasion into the newly denervated area and resulted in a rapid clearing of degeneration and/or release of trophic factors; or (iii) some combination of the two. It remains to be seen whether the sequence of entorhinal ablation, resulting in a differential spatial pattern of dendritic deafferentation, is of relevance to the catalytic effect of the priming lesion.
In any anatomical investigation wherein reinnervation of a deafferented structure is to be qualitatively or quantitatively analyzed, one must recognize the possibility that the shrinkage of the deafferented target structure could confound the analysis. Shrinkage of the molecular layer resulting from the deafferentation associated with unilateral entorhinal lesions has been discussed in several contexts (20, 28, 29). On the basis of measurements reported earlier (20, 28, 29), we assume that shrinkage in the denervated zone of the molecular layer for 4 days is 5%, 6 days is 10%, 8 days is 15%, 10 days is 20%, 12 days is 25%, and 14 days is 30%. Even after taking this shrinkage into account, our results indicate that the rate of sprouting after progressive lesions is nevertheless dramatically accelerated relative to the one-stage preparation. At the 4-day time point, for example, whereas the C/I ratio of the One-stage group for the dorsal leaf is 33% larger and for the ventral leaf 40% larger than that of the Control group, the Prog group’s ratio for the dorsal leaf is 83% larger and for the ventral leaf 99% larger than the corresponding control figures. After the values have been corrected for shrinkage, the C/I ratio of the Prog group is 74% greater in the dorsal leaf and 89% greater in the ventral leaf than the controls, while the One-stage group is 26% greater in the dorsal leaf and 33% greater in the ventral leaf (the data are not shown).
Although axonal sprouting may contribute to certain pathologies—e.g., epilepsy—by scrambling the processing of information as is frequently suggested (30), our findings indicate that homotypic sprouting may provide a compensatory mechanism for the loss of neurons in the CNS. They therefore support the postulate that sprouting may be a mechanism by which functional reorganization is mediated in an injured CNS. Moreover, our observations underscore the potential behavioral importance of sprouting by structures homologous to the injured afferents. Indeed, accelerating homotypic sprouting may be one strategy by which to ameliorate the consequences of human brain trauma or degenerative CNS diseases. Hippocampal deafferentation resulting from uncal herniation or the deterioration of the entorhinal area in Alzheimer disease has been shown to induce synaptic reorganization in the human hippocampus similar to that observed in the rat (31–34). The functional consequences of these alterations in connectivity are unknown, but our results raise the possibility that homotypic sprouting may have a beneficial behavioral outcome. Conceivably, homotypic sprouting may attenuate the deficits that in its absence might otherwise be manifested in these or similar disorders of the CNS. Thus, in combination with efforts to promote the survival of neurons that are either traumatized or vulnerable to degenerative diseases (e.g., refs. 35–37), one therapeutic approach would be to develop neurotrophic agents that enhance the axonal sprouting of homotypic inputs that remain viable. In so doing, the intrinsic restorative processes of these inputs would be facilitated.
Acknowledgments
We thank Ruth Ault, Michael Borenstein, Pamela Hay, Donald G. Stein, and Richard Tomasulo for critical readings of the manuscript. This work was supported by grants from the National Institute of Mental Health (MH47895), the National Institute of Neurological Disorders and Stroke (NS31740), and the National Science Foundation (BNS9020151) to J.J.R.
Footnotes
Abbreviations: C/A, commissural/associational; C/I ratio, ratio of contralateral to ipsilateral grain counts; CNS, central nervous system; CTD, crossed temporodentate pathway; DG, dentate gyrus; EC, entorhinal cortex; DP, dorsal psalterium; IOI, interoperation interval.
References
- 1.Laurence S, Stein D G. In: Recovery from Brain Damage: Research and Theory. Finger S, editor. New York: Plenum; 1978. pp. 369–407. [Google Scholar]
- 2.Waxman S G. Adv Neurol. 1988;47:1–7. [PubMed] [Google Scholar]
- 3.Stein D G, Brailowsky S, Will B. Brain Repair. New York: Oxford Univ. Press; 1995. [Google Scholar]
- 4.Cotman C W, Nieto-Sampedro M, Harris E W. Physiol Rev. 1981;61:684–784. doi: 10.1152/physrev.1981.61.3.684. [DOI] [PubMed] [Google Scholar]
- 5.Steward O. J Neurotrauma. 1989;6:99–152. doi: 10.1089/neu.1989.6.99. [DOI] [PubMed] [Google Scholar]
- 6.Loesche J, Steward O. Brain Res Bull. 1977;2:31–39. doi: 10.1016/0361-9230(77)90022-3. [DOI] [PubMed] [Google Scholar]
- 7.Reeves T M, Smith D C. Behav Neurosci. 1987;101:179–186. doi: 10.1037//0735-7044.101.2.179. [DOI] [PubMed] [Google Scholar]
- 8.Squire L R. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 9.Jarrard L E. Behav Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- 10.Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio A R. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- 11.Steward O, Cotman C W, Lynch G S. Brain Res. 1976;114:181–200. doi: 10.1016/0006-8993(76)90665-x. [DOI] [PubMed] [Google Scholar]
- 12.Harris E W, Lasher S S, Steward O. Brain Res. 1978;151:623–631. doi: 10.1016/0006-8993(78)91098-3. [DOI] [PubMed] [Google Scholar]
- 13.Wilson R C, Levy W B, Steward O. Brain Res. 1979;176:65–78. doi: 10.1016/0006-8993(79)90870-9. [DOI] [PubMed] [Google Scholar]
- 14.Scheff S, Benardo L, Cotman C. Science. 1977;197:795–797. doi: 10.1126/science.887924. [DOI] [PubMed] [Google Scholar]
- 15.Scheff S, Benardo L, Cotman C. Brain Res. 1978;150:45–53. doi: 10.1016/0006-8993(78)90652-2. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez J J, Stein D G. Behav Brain Res. 1984;13:55–61. doi: 10.1016/0166-4328(84)90029-9. [DOI] [PubMed] [Google Scholar]
- 17.Steward O, Loesche J. Brain Res. 1977;125:11–21. doi: 10.1016/0006-8993(77)90356-0. [DOI] [PubMed] [Google Scholar]
- 18.Steward O. Science. 1976;194:426–428. doi: 10.1126/science.982024. [DOI] [PubMed] [Google Scholar]
- 19.Ramirez J J, Labbe R, Stein D G. Brain Res. 1988;459:153–156. doi: 10.1016/0006-8993(88)90296-x. [DOI] [PubMed] [Google Scholar]
- 20.Lynch G, Rose G, Gall C, Cotman C W. In: Golgi Centennial Symposium Proceedings. Santini M, editor. New York: Raven; 1975. pp. 305–317. [Google Scholar]
- 21.Steward O. Exp Neurol. 1992;118:340–351. doi: 10.1016/0014-4886(92)90192-s. [DOI] [PubMed] [Google Scholar]
- 22.Fagan A M, Gage F H. Exp Neurol. 1990;110:105–120. doi: 10.1016/0014-4886(90)90055-w. [DOI] [PubMed] [Google Scholar]
- 23.Gómez-Pinilla F, Lee J W-K, Cotman C W. J Neurosci. 1992;12:345–355. doi: 10.1523/JNEUROSCI.12-01-00345.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kar S, Baccichet A, Quirion R, Poirer J. Neuroscience. 1993;55:69–80. doi: 10.1016/0306-4522(93)90455-o. [DOI] [PubMed] [Google Scholar]
- 25.Conner J M, Fass-Holmes B, Varon S. J Comp Neurol. 1994;345:409–418. doi: 10.1002/cne.903450307. [DOI] [PubMed] [Google Scholar]
- 26.Steward O. J Comp Neurol. 1976;167:285–314. doi: 10.1002/cne.901670303. [DOI] [PubMed] [Google Scholar]
- 27.Witter M P, Groenewegen H J, Lopes da Silva F H, Lohman A H M. Prog Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- 28.Steward O, Vinsant S L. J Comp Neurol. 1983;214:370–386. [Google Scholar]
- 29.Scheff S W. In: Neural Regeneration and Transplantation. Seil F J, editor. New York: Liss; 1989. pp. 137–156. [Google Scholar]
- 30.Babb T L, Kupfer W R, Pretorious J K, Crandall P H, Levesque M F. Neuroscience. 1991;42:351–363. doi: 10.1016/0306-4522(91)90380-7. [DOI] [PubMed] [Google Scholar]
- 31.Geddes J W, Monaghan D T, Cotman C W, Lott I T, Kim R C, Chui H-C. Science. 1985;230:1179–1181. doi: 10.1126/science.4071042. [DOI] [PubMed] [Google Scholar]
- 32.Geddes J W, Cotman C W. Neurosci Res. 1986;3:672–678. doi: 10.1016/0168-0102(86)90062-3. [DOI] [PubMed] [Google Scholar]
- 33.Hyman B T, Kromer L J, Van Hoesen G W. Ann Neurol. 1987;21:259–267. doi: 10.1002/ana.410210307. [DOI] [PubMed] [Google Scholar]
- 34.Grady M S, Jane J A, Steward O. J Neurosurg. 1989;71:534–537. doi: 10.3171/jns.1989.71.4.0534. [DOI] [PubMed] [Google Scholar]
- 35.Chen L S, Ray J, Fisher L J, Kawaja M D, Schinstine M, Kang U J, Gage F H. J Cell Biochem. 1991;45:252–257. doi: 10.1002/jcb.240450305. [DOI] [PubMed] [Google Scholar]
- 36.Varon S, Conner J M. J Neurotrauma. 1994;11:473–486. doi: 10.1089/neu.1994.11.473. [DOI] [PubMed] [Google Scholar]
- 37.Chen K S, Gage F H. J Neurosci. 1995;15:2819–2825. doi: 10.1523/JNEUROSCI.15-04-02819.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


