Abstract
The transcription elongation complexes yFACT, Spt4/Spt5, and Spt6/Iws1 were previously shown to follow similar patterns of association across transcribed genes in Saccharomyces cerevisiae. Using a histone H3 mutant, we now provide evidence that the mechanism of association of yFACT across genes is separable from that adopted by Spt4/Spt5 and Spt6/Iws1.
In recent years, it has become clear that the transcription elongation process in eukaryotic cells is highly dynamic, requiring the contributions of many elongation factors possessing a variety of biochemical activities. Many of these factors have been shown to promote changes, either directly or indirectly, in the structure of chromatin to facilitate passage of RNA polymerase II across transcribed genes and, in certain cases, to prevent the inappropriate initiation of transcription within open reading frames (ORFs) (6, 10, 26). Although a number of studies have defined some of the physical interactions that occur between elongation factors (for some examples, see references 14, 21, and 23, and for a review, see reference 15), little is known about the mechanisms that control how these factors are recruited to, retained on, and removed from transcribed units in vivo. In particular, the interdependent relationships between different factors for chromatin association, especially in cases involving factors that are essential for cell viability, still remain to be elucidated.
We recently showed that the distribution of the Spt16 component of the Saccharomyces cerevisiae FACT complex (yFACT) across transcribed genes is affected by the histone H3 mutant H3-L61W, resulting in lower Spt16 occupancy at 5′ regions of ORFs and in a marked accumulation at 3′ regions of transcribed units (4). To determine if H3-L61W affects chromatin association of Spt16 in the context of yFACT, we performed chromatin immunoprecipitation (ChIP) experiments directed against the Spt16 and Pob3 subunits of yFACT and assessed their levels of binding to three regions across the constitutively expressed PMA1 and ADH1 genes in wild-type and H3-L61W cells. ChIP experiments were carried out as described previously (17). Spt16 precipitation was carried out using polyclonal rabbit antibodies specific to Spt16 (a gift from Tim Formosa), and Pob3 precipitation was performed using immunoglobulin G (IgG) Sepharose (GE Healthcare) since Pob3 harbored a tandem affinity purification (TAP) tag containing two IgG binding domains of Staphylococcus aureus protein A (Open Biosystems). Control experiments were carried out to ensure that the ChIP signals observed were dependent on the specific antibodies used or on the presence of the TAP tag (data not shown). The amount of DNA was quantified by quantitative PCR, using a MiniOpticon system from Bio-Rad. Since we were interested in interactions between genotypes (wild type versus H3-L61W) and between different locations across genes, we analyzed the results by two-way analysis of variance. Data sets that did not show homogeneity of variances were log10 transformed prior to analysis. A statistically significant genotype X location interaction indicates a change in the distributional pattern of a particular elongation factor across the gene analyzed.
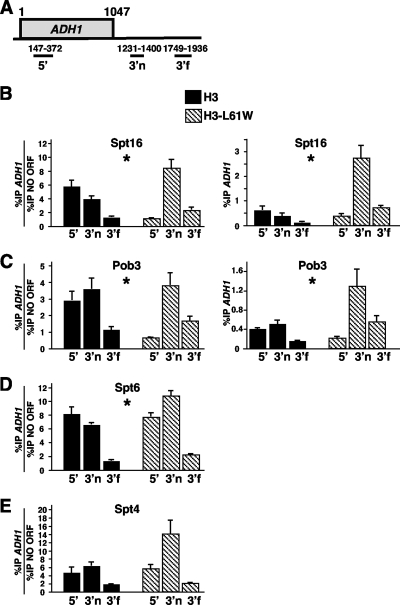
As expected, and consistent with previous reports (4, 13, 18), in wild-type cells we detected strong binding of Spt16 to the 5′ regions of both ORFs and no binding to a region significantly downstream from the end of each ORF, whereas in H3-L61W cells we observed a dramatic shift in Spt16 distribution toward the 3′ end of each gene (Fig. 1B, left panel [F2,30 = 129.9; P < 0.001] and right panel [F2,30 = 54.49; P < 0.001], and Fig. 2B, left panel [F2,30 = 40.18; P < 0.001] and right panel [F2,30 = 24.15; P < 0.001]). We now show that the distribution of Pob3 across both loci is also greatly shifted toward the 3′ regions of both ORFs in the context of H3-L61W (Fig. 1C, left panel [F2,12 = 23.08; P < 0.001] and right panel [F2,12 = 12.13; P = 0.001], and Fig. 2C, left panel [F2,12 = 3.50; P = 0.024] and right panel [F2,12 = 6.60; P = 0.012]). Although the magnitude of the 3′ shift for Pob3 was lower than that seen for Spt16, our data, combined with the fact that Spt16 and Pob3 are components of the yFACT complex (3, 9), support the notion that at least to a large extent, yFACT as a whole is affected in the H3-L61W mutant. Interestingly, we routinely observed increased levels of Spt16 and Pob3 binding to a nontranscribed region on chromosome V (4; this study) as well as increased Spt16 binding to a telomeric region on chromosome VI not associated with transcription in H3-L61W cells compared to the case in wild-type cells (data not shown), suggesting that yFACT might bind abnormally to nontranscribed regions throughout the genome in H3-L61W cells.
FIG. 1.
H3-L61W confers a marked 3′ shift in the distribution patterns of components of yFACT across PMA1 but causes little and no change in distribution patterns of Spt6 and Spt4, respectively. (A) Schematic representation of the PMA1 gene (drawn to scale) showing the three regions (5′, 3′n [3′ near], and 3′f [3′ far]) used for ChIP experiments. The primer sequences used for these experiments are available upon request. (B to E) Results of Spt16, Pob3, Spt6, and Spt4 ChIP assays across the PMA1 gene. ChIP experiments were carried out using the strains listed in Table 1 (except for strain yAAD1128). To normalize for variations in overall IP efficiency between different experiments, the level of binding of a factor to each of the three regions across PMA1 is reported as a ratio of the %IP for that region to the %IP for a genomic region devoid of ORFs (no-ORF region) (4). For the Spt16 and Pob3 experiments, we also show the data solely as %IP for the region of interest (panels B and C, right panels). This allows for a more direct comparison between wild-type and H3-L61W cells in these cases, since we detected a significant increase in Spt16 and Pob3 (but not in Spt4 or Spt6) binding to the no-ORF region in H3-L61W cells compared to that in wild-type cells (see the text). In all cases, the results shown are the means with corresponding standard errors for at least three independent experiments. Asterisks indicate statistically significant changes in the distribution pattern of the elongation factor analyzed as a result of the H3-L61W mutation (see the text).
FIG. 2.
H3-L61W confers a marked 3′ shift in the distribution patterns of components of yFACT across ADH1 but causes little and no change in distribution patterns of Spt6 and Spt4, respectively. (A) Schematic representation of the ADH1 gene (drawn to scale) showing the three regions (5′, 3′n [3′ near], and 3′f [3′far]) used for the ChIP experiments. The primer sequences used for these experiments are available upon request. (B to E) Results of Spt16, Pob3, Spt6, and Spt4 ChIP assays across the ADH1 gene. The data are displayed as described in the legend to Fig. 1. In all cases, the graphs show the means with corresponding standard errors for at least three independent experiments. Asterisks indicate statistically significant changes in the distribution pattern of the elongation factor analyzed as a result of the H3-L61W mutation (see the text).
The Spt4, Spt6, and Spt16 elongation factors were originally identified through similar genetic selection experiments and are thought to participate in the transcription elongation process through interactions with chromatin (7, 16, 20, 25). Whereas genetic and biochemical studies indicate that Spt4, Spt6, and Spt16 share some common functional features with each other (11), these three factors are found in different complexes, namely, Spt4/Spt5 (DSIF in humans), Spt6/Iws1, and yFACT, respectively, with distinct biochemical properties (1, 2, 24). Consistent with the idea that the functions of these complexes are to some degree related with each other, a recent study has shown that Spt4/Spt5, Spt6/Iws1, and yFACT display the same pattern of association across PMA1, ADH1, and several other transcribed genes (13). However, it is not clear whether these complexes utilize the same or different mechanisms to regulate their association with transcribed genes and whether there is an interdependent relationship in their ability to interact with chromatin.
We took advantage of the H3-L61W mutant to obtain some insights into these questions. For these studies, we performed ChIPs to determine whether the patterns of association of Spt6 and Spt4 across the PMA1 and ADH1 genes are affected in H3-L61W cells. The experiments were carried out as described above, but using polyclonal rabbit antibodies specific to Spt6 (a gift from Tim Formosa) and IgG Sepharose for Spt4 (as this protein was tagged with a TAP tag). We found that Spt6 displayed a minor but statistically significant 3′ shift at both genes in the context of H3-L61W (Fig. 1D [F2,18 = 6.70; P = 0.007] and 2D [F2,18 = 11.43; P = 0.002]). However, the pattern of Spt4 association across either gene was not significantly perturbed by the H3-L61W mutant (Fig. 1E [F2,12 = 3.16; P = 0.08] and 2E [F2,12 = 1.99; P = 0.18]). The observation that the pattern of chromatin association of yFACT at PMA1 and ADH1 can be uncoupled from that of Spt4 and Spt6 by a mutation (i.e., H3-L61W) provides evidence that (i) there is no strict requirement for the presence of Spt4 or Spt6 for yFACT association with chromatin, since high levels of yFACT are seen at regions of genes (3′ ends) that are not or only poorly occupied by Spt4 and Spt6; and (ii) the mechanisms that regulate yFACT interaction with genes in vivo are distinct from those regulating chromatin association of Spt4 and Spt6.
Spt16 and Spt6 both have histone chaperoning activity, and defects in these factors have been shown to result in cryptic transcription from within ORFs, presumably due to defects in nucleosome assembly in the wake of RNA polymerase II passage (12, 18). Given these shared characteristics, we entertained the possibility that the cause for the minor distribution shift observed for Spt6 across genes in H3-L61W cells could be a secondary effect of abnormal Spt16-histone H3 interactions and that suppressor mutations that improve Spt16-histone H3 interactions might in turn also suppress the Spt6 distribution defects. To test this possibility, we performed ChIP experiments with H3-L61W cells that also express a previously isolated Spt16 mutant, Spt16-790, that moderately suppresses the Spt16 3′-shift phenotype at PMA1 and other genes (4). We found that whereas Spt16-790 significantly suppressed the Spt16 3′-shift phenotype at PMA1 in H3-L61W cells (Fig. 3A) (F2,21 = 16.05; P < 0.001), the same mutation did not suppress the defect in Spt6 distribution over PMA1 in the context of H3-L61W cells (Fig. 3B) (F2,15 = 0.57; P = 0.58). These data support the notion that H3-L61W causes its effects on the patterns of chromatin association of Spt16 and Spt6 across genes through two distinct, possibly unrelated mechanisms.
FIG. 3.
The Spt16-790 mutant does not ameliorate the minor defect in Spt6 distribution across PMA1 seen in H3-L61W cells. Spt16 and Spt6 ChIP assays were carried out on strain yAAD1128 (H3-L61W Spt16-790) and compared to the data for H3-L61W strains (as already reported in Fig. 1 and provided here to facilitate direct comparison). The regions assayed are the same as those shown in Fig. 1. The data are presented in the same manner as that in Fig. 1. The values shown are the means with corresponding standard errors for at least three independent experiments. Statistically significant changes in elongation factor distribution across PMA1 in H3-L61W Spt16-790 cells compared to H3-L61W cells are indicated with an asterisk (see the text).
We previously hypothesized that the presence of H3-L61W may prevent a 3′-untranslated region dissociation signal from reaching Spt16 at the end of the transcription process, thereby resulting in a higher abundance of Spt16 at 3′ versus 5′ regions of genes (4). Given recent data showing that Spt16 directly interacts with the globular regions of histones H3 and H4 (22), this putative signal is likely to involve direct interactions between Spt16 and these histones. The observation that the H3-L61W mutant has only a modest effect on Spt6 and no effect on Spt4 distribution across the genes assayed here suggests that these elongation factors rely on 3′-untranslated region dissociation signals that are, at least to some degree, separable from those used by yFACT (i.e., signals that are mostly H3-L61W insensitive). Future work will focus on elucidating the nature of the mechanisms that regulate the dynamic association of yFACT with chromatin during the transcription process. Of particular interest in this regard will be determining the role of posttranslational modifications of histones, such as H2B ubiquitylation, which was recently shown to affect Spt16 function and chromatin association (8, 19), in regulating the proper departure of yFACT following transcription. In addition, the recent finding that conditions that promote transcriptional stress cause increased levels of histone occupancy and a Chd1-dependent increase in histone H3-K4 methylation at 3′ ends of genes (27) will be investigated in the context of our model, particularly since Chd1 and yFACT have been shown to interact with each other (14, 21).
TABLE 1.
Saccharomyces cerevisiae strains used in this study
| Straina | Genotype |
|---|---|
| yAAD1048 | MATα his3Δ200 leu2Δ1 ura3-52 lys2-128Δ (hht1-hhf1)Δ::LEU2 hht2-11 |
| yAAD1049 | MATahis3Δ200 leu2Δ1 ura3-52 lys2-128Δ (hht1-hhf1)Δ::LEU2 hht2-11 |
| yAAD1052 | MATahis3Δ200 leu2Δ1 ura3-52 lys2-128Δ (hht1-hhf1)Δ::LEU2 |
| yAAD1053 | MATα his3Δ200 leu2Δ1 ura3-52 lys2-128Δ (hht1-hhf1)Δ::LEU2 |
| yAAD1128 | MATahis3Δ200 leu2Δ1 ura3-52 lys2-128Δ (hht1-hhf1)Δ::LEU2 hht2-11 SPT16-790 |
| yAAD2214 | MATahis3bleu2Δcura3dlys2-128Δ (hht1-hhf1)Δ::LEU2 POB3-TAP::HIS3MX6 |
| yAAD2215 | MATahis3bleu2Δcura3d (hht1-hhf1)Δ::LEU2 hht2-11 POB3-TAP::HIS3MX6 |
| yAAD2220e | MATahis3bleu2Δcura3dlys2-128Δ (hht1-hhf1)Δ::LEU2 hht2-11SPT4-TAP::HIS3MX6 |
| yAAD2223 | MATα his3bleu2Δcura3d met15Δ0 (hht1-hhf1)Δ::LEU2 hht2-11SPT4-TAP::HIS3MX6 |
| yAAD2224e | MATα his3bleu2Δcura3dmet15Δ0 (hht1-hhf1)Δ::LEU2 SPT4-TAP::HIS3MX6 |
| yAAD2226 | MATahis3bleu2Δcura3dmet15Δ0 (hht1-hhf1)Δ::LEU2 SPT4-TAP::HIS3MX6 |
| yAAD2241 | MATahis3bleu2Δcura3dlys2-128Δ (hht1-hhf1)Δ::LEU2 hht2-11 POB3-TAP::HIS3MX6 |
| yAAD2242 | MATahis3bleu2Δcura3dlys2-128Δ (hht1-hhf1)Δ::LEU2 hht2-11 POB3-TAP::HIS3MX6 |
| yAAD2243 | MATahis3bleu2Δcura3dlys2-128Δ (hht1-hhf1)Δ::LEU2 POB3-TAP::HIS3MX6 |
| yAAD2244 | MATahis3bleu2Δcura3dlys2-128Δ (hht1-hhf1)Δ::LEU2 POB3-TAP::HIS3MX6 |
All strains were derived from the S288C background. The generation of the hht2-11 and (hht1-hhf1)Δ::LEU2 mutations has been described previously (5). Strains containing the SPT4-TAP::HIS3MX6 and POB3-TAP::HIS3MX6 alleles were generated by crossing commercially available strains harboring the TAP::HIS3MX6 fusions (Open Biosystems) with strains with the appropriate genotypes.
The allele at this locus is either his3Δ200 or his3Δ1.
The allele at this locus is either leu2Δ1 or leu2Δ0.
The allele at this locus is either ura3-52 or ura3Δ0.
The original strains yAAD2220 and yAAD2224 also contain a URA3-marked plasmid harboring the wild-type HHT1-HHF1 locus. The strains used in these studies were selected for loss of this plasmid and therefore contain only genomic HHT2 or hht2-11 (as indicated) as the sole source of H3 protein.
Acknowledgments
We are grateful to Joseph Martens, Richard Murray, and Reine Protacio for helpful comments on the manuscript and to Kacey Swindle and Landon Reeves for technical assistance. We thank Tim Formosa for antibodies against the Spt16 and Spt6 proteins. We also thank Amine Nourani, Mary Bryk, and Varsha Kaushal for assistance on real-time PCR analysis.
This material is based upon work supported by the National Science Foundation under grant 0543412, by NIH grant P20 RR16460-03 from the IDeA Networks of Biomedical Research Excellence (INBRE) Program of the National Center for Research Resources, and by start-up funds from Hendrix College to A.A.D. K.P. was supported by a Student Undergraduate Research Fellowship (SURF) grant from the Arkansas Department of Higher Education.
Footnotes
Published ahead of print on 1 December 2008.
REFERENCES
- 1.Belotserkovskaya, R., S. Oh, V. A. Bondarenko, G. Orphanides, V. M. Studitsky, and D. Reinberg. 2003. FACT facilitates transcription-dependent nucleosome alteration. Science 3011090-1093. [DOI] [PubMed] [Google Scholar]
- 2.Bortvin, A., and F. Winston. 1996. Evidence that Spt6p controls chromatin structure by a direct interaction with histones. Science 2721473-1476. [DOI] [PubMed] [Google Scholar]
- 3.Brewster, N. K., G. C. Johnston, and R. A. Singer. 1998. Characterization of the CP complex, an abundant dimer of Cdc68 and Pob3 proteins that regulates yeast transcriptional activation and chromatin repression. J. Biol. Chem. 27321972-21979. [DOI] [PubMed] [Google Scholar]
- 4.Duina, A. A., A. Rufiange, J. Bracey, J. Hall, A. Nourani, and F. Winston. 2007. Evidence that the localization of the elongation factor Spt16 across transcribed genes is dependent upon histone H3 integrity in Saccharomyces cerevisiae. Genetics 177101-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duina, A. A., and F. Winston. 2004. Analysis of a mutant histone H3 that perturbs the association of Swi/Snf with chromatin. Mol. Cell. Biol. 24561-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eissenberg, J. C., and A. Shilatifard. 2006. Leaving a mark: the many footprints of the elongating RNA polymerase II. Curr. Opin. Genet. Dev. 16184-190. [DOI] [PubMed] [Google Scholar]
- 7.Fassler, J. S., and F. Winston. 1988. Isolation and analysis of a novel class of suppressor of Ty insertion mutations in Saccharomyces cerevisiae. Genetics 118203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming, A. B., K. Cheng-Fu, C. Hillyer, M. Pikaart, and M. A. Osley. 2008. H2B ubiquitylation plays a role in nucleosome dynamics during transcription elongation. Mol. Cell 3157-66. [DOI] [PubMed] [Google Scholar]
- 9.Formosa, T., P. Eriksson, J. Wittmeyer, J. Ginn, Y. Yu, and D. J. Stillman. 2001. Spt16-Pob3 and the HMG protein Nhp6 combine to form the nucleosome-binding factor SPN. EMBO J. 203506-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartzog, G. A. 2003. Transcription elongation by RNA polymerase II. Curr. Opin. Genet. Dev. 13119-126. [DOI] [PubMed] [Google Scholar]
- 11.Hartzog, G. A., J. L. Speer, and D. L. Lindstrom. 2002. Transcript elongation on a nucleoprotein template. Biochim. Biophys. Acta 1577276-286. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan, C. D., L. Laprade, and F. Winston. 2003. Transcription elongation factors repress transcription initiation from cryptic sites. Science 3011096-1099. [DOI] [PubMed] [Google Scholar]
- 13.Kim, M., S. H. Ahn, N. J. Krogan, J. F. Greenblatt, and S. Buratowski. 2004. Transitions in RNA polymerase II elongation complexes at the 3′ ends of genes. EMBO J. 23354-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krogan, N. J., M. Kim, S. H. Ahn, G. Zhong, M. S. Kobor, G. Cagney, A. Emili, A. Shilatifard, S. Buratowski, and J. F. Greenblatt. 2002. RNA polymerase II elongation factors of Saccharomyces cerevisiae: a targeted proteomics approach. Mol. Cell. Biol. 226979-6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, B., M. Carey, and J. L. Workman. 2007. The role of chromatin during transcription. Cell 128707-719. [DOI] [PubMed] [Google Scholar]
- 16.Malone, E. A., C. D. Clark, A. Chiang, and F. Winston. 1991. Mutations in SPT16/CDC68 suppress cis- and trans-acting mutations that affect promoter function in Saccharomyces cerevisiae. Mol. Cell. Biol. 115710-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martens, J. A., and F. Winston. 2002. Evidence that Swi/Snf directly represses transcription in S. cerevisiae. Genes Dev. 162231-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason, P. B., and K. Struhl. 2003. The FACT complex travels with elongating RNA polymerase II and is important for the fidelity of transcriptional initiation in vivo. Mol. Cell. Biol. 238323-8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavri, R., B. Zhu, G. Li, P. Trojer, S. Mandal, A. Shilatifard, and D. Reinberg. 2006. Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II. Cell 125703-717. [DOI] [PubMed] [Google Scholar]
- 20.Rowley, A., R. A. Singer, and G. C. Johnston. 1991. CDC68, a yeast gene that affects regulation of cell proliferation and transcription, encodes a protein with a highly acidic carboxyl terminus. Mol. Cell. Biol. 115718-5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simic, R., D. L. Lindstrom, H. G. Tran, K. L. Roinick, P. J. Costa, A. D. Johnson, G. A. Hartzog, and K. M. Arndt. 2003. Chromatin remodeling protein Chd1 interacts with transcription elongation factors and localizes to transcribed genes. EMBO J. 221846-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuwe, T., M. Hothorn, E. Lejeune, V. Rybin, M. Bortfeld, K. Scheffzek, and A. G. Ladurner. 2008. The FACT Spt16 “peptidase” domain is a histone H3-H4 binding module. Proc. Natl. Acad. Sci. USA 1058884-8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tardiff, D. F., K. C. Abruzzi, and M. Rosbash. 2007. Protein characterization of Saccharomyces cerevisiae RNA polymerase II after in vivo cross-linking. Proc. Natl. Acad. Sci. USA 10419948-19953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wada, T., T. Takagi, Y. Yamaguchi, A. Ferdous, T. Imai, S. Hirose, S. Sugimoto, K. Yano, G. A. Hartzog, F. Winston, S. Buratowski, and H. Handa. 1998. DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity, is composed of human Spt4 and Spt5 homologs. Genes Dev. 12343-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winston, F., D. T. Chaleff, B. Valent, and G. R. Fink. 1984. Mutations affecting Ty-mediated expression of the HIS4 gene of Saccharomyces cerevisiae. Genetics 107179-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Workman, J. L. 2006. Nucleosome displacement in transcription. Genes Dev. 202009-2017. [DOI] [PubMed] [Google Scholar]
- 27.Zhang, L., S. Schroeder, N. Fong, and D. L. Bentley. 2005. Altered nucleosome occupancy and histone H3K4 methylation in response to ′transcriptional stress.’ EMBO J. 242379-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]