Abstract
Toxoplasma gondii motility is powered by the myosin XIV motor complex, which consists of the myosin XIV heavy chain (MyoA), the myosin light chain (MLC1), GAP45, and GAP50, the membrane anchor of the complex. MyoA, MLC1, and GAP45 are initially assembled into a soluble complex, which then associates with GAP50, an integral membrane protein of the parasite inner membrane complex. While all proteins in the myosin XIV motor complex are essential for parasite survival, the specific role of GAP45 remains unclear. We demonstrate here that final assembly of the motor complex is controlled by phosphorylation of GAP45. This protein is phosphorylated on multiple residues, and by using mass spectroscopy, we have identified two of these, Ser163 and Ser167. The importance of these phosphorylation events was determined by mutation of Ser163 and Ser167 to Glu and Ala residues to mimic phosphorylated and nonphosphorylated residues, respectively. Mutation of Ser163 and Ser167 to either Ala or Glu residues does not affect targeting of GAP45 to the inner membrane complex or its association with MyoA and MLC1. Mutation of Ser163 and Ser167 to Ala residues also does not affect assembly of the mutant GAP45 protein into the myosin motor complex. Mutation of Ser163 and Ser167 to Glu residues, however, prevents association of the MyoA-MLC1-GAP45 complex with GAP50. These observations indicate that phosphorylation of Ser163 and Ser167 in GAP45 controls the final step in assembly of the myosin XIV motor complex.
Toxoplasma gondii and other apicomplexan parasites must be motile at many stages in their life cycle, including invasion and escape from host cells. These parasites lack structures commonly used by other cells for motility (e.g., pseudopods, cilia, and flagella), instead relying on a unique, substrate-dependent mechanism termed gliding motility (1). This complex process involves not only an actin-myosin motor but also proteins that connect the F-actin to extracellular ligands and that anchor the myosin motor in the inner membrane complex (9, 15). The motility of Toxoplasma tachyzoites is activated by the decrease in potassium concentration in their immediate environment that follows disruption of an infected host cell (20). This, in turn, results in the activation of phospholipase C activity in the parasite, which results in an increase in cytoplasmic calcium (20). The latter appears to be a trigger for the fusion of small secretory organelles, i.e., micronemes, with the parasite plasma membrane and the subsequent secretion of micronemal adhesins that mediate parasite attachment to host cells (25). The cytoplasmic domain of one of these adhesins, MIC2, has been found to interact with cytoplasmic aldolase, which in turn associates with F-actin (14).
Regulation of parasite motility can occur at several levels, including the secretion of adhesins (26), actin polymerization (24), and motor activity. Inhibitor studies have implicated protein phosphorylation as one regulator of motility and invasion. Specifically, a calmodulin domain protein kinase (CDPK1) may play a critical role, as its inhibition blocks both parasite motility and host cell attachment (8, 16). An inhibitor of cyclic GMP-dependent protein kinase similarly blocks motility and invasion (27). Toxofilin, an actin-binding protein, is regulated through a phosphorylation-dephosphorylation cycle by a casein kinase II-type activity and a 2C serine/threonine phosphatase-like activity (6). Other than toxofilin, no kinase/phosphatase targets involved in motility have been identified.
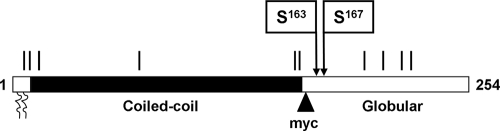
Parasite motility is powered by a class XIV myosin, myosin A (MyoA) (19). The motor domain lacks the TEDS consensus site for Ser/Thr phosphorylation that controls mechanochemical function in other myosins (2, 21). Another striking feature of class XIV myosins is an extremely short tail, the domain that typically determines function by mediating binding to organelles or other myosin subunits (7). These unique features of apicomplexan class XIV myosins suggest a novel method of regulation. MyoA is found in a complex with an atypical myosin light chain (MLC1) and two novel proteins, GAP50 and GAP45 (9). GAP50 is an integral membrane protein of the inner membrane complex (IMC) that anchors and immobilizes the motor complex in the plane of the IMC membrane in a cholesterol-dependent manner (15). The function of the fourth subunit of the motor complex, GAP45, is less clear. It is highly conserved among apicomplexans but has no sequence homology to known proteins (9). GAP45 is a 254-residue protein with N-terminal myristoylation and palmitoylation sites that anchor the protein in the IMC. The remainder of the protein is composed of two domains, an N-terminal domain predicted to form a coiled-coil and a C-terminal domain that is predicted to have a globular structure.
We have previously shown that the myosin XIV motor complex is assembled in two stages (9). MyoA, MLC1, and GAP45 are initially assembled into a soluble complex in the parasite cytoplasm. The glycoprotein GAP50 is inserted cotranslationally into the endoplasmic reticulum and transported to the IMC, presumably by vesicular trafficking, where it associates with the MyoA-MLC1-GAP45 complex.
Given the critical role of the myosin XIV motor complex in parasite motility and host cell invasion, it is likely that its activity and/or assembly is tightly controlled at the various stages of parasite-host cell interaction. Here we demonstrate that GAP45 is phosphorylated on multiple residues and that phosphorylation of Ser163 and Ser167 plays an important role in controlling the final assembly of the myosin XIV motor complex.
MATERIALS AND METHODS
Materials.
All chemicals were from Sigma (St. Louis, MO), unless otherwise noted. Protease inhibitor stocks contained 104 mM 4-(2-aminoethyl)-benzene-sulfonyl fluoride, 0.08 mM aprotinin, 2.1 mM leupeptin, 3.6 mM bestatin, 1.5 mM pepstatin A, and 1.4 mM E-64 in dimethyl sulfoxide. All materials used for two-dimensional (2D) gel electrophoresis were of ultrapure quality.
Parasite culture.
The T. gondii RH(EP) wild-type strain was maintained by serial passage in confluent cultures of human foreskin fibroblasts (HFFs) as previously described (23). Parasites released from freshly lysed HFFs were filtered through 3-μm Nuclepore syringe filters (Whatman, Clifton, NJ) to remove host cell debris and washed twice by centrifugation (5 min, 1,000 × g) in cold phosphate-buffered saline prior to use. Transfections were done as previously described (23), and stable parasites were selected with chloramphenicol (17).
Metabolic labeling and immunoprecipitation.
For metabolic labeling with [35S]methionine-cysteine, 107 parasites were added to a confluent HFF monolayer in a T25 flask. After 14 to 16 h, cells were incubated for 1 h in methionine- and cysteine-free Dulbecco's modified Eagle's medium (HyClone, Logan, UT) containing 1% (vol/vol) fetal bovine serum prior to the addition of 0.7 mCi [35S]methionine-cysteine (GE Biosciences, Piscataway, NJ).
For metabolic labeling with [32P]orthophosphate, a T75 flask with a confluent monolayer of HFF cells was infected with 3 × 107 parasites. After 14 to 16 h, cells were incubated for 1 h in phosphate-free Dulbecco's modified Eagle's medium (HyClone) containing 5% fetal bovine serum, followed by the addition of 1 mCi [32P]orthophosphate. Parasites were collected after being labeled for 20 to 24 h and extracted in TX/TEN buffer (1% Triton X-100, 10 mM Tris, pH 8, 150 mM NaCl, 2 mM EDTA, 1:100 protease inhibitors). Immunoprecipitation was carried out as previously described (9), using anti-GAP45 rabbit serum (1:1,000) or anti-myc monoclonal antibody 9E10 (1:500), followed by incubation with protein A-Sepharose or protein G-Sepharose, respectively (Zymed, San Francisco, CA), for 1 h at 4°C. The beads were washed four times in Triton X-100 buffer, and bound proteins were eluted in reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 4% (vol/vol) β-mercaptoethanol.
Association of mycGAP45 and the mycGAP45 phosphorylation site mutants with the other subunits of the myosin motor complex was determined by immunoprecipitating mycGAP45 from parasites 24 h after transfection. Samples were fractionated by SDS-PAGE under nonreducing conditions, transferred to nitrocellulose, and analyzed by immunoblotting with monospecific antisera to MyoA (a generous gift from D. Soldati), GAP50, GAP45, and MLC1.
GAP45 phosphorylation analysis by 2D gel electrophoresis.
Parasites (107) were disrupted by a single freeze-thaw step in 20 μl 50 mM Tris-HCl, pH 9.3, 1 mM MgCl2, and 0.1 mM ZnCl2 and incubated for 1 h at 37°C in the presence or absence of 2.5 units of calf intestinal phosphatase (Promega, Madison, WI). The reaction was stopped by the addition of isoelectric focusing (IEF) sample buffer (7 M urea, 2 M thiourea, 1% ASB-14, 40 mM Tris base, 0.5% Biolytes 3-10, 2 mM tributylphosphine). Samples were analyzed by 2D gel electrophoresis using 7-cm pH 4.5 to 5.2 or 18-cm pH 4.5 to 5.5 IPG ReadyStrips (Bio-Rad, Carlsbad, CA) in the first dimension and a 12% polyacrylamide gel in the second dimension. Following transfer to nitrocellulose, Western blotting with rabbit anti-GAP45 was done as previously described (9), and gels were silver stained.
Purification and mass spectrometry analysis to identify phosphorylation sites.
Parasites (5 × 108) were extracted on ice in 150 mM NaCl, Tris-HCl, pH 7.4, containing 0.5% Triton X-114 and protease inhibitors. Detergent-insoluble material was removed by centrifugation twice for 5 min each time at 14,000 × g at 4°C. The detergent-soluble fraction was subjected to phase separation by incubation for 5 min at 37°C, and the detergent phase was collected by centrifugation for 5 min at 300 × g (3). The detergent pellet was subjected to acetone precipitation and focused on 18-cm pH 4.4 to 5.5 IPG strips as previously described (10). The second-dimension SDS-PAGE gel was stained with SimplyBlue colloidal Coomassie blue stain (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions; spots corresponding to the second, third, and fourth phosphoforms (where the most basic phosphoform is spot 1) were pooled from three separate gels, for a total of nine spots, and analyzed by liquid chromatography-tandem mass spectrometry (Taplin Biological Mass Spectrometry Facility, Harvard University, Boston, MA).
Cloning of mycGAP45 and phosphorylation site mutants.
The GAP45 open reading frame was cloned into pBluescript and an internal myc tag was inserted into GAP45 after amino acid 157 to create pBluescript-mycGAP45 through inverse PCR using the following primers (myc tag sequence is capitalized): forward, ATATCTGAAGAGGATCTGaagtacgacaagttagccagc; and reverse, CAGTTTCTGTTCctctctcggtgacatttcctc. Single phosphorylation site mutants were generated by site-directed mutagenesis with the following primer pairs (mutated residues are underlined): for S163A mutation, CGACAAGTTAGCCGCCCCCGAAGACTCCG and CGGAGTCTTCGGGGGCGGCTAACTTGTCG; for S163E mutation, GAAGTACGACAAGTTAGCCGAGCCCGAAGACTCCGCATC and GATGCGGAGTCTTCGGGCTCGGCTAACTTGTCGTACTTC; for S167A mutation, GCCCCGAAGACGCCGCATCCGAGAC and GTCTCGGATGCGGCGTCTTCGGGGC; and for S167E mutation, GCCAGCCCCGAAGACGAAGCATCCGAGACCACG and CGTGGTCTCGGATGCTTCGTCTTCGGGGCTGGC. The S163A/S167A double mutant was created using the primers CCCCCGAAGACGCAGCATCCGAGAC and GTCTCGGATGCTGCGTCTTCGGGGG and the S163A mutant as a template. The S163E/S167E double mutant was created using the primers GTACGACAAGTTAGCCGAACCCGAAGACGAAGCATC and GATGCTTCGTCTTCGGGTTCGGCTAACTTGTCGTAC and the S167E mutant as a template. Inserts were then cloned into pTUB-YFP/CAM (12) and confirmed by sequencing.
Immunofluorescence microscopy.
Approximately 24 h prior to use, parasites were added to confluent HFF monolayers on glass coverslips. Following fixation (3% paraformaldehyde for 10 min at room temperature), parasites were blocked and permeabilized in 3% (wt/vol) bovine serum albumin-0.25% (vol/vol) Triton X-100 in phosphate-buffered saline for 10 min, followed by incubation with primary and Alexa Fluor-conjugated secondary antibodies as previously described (18). Primary antibodies used were mouse monoclonal anti-IMC1, rabbit anti-IMC1 (18), rabbit anti-GAP45 (9), and mouse anti-myc monoclonal antibody 9E10 (ATCC, Manassas, VA).
Gel filtration analysis.
Extracellular parasites expressing myc-tagged GAP45 were extracted for 10 min on ice in 1% Triton X-100 in 150 mM NaCl, 5 mM EDTA, and 25 mM Tris-HCl, pH 7.6, in the presence of protease inhibitors. Insoluble material was removed by centrifugation for 10 min at 14,000 × g at 4°C. The soluble material was filtered through a 0.2-μm filter and fractionated by size-exclusion chromatography on a Superdex 200 column (GE Healthcare, Piscataway, NJ) that had been calibrated with size standards (GE Healthcare). Fractions (0.5 ml) were collected, precipitated with trichloroacetic acid, and analyzed by SDS-PAGE and immunoblotting with antibodies specific to the myc epitope, myosin A, GAP50, GAP45, and MLC1.
RESULTS
GAP45 is phosphorylated on multiple residues.
During our previous analysis of GAP45 (9), we noted that this protein does not migrate as a single species on 2D gels, appearing instead as a series of spots with nearly identical molecular weights but different isoelectric points (Fig. 1A). To determine whether phosphorylation contributes to this charge heterogeneity, we treated protein samples with alkaline phosphatase prior to electrophoresis. This resulted in a collapse of the GAP45 spots, confirming that the charge heterogeneity of GAP45 is indeed caused by phosphorylation (Fig. 1A). This conclusion was confirmed by the immunoprecipitation of GAP45 from parasites incubated with [32P]orthophosphate (Fig. 1B).
FIG. 1.
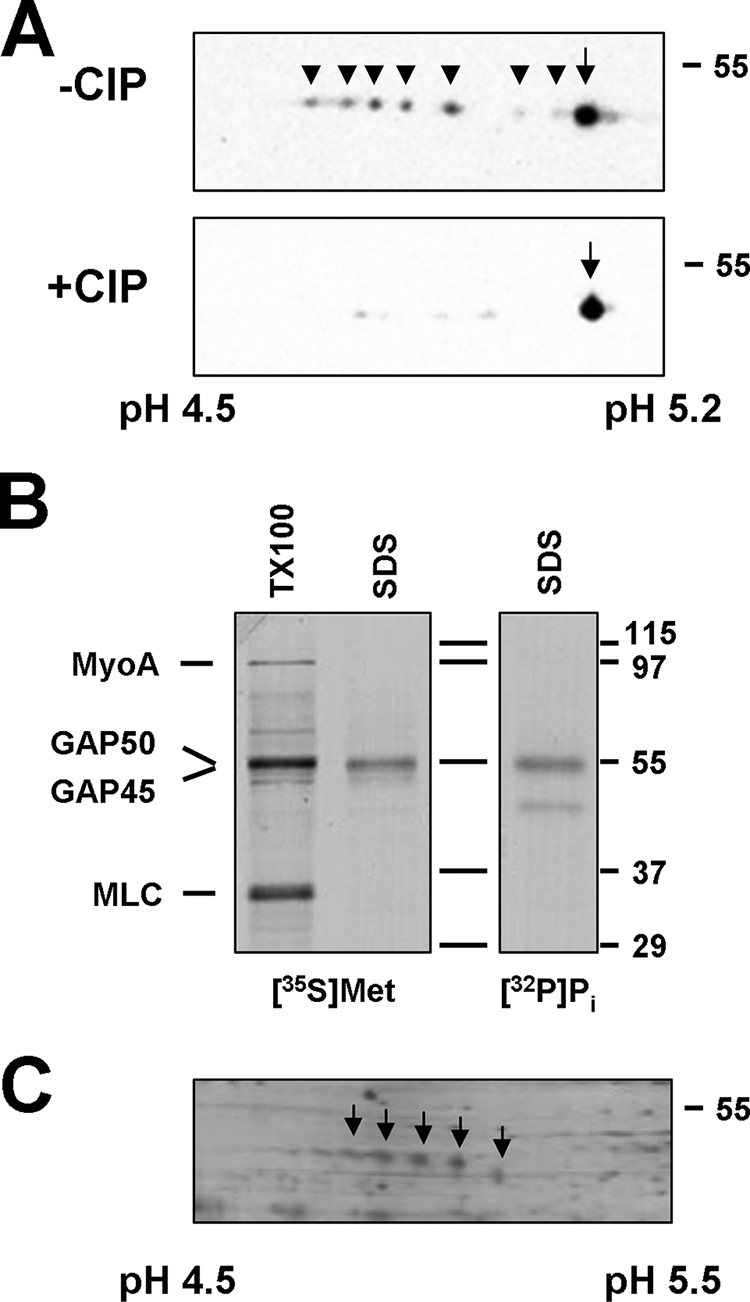
GAP45 is phosphorylated on multiple residues. (A) 2D gel electrophoresis reveals the presence of up to eight GAP45 species with different isoelectric points in untreated parasite lysates (−CIP). Following treatment with calf intestine phosphatase (+CIP), only a single species was observed, indicating that the observed heterogeneity is due to phosphorylation. Samples were separated by IEF on pH 4.5 to 5.2 IPG strips, followed by SDS-PAGE on 12% gels and anti-GAP45 immunoblotting. The phosphorylated GAP45 species are labeled with arrowheads, and the nonphosphorylated species are labeled with arrows. The acidic and basic ends of the IEF strips are indicated. (B) Immunoprecipitation of GAP45 from an SDS lysate of [32P]orthophosphate-labeled tachyzoites demonstrates that GAP45 is phosphorylated. GAP45 immunoprecipitates from an SDS lysate and a Triton X-100 lysate of [35S]methionine-cysteine-labeled parasites were prepared in parallel. In lysates generated with Triton X-100, all subunits of the myosin XIV motor complex were immunoprecipitated by the anti-GAP45 antiserum. (C) Toxoplasma GAP45 is an abundant protein. A parasite lysate was separated by IEF using pH 4.5 to 5.5 IPG strips, followed by SDS-PAGE and silver staining. The different GAP45 species are marked with arrows.
Upon 2D gel electrophoresis of parasite lysates, we routinely observed up to seven phosphorylated species of GAP45 in addition to the nonphosphorylated form (Fig. 1A). This observation suggests that GAP45 is phosphorylated on at least seven residues. The relative intensities of the individual spots varied between preparations, but we have not found any obvious correlations between this and any specific aspect of parasite preparation.
Identification of phosphorylation sites on GAP45.
Since the Toxoplasma genome does not appear to encode tyrosine kinases and GAP45 is not exposed to host cell protein kinases, we analyzed the predicted sequence of GAP45 for the presence of a variety of potential phosphorylation sites for Ser/Thr-specific protein kinases (Fig. 2). Given the limited reliability of the algorithms used for such analyses, we decided to identify actual GAP45 phosphorylation sites used in vivo. When parasite lysates were separated by 2D gel electrophoresis and stained, several GAP45 species were readily detected (Fig. 1C). Since the most basic GAP45 species (far right spot in Fig. 1C) likely corresponds to the nonphosphorylated form of the protein, this was not analyzed further. The three major GAP45 spots corresponding to phosphorylated forms were excised from the gels and analyzed by mass spectroscopy, resulting in approximately 50% coverage. One peptide, LASPEDSASETTMATQPQK, was found in three forms, namely, nonphosphorylated, phosphorylated on a single residue, and phosphorylated on two residues. The first two serines of this peptide, Ser163 and Ser167 of the full-length protein, were identified as the phosphorylated residues.
FIG. 2.
GAP45 domain structure and posttranslational modifications. The GAP45 domain structure consists of an N-terminal coiled-coil domain and a C-terminal globular domain. Predicted serine/threonine phosphorylation sites are indicated by vertical lines; the two identified phosphorylation sites, Ser163 and Ser167, are indicated by arrows. The location of the myc tag between residues 157 and 158 is also indicated, along with the N-terminal myristoylation and palmitoylation sites (squiggly lines).
Although at this time we cannot be certain of the total number of GAP45 phosphorylation sites used in vivo, the two sites identified here plus the nonphosphorylated form are theoretically sufficient to account for three of the major species seen in Fig. 1A. It is interesting that the identified phosphorylation sites are located close to the boundary between the predicted N-terminal coiled-coil domain and the C-terminal globular domain (Fig. 2), suggesting that changes in their phosphorylation state could have a pronounced effect on the overall structure of the protein.
Expression of epitope-tagged GAP45 in Toxoplasma.
To examine the role of phosphorylation of Ser163 and Ser167, we determined the impact of mutating these two residues on assembly and function of the myosin XIV motor complex. In order to differentiate between endogenous and mutated GAP45 proteins, we generated several versions of GAP45 where a myc epitope was inserted at either terminus or at various internal positions. In most cases, epitope insertion disrupted expression of the tagged GAP45 protein, targeting to the IMC, or assembly of the myosin XIV complex (data not shown). Correct targeting of a tagged GAP45 protein (mycGAP45) along with incorporation into the myosin XIV motor complex and a good level of expression was obtained only when the epitope tag was inserted between the predicted coiled-coil and globular domains. Parasites expressing this construct did not show any obvious defects in motility or host cell invasion (data not shown), indicating that mycGAP45 is not detrimental to Toxoplasma viability.
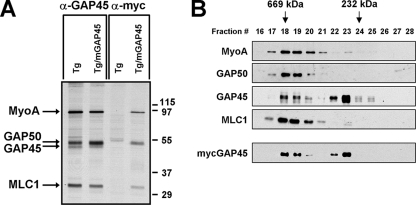
When mycGAP45 was immunoprecipitated from [35S]methionine-cysteine-labeled parasites, MyoA and MLC1 were clearly immunoprecipitated along with mycGAP45 (Fig. 3A). Since mycGAP45 and GAP50 migrate with the same apparent molecular weight during SDS-PAGE, it was not clear whether GAP50 was present in the complex and therefore whether mycGAP45 was incorporated into the mature myosin XIV motor complex or not. Immunoprecipitation of mycGAP45 followed by immunoblotting with antisera to MyoA, GAP50, and MLC1 clearly demonstrated that mycGAP45 was in fact associated with these three proteins, confirming that it is incorporated into the complete myosin XIV motor complex (see Fig. 5). This conclusion was confirmed by gel filtration analysis of mycGAP45-expressing parasites (Fig. 3B). Like wild-type GAP45, mycGAP45 is present in the mature myosin XIV complex, indicating that mycGAP45 does indeed behave like endogenous GAP45. It should be noted that the apparent molecular size of the myosin XIV motor complex (∼730 kDa) is much larger than the predicted size (∼200 kDa), suggesting that one or more of its subunits have an extended shape.
FIG. 3.
mycGAP45 associates with other members of the myosin XIV motor complex. (A) GAP45 antiserum immunoprecipitates a complex of MyoA, MLC1, GAP45, and GAP50 from [35S]methionine-cysteine-labeled wild-type parasites. With parasites expressing mycGAP45, immunoprecipitation with anti-myc pulls down mycGAP45, MyoA, and MLC1, demonstrating that addition of the myc tag does not interfere with assembly of the myosin XIV motor complex. GAP50 and GAP45/mycGAP45 migrate at the same molecular weight on SDS-PAGE gels. (B) Nontransfected parasites and parasites expressing mycGAP45 were analyzed by gel filtration chromatography on Superdex 200 columns, and the fractions were analyzed by immunoblotting for the various members of the myosin XIV motor complex. The subunits of the mature complex, MyoA, GAP50, GAP45/mycGAP45, and MLC1, all coeluted in the higher-molecular-size fractions, peaking in fractions 18 and 19. The fractions containing thyroglobulin (669 kDa) and catalase (232 kDa) are indicated by arrows.
FIG. 5.

GAP45 phosphorylation is involved in assembly of the myosin XIV motor complex. Parasites transiently expressing mycGAP45 (wt), mycGAP45(Ser163Ala), mycGAP45(Ser163Glu), mycGAP45(Ser167Ala), mycGAP45(Ser167Glu), mycGAP45(Ser163Ala, Ser167Ala), and mycGAP45(Ser163Glu, Ser167Glu) were immunoprecipitated with a monoclonal antibody to the myc epitope. Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with monospecific rabbit antisera to MyoA, GAP50, GAP45, and MLC1.
Phosphorylation of Ser163 and Ser167 blocks assembly of the mature myosin XIV complex.
To test the role of phosphorylation of Ser163 and Ser167 on GAP45 function, we altered these residues in mycGAP45 individually or together to either alanine or glutamate to mimic nonphosphorylated or phosphorylated serine, respectively. As shown in Fig. 4A and B, all six GAP45 mutants were expressed and targeted correctly to the Toxoplasma pellicle, indicating that phosphorylation of these residues does not play a role in targeting to the IMC. Expression of the GAP45 mutants did not have any obvious deleterious effects on parasite motility or host cell invasion immediately after transfection. Stable parasite lines expressing the mycGAP45(Ser163Glu) and mycGAP45(Ser167Glu) mutants were readily obtained, and these parasites did not demonstrate any noticeable defects in motility or host cell invasion. It should be noted that the levels of expression of these mutants were <1% of the wild-type GAP45 level, as judged by immunoprecipitation (data not shown). We were not successful in generating parasite lines stably expressing the mycGAP45(Ser163Ala) and mycGAP45(Ser167Ala) mutants or either double mutant, however, indicating that their expression confers a selective disadvantage to parasites.
FIG. 4.
GAP45 phosphorylation site mutants localize to the parasite periphery. mycGAP45, mycGAP45(Ser163Ala), mycGAP45(Ser163Glu), mycGAP45(Ser167Ala), and mycGAP45(Ser167Glu) (A) and mycGAP45(Ser163Ala, Ser167Ala) and mycGAP45(Ser163Glu, Ser167Glu) (B) are targeted correctly to the Toxoplasma pellicle, as judged by their colocalization with the IMC marker protein IMC1.
When we tested the ability of mycGAP45 phosphorylation site mutants to associate with other subunits of the myosin XIV motor complex, it was clear that mycGAP45(Ser163Ala), mycGAP45(Ser167Ala), and mycGAP45(Ser163Ala, Ser167Ala) were associated with MyoA, MLC1, and GAP50 by coimmunoprecipitation (Fig. 5). The mycGAP45(Ser163Glu), mycGAP45(Ser167Glu), and mycGAP45(Ser163Glu, Ser167Glu) mutants, on the other hand, associated with MyoA and MLC1 but failed to associate with GAP50 (Fig. 5).
Together, these data suggest that the phosphorylation state of both Ser163 and Ser167 in GAP45 controls assembly of the myosin motor complex. Specifically, they indicate that dephosphorylation of both residues is required for the final assembly of the MyoA-MLC1-GAP45 complex with GAP50 into the fully active myosin XIV motor complex.
DISCUSSION
The assembly of protein complexes is a carefully controlled process which frequently occurs during or immediately after synthesis of the subunits, but it can also occur at specific stages in the life of a cell or in response to specific stimuli. We have shown previously that the myosin XIV motor complex of Toxoplasma gondii consists of four proteins, namely, the myosin XIV heavy chain (MyoA), MLC1, the membrane anchor GAP50, and GAP45 (9). This complex is assembled in two stages. First, a complex of MyoA, MLC1, and GAP45 is assembled in the cytoplasm, while GAP50 is inserted into the parasite endoplasmic reticulum and trafficked from there to the IMC. Final assembly occurs at the IMC (9). It was not clear, however, which mechanism(s) controls the final assembly of the myosin XIV motor complex and how assembly is confined to the IMC. Whereas specific functions have been assigned to the other complex members (15, 19), the role of GAP45 has proven elusive. This protein appears to be essential for parasite survival, as attempts to generate a knockout have proven unsuccessful (9). GAP45 is also highly conserved among other members of the phylum Apicomplexa, but it has no obvious homologs in other eukaryotes.
We have shown here that GAP45 is phosphorylated on multiple residues and that its phosphorylation plays an important role in assembly of the myosin XIV motor complex. GAP45 is predicted to contain more than 10 potential phosphorylation sites for Ser/Thr-specific kinases (Fig. 2), but as we typically detect up to eight species on 2D gels (Fig. 1A), it is possible that as few as seven of the predicted phosphorylation sites are actually used in vivo. The prediction of phosphorylation sites in proteins is not accurate at this time (5), and in the case of understudied organisms such as apicomplexans, this problem is compounded by the presence of protein kinases whose sequence specificity is not known (16). Direct analysis of phosphorylation sites in GAP45 by mass spectroscopy revealed that Ser163 and Ser167 are phosphorylated in vivo. As indicated above, 2D gel electrophoresis identified at least seven phosphorylated GAP45 species, suggesting that at least five additional Ser or Thr residues are phosphorylated in vivo. There are several possible explanations for our failure to detect the additional phosphorylation sites, but we believe that the use of other proteases is likely to reveal additional phosphorylation sites by mass spectroscopy.
It is unclear which protein kinases phosphorylate these particular residues in Toxoplasma. Various prediction algorithms (e.g., NetPhos [http://www.cbs.dtu.dk/services/NetPhos/] and group-based phosphorylation scoring [http://bioinformatics.lcd-ustc.org/gps_web]) suggest multiple (more than five) classes of protein kinases that might phosphorylate either Ser163 or Ser167, but it is not clear at this time whether these particular enzymes are in fact present in Toxoplasma. Interestingly, Ser163 and Ser167 are found near the boundary of the predicted N-terminal coiled-coil domain and the C-terminal globular domain, suggesting that the presence or absence of phosphorylated residues may have an impact on the overall structure of the GAP45 protein and therefore on assembly or activity of the myosin XIV motor complex.
In order to determine whether the phosphorylation state of Ser163 and Ser167 of GAP45 does in fact have an impact on assembly or activity of the myosin XIV motor complex, we mutated these residues to alanines, to mimic nonphosphorylated serines, and to glutamates, to mimic phosphorylated serines. All GAP45 mutants localized normally to the parasite pellicle, indicating that the phosphorylation state of Ser163 and Ser167 does not affect the subcellular targeting of the protein (Fig. 4A and B). Phosphorylation of Ser163 and Ser167 also does not appear to be necessary for assembly of the myosin XIV motor complex, as the individual Ser163Ala and Ser167Ala mutants and the Ser163Ala/Ser167Ala double mutant of mycGAP45 associated normally with MyoA, MLC1, and GAP50 (Fig. 5). The Ser163Glu and Ser167Glu mutants and the Ser163Glu/Ser167Glu double mutant of GAP45 also associated normally with MyoA and MLC1, but the resulting complex did not associate with GAP50, suggesting that the final assembly step of the myosin XIV motor complex requires dephosphorylation of Ser163 and Ser167 of GAP45. Specifically, we propose that phosphorylation of these residues is required to prevent association of the newly synthesized MyoA-MLC1-GAP45 precursor complex with GAP50 until the latter reaches its final destination in the IMC, where dephosphorylation of Ser163 and Ser167 enables assembly of the full complex.
Apart from controlling assembly of the myosin XIV motor complex, protein phosphorylation and dephosphorylation are likely to affect Toxoplasma motility at several other steps, such as controlling the activity of the myosin XIV motor complex and polymerization of F-actin. The protein kinase CDPK1 is known to control Toxoplasma motility (16), but this enzyme does not phosphorylate any of the motor complex subunits in vitro (data not shown), suggesting that it acts on other proteins critical for motility. This is in contrast to recent observations in Plasmodium falciparum, where a CDPK1 homolog was found to phosphorylate GAP45 on Ser or Thr residues between positions 97 and 122 and positions 141 and 155 in vivo (11). The two CDPK1 homologs also differ in that, unlike the Plasmodium enzyme, Toxoplasma CDPK1 is not sensitive to K252a (16) and is a cytoplasmic rather than a pellicle-associated enzyme (22). The genomes of both Toxoplasma and Plasmodium encode multiple CDPK homologs, however, so it is possible that a different CDPK1-like enzyme can in fact phosphorylate GAP45 in Toxoplasma. It is also worth noting that based on 2D gel analysis, Toxoplasma GAP45 is clearly phosphorylated on at least seven sites (Fig. 1), whereas the extent of Plasmodium GAP45 phosphorylation is clearly more limited (11).
In addition to CDPKs, a casein kinase 2-like activity has also been implicated in the control of Toxoplasma motility, although the enzyme involved has not been identified to date (6). Our observations indicate that protein phosphatases are required for assembly of the myosin XIV motor complex. We described previously that the protein phosphatase 2B (PP2B) inhibitor cyclosporine A is a potent inhibitor of Toxoplasma motility (20). Its effect is observed after only a brief preincubation of parasites, however, suggesting that it does not affect assembly of the motor complex but acts by blocking dephosphorylation of other phosphorylation sites in GAP45, other subunits of the motor complex, or another protein required for motility. PP2C has also been implicated in the control of Toxoplasma motility (6). Initially, it was believed that Toxoplasma PP2C binds to and acts on toxofilin, a parasite protein that binds to G-actin (6). More recently, it was found that toxofilin is most likely secreted into the host cell during infection (4), where it interacts with host rather than parasite actin (13). It is not clear at this time, however, if Toxoplasma PP2C is secreted along with toxofilin into the host cell, or if a host cell PP2C acts on toxofilin instead. The possibility therefore remains that Toxoplasma PP2C can also dephosphorylate other parasite proteins such as GAP45.
Identification of additional GAP45 phosphorylation sites as well as identification of the specific protein kinases and phosphatases that act on GAP45 will shed more light on the control of assembly and activity of this complex and on parasite motility in general.
Acknowledgments
We thank Dominique Soldati for the generous gift of antisera to myosin A. We also thank members of the Beckers lab for thoughtful discussions and critical readings of the manuscript.
This work was supported by PHS grant AI041765 (C.J.B.), The Burroughs Wellcome Fund (G.E.W.), and PHS grants AI054961 and AI01719 (G.E.W.).
Footnotes
Published ahead of print on 1 December 2008.
REFERENCES
- 1.Baum, J., A. T. Papenfuss, B. Baum, T. P. Speed, and A. F. Cowman. 2006. Regulation of apicomplexan actin-based motility. Nat. Rev. Microbiol. 4621-628. [DOI] [PubMed] [Google Scholar]
- 2.Bement, W. M., and M. S. Mooseker. 1995. TEDS rule: a molecular rationale for differential regulation of myosins by phosphorylation of the heavy chain head. Cell. Motil. Cytoskeleton 3187-92. [DOI] [PubMed] [Google Scholar]
- 3.Bordier, C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 2561604-1607. [PubMed] [Google Scholar]
- 4.Bradley, P. J., C. Ward, S. J. Cheng, D. L. Alexander, S. Coller, G. H. Coombs, J. D. Dunn, D. J. Ferguson, S. J. Sanderson, J. M. Wastling, and J. C. Boothroyd. 2005. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J. Biol. Chem. 28034245-34258. [DOI] [PubMed] [Google Scholar]
- 5.Chang, E. J., R. Begum, B. T. Chait, and T. Gaasterland. 2007. Prediction of cyclin-dependent kinase phosphorylation substrates. PLoS ONE 2e656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delorme, V., X. Cayla, G. Faure, A. Garcia, and I. Tardieux. 2003. Actin dynamics is controlled by a casein kinase II and phosphatase 2C interplay on Toxoplasma gondii toxofilin. Mol. Biol. Cell 141900-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DePina, A. S., and G. M. Langford. 1999. Vesicle transport: the role of actin filaments and myosin motors. Microsc. Res. Tech. 4793-106. [DOI] [PubMed] [Google Scholar]
- 8.Dobrowolski, J. M., V. B. Carruthers, and L. D. Sibley. 1997. Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii. Mol. Microbiol. 26163-173. [DOI] [PubMed] [Google Scholar]
- 9.Gaskins, E., S. D. Gilk, N. DeVore, T. Mann, L. Peck, G. E. Ward, and C. Beckers. 2004. Identification of the membrane receptor of a class XIV myosin in Toxoplasma gondii. J. Cell Biol. 165383-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilk, S. D., Y. Raviv, K. Hu, J. M. Murray, C. J. Beckers, and G. E. Ward. 2006. Identification of PhIL1, a novel cytoskeletal protein of the Toxoplasma gondii pellicle, through photosensitized labeling with 5-[125I]iodonaphthalene-1-azide. Eukaryot. Cell 51622-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green, J. L., R. R. Rees-Channer, S. A. Howell, S. R. Martin, E. Knuepfer, H. M. Taylor, M. Grainger, and A. A. Holder. 2008. The motor complex of Plasmodium falciparum: phosphorylation by a calcium-dependent protein kinase. J. Biol. Chem. 28330980-30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu, K., T. Mann, B. Striepen, C. J. Beckers, D. S. Roos, and J. M. Murray. 2002. Daughter cell assembly in the protozoan parasite Toxoplasma gondii. Mol. Biol. Cell 13593-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jan, G., V. Delorme, V. David, C. Revenu, A. Rebollo, X. Cayla, and I. Tardieux. 2007. The toxofilin-actin-PP2C complex of Toxoplasma: identification of interacting domains. Biochem. J. 401711-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jewett, T. J., and L. D. Sibley. 2003. Aldolase forms a bridge between cell surface adhesins and the actin cytoskeleton in apicomplexan parasites. Mol. Cell 11885-894. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, T. M., Z. Rajfur, K. Jacobson, and C. J. Beckers. 2007. Immobilization of the type XIV myosin complex in Toxoplasma gondii. Mol. Biol. Cell 183039-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kieschnick, H., T. Wakefield, C. A. Narducci, and C. Beckers. 2001. Toxoplasma gondii attachment to host cells is regulated by a calmodulin-like domain protein kinase. J. Biol. Chem. 27612369-12377. [DOI] [PubMed] [Google Scholar]
- 17.Kim, K., D. Soldati, and J. C. Boothroyd. 1993. Gene replacement in Toxoplasma gondii with chloramphenicol acetyltransferase as selectable marker. Science 262911-914. [DOI] [PubMed] [Google Scholar]
- 18.Mann, T., and C. Beckers. 2001. Characterization of the subpellicular network, a filamentous membrane skeletal component in the parasite Toxoplasma gondii. Mol. Biochem. Parasitol. 115257-268. [DOI] [PubMed] [Google Scholar]
- 19.Meissner, M., D. Schluter, and D. Soldati. 2002. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science 298837-840. [DOI] [PubMed] [Google Scholar]
- 20.Moudy, R., T. J. Manning, and C. J. Beckers. 2001. The loss of cytoplasmic potassium upon host cell breakdown triggers egress of Toxoplasma gondii. J. Biol. Chem. 27641492-41501. [DOI] [PubMed] [Google Scholar]
- 21.Novak, K. D., and M. A. Titus. 1998. The myosin I SH3 domain and TEDS rule phosphorylation site are required for in vivo function. Mol. Biol. Cell 975-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pomel, S., F. C. Y. Luk, and C. J. M. Beckers. 2008. Host cell egress and invasion induce marked relocations of glycolytic enzymes in Toxoplasma gondii tachyzoites. PLoS Pathog. 4e1000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roos, D. S., R. G. Donald, N. S. Morrissette, and A. L. Moulton. 1994. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 4527-63. [DOI] [PubMed] [Google Scholar]
- 24.Schuler, H., and K. Matuschewski. 2006. Plasmodium motility: actin not actin' like actin. Trends Parasitol. 22146-147. [DOI] [PubMed] [Google Scholar]
- 25.Soldati, D., J. F. Dubremetz, and M. Lebrun. 2001. Microneme proteins: structural and functional requirements to promote adhesion and invasion by the apicomplexan parasite Toxoplasma gondii. Int. J. Parasitol. 311293-1302. [DOI] [PubMed] [Google Scholar]
- 26.Wetzel, D. M., L. A. Chen, F. A. Ruiz, S. N. Moreno, and L. D. Sibley. 2004. Calcium-mediated protein secretion potentiates motility in Toxoplasma gondii. J. Cell Sci. 1175739-5748. [DOI] [PubMed] [Google Scholar]
- 27.Wiersma, H. I., S. E. Galuska, F. M. Tomley, L. D. Sibley, P. A. Liberator, and R. G. Donald. 2004. A role for coccidian cGMP-dependent protein kinase in motility and invasion. Int. J. Parasitol. 34369-380. [DOI] [PubMed] [Google Scholar]