Abstract
In this paper, we describe the range of N-linked glycan structures produced by wild-type and glucosidase II null mutant bloodstream form Trypanosoma brucei parasites and the creation and characterization of a bloodstream form Trypanosoma brucei UDP-glucose:glycoprotein glucosyltransferase null mutant. These analyses highlight peculiarities of the Trypanosoma brucei UDP-glucose:glycoprotein glucosyltransferase, including an unusually wide substrate specificity, ranging from Man5GlcNAc2 to Man9GlcNAc2 glycans, and an unusually high efficiency in vivo, quantitatively glucosylating the Asn263 N-glycan of variant surface glycoprotein (VSG) 221 and 75% of all non-VSG N glycosylation sites. We also show that although Trypanosoma brucei UDP-glucose:glycoprotein glucosyltransferase is not essential for parasite growth at 37°C, it is essential for parasite growth and survival at 40°C. The null mutant was also shown to be hypersensitive to the effects of the N glycosylation inhibitor tunicamycin. Further analysis of bloodstream form Trypanosoma brucei under normal conditions and stress conditions suggests that it does not have a classical unfolded protein response triggered by sensing unfolded proteins in the endoplasmic reticulum. Rather, judging by its uniform Grp78/BiP levels, it appears to have an unregulated and constitutively active endoplasmic reticulum protein folding system. We suggest that the latter may be particularly appropriate for this organism, which has an extremely high flux of glycoproteins through its secretory pathway.
Trypanosoma brucei is a protozoan parasite with two main proliferative stages in its life cycle: the procyclic form that grows in the tsetse fly midgut, and the bloodstream form that causes African sleeping sickness in humans and nagana in cattle. The bloodstream form is covered in a densely packed layer of 5 × 106 glycosylphosphatidylinositol (GPI)-anchored variant surface glycoprotein (VSG) dimers. This coat protects the parasites from the alternative pathway of complement-mediated lysis, shields other cell surface proteins from the host immune system, and by the process of antigenic variation allows these parasites to persist for long periods in the host bloodstream (16, 54). The trypanosome genome contains several hundreds of silent VSG genes, most of which are pseudogenes in subtelomeric arrays (40). T. brucei evades host-acquired immunity through differential activation of these genes, which encode immunologically distinct GPI-anchored glycoproteins with one to three N glycosylation sites (27, 43).
Protein N glycosylation is the most common covalent protein modification in eukaryotic cells (25). N-glycans contribute to “quality control” in the endoplasmic reticulum (ER) through a series of oligosaccharide-processing and lectin-binding reactions that contribute to protein folding and the targeting of misfolded glycoproteins for degradation (24, 47, 58, 65). As nascent protein chains enter the ER lumen, they are modified covalently in most eukaryotes by the addition of the Glc3Man9GlcNAc2 core glycan via the action of oligosaccharyltransferase (OST). After deglucosylation by α-glucosidases I (GI) and II (GII), misfolded glycoproteins can be reglucosylated in the ER by the UDP-Glc:glycoprotein glucosyltransferase (UGGT), recreating the same monoglucosylated trimming intermediate generated by GII (9, 64, 66). UGGT behaves as a sensor of glycoprotein conformation and is a key constituent of ER quality control (50, 61). Calnexin and calreticulin are ER-resident lectin-like quality control chaperones that recognize the monoglucosylated glycans on glycoproteins and help them to fold properly through their close association with the oxidoreductase ERp57 (49). On reaching the proper tertiary structure, the glycoproteins are still substrates of GII but no longer of UGGT. Properly folded molecules, thus liberated from the lectins, are then free to continue their transit to the Golgi apparatus (64). When exposure to the folding machinery in the ER is not sufficient to promote a native conformation, proteins are eventually degraded by ER-associated degradation (49, 64).
Most eukaryotes under conditions of stress, such as heat shock, undergo an unfolded protein response (UPR) that is triggered by sensing unfolded proteins in the ER. The UPR typically leads to increased expression of ER quality control components, such as calnexin and calreticulin and the ER chaperone Gpr78/BiP, as well inhibition of protein synthesis and cell cycle arrest (53, 57, 60).
In contrast to the situation in most other eukaryotes, none of the trypanosomatid dolichol-linked oligosaccharides are capped with glucose residues, as these parasites do not synthesize the sugar donor dolichol-phosphate-glucose for these reactions (41, 59). The mature dolichol-phosphate-oligosaccharide species used for transfer to protein vary according to trypanosomatid species (17, 51, 52, 56). Therefore, in these organisms, monoglucosylated glycans are exclusively formed through UGGT-dependent glucosylation (12). Furthermore, trypanosomatids lack calnexin, which binds and participates in the refolding of glucosylated proteins, and it has been suggested that differences in the N-glycan precursor have profound effects on N-glycan-dependent quality control of glycoprotein folding and ER-associated degradation (4). These protozoa do not present a conventional OST complex and express only the catalytic stt3 protein subunit that, at least for the Trypanosoma cruzi and Leishmania major enzymes, shows little specificity toward the structure of the dolichol-phosphate-oligosaccharide donor (4, 11, 26, 31, 32, 45). In the case of T. brucei, while the insect-dwelling procyclic form makes and transfers Man9GlcNAc2-phosphate-dolichol (1), previous work from our group showed that the bloodstream form of the parasite transfers both Man9GlcNAc2 and Man5GlcNAc2 to VSG in a site-specific manner (29). Regarding ER folding and quality control, although in vitro assays have shown that T. cruzi and higher eukaryotic UGGTs exclusively glucosylate high-mannose glycans in misfolded glycoproteins (66), in T. brucei the UGGT and GII enzymes use Man5GlcNAc2 and Glc1Man5GlcNAc2, respectively, as their substrates in the processing of VSG variant 221 (VSG221) (29). However, it could not be concluded from that study whether this apparent preference for atypical biantennary Man5GlcNAc2 and Glc1Man5GlcNAc2 structures reflected the substrate specificity of the enzymes or the location of the glycosylation site in the VSG polypeptide chain (30).
In this work, we further analyze the specificity and function of the UGGT/GII quality control system of T. brucei by analyzing the non-VSG N-glycans of our α-GII null mutant and creating and characterizing a T. brucei UGGT null mutant.
MATERIALS AND METHODS
Extraction of ricin binding glycoproteins and N-glycan release and purification.
T. brucei GII null mutant parasites (29) were isolated from infected rats and purified over DEAE-cellulose. Glycoprotein extraction and purification were carried out as previously described (3). N-glycans were released from proteins and separated on Bio-Gel P-4 as previously reported (3). Low-molecular-weight fractions were pooled and desalted using a small column containing Dowex AG50 (H+) over AG3 (OH−). Desalted glycans were freeze-dried, redissolved in water, and further fractionated by high-pH anion-exchange chromatography (HPAEC) using a Dionex CarboPac PA-100 column (2 mm by 250 mm). The column was equilibrated with 98% buffer A (100 mM NaOH) and 2% buffer B (380 mM sodium acetate in 100 mM NaOH) for 20 min at a flow rate of 0.25 ml/min. N-glycans were separated using a linear gradient of 2 to 25% buffer B over 40 min at 0.6 ml/min. N-glycans were detected with a pulse-amperometric detector, and sodium ions were removed from the eluate using an online Dionex ARRS unit. Glycans were collected individually at the detector outlet and desalted by passage through a column of 0.5 ml Dowex AG50 (H+) over 0.5 ml Dowex AG3 (OH−) and elution with 4 ml water. The eluates were freeze-dried and redissolved in water.
Permethylation and methylation linkage analysis.
Methylation analysis was carried out on the whole low-molecular-weight N-glycan fractions from both wild-type and GII null mutant cells. Approximately 5 nmol of material was used for methylation analysis as previously described (21). An aliquot (2%) of the permethylated glycan mixture was taken for electrospray-mass spectrometry (ES-MS) and ES-tandem MS (ES-MS-MS) analysis before the remainder was processed to partially methylated alditol acetates that were analyzed by gas chromatography-MS (GC-MS), using an HP-5 (30 m by 0.25 mm; Agilent) and a Supelco SP 2380 column (the latter to allow resolution of the nonreducing terminal Man and nonreducing terminal Glc partially methylated alditol acetates).
ES-MS analysis of permethylated and native N-glycans.
The whole permethylated N-glycan fraction was dissolved in 80% acetonitrile, and aliquots (2 μl) were mixed with an equal volume of 80% acetonitrile containing 1 mM sodium acetate prior to loading into nanotips (Micromass type F) for positive-ion ES-MS and ES-MS-MS on a Micromass Q-TOF2 orthogonal quadrupole-time of flight MS (Micromass United Kingdom). Tip and cone voltages were 1 kV and 40 V, respectively, and the collision energy was 45 to 90 V. Native N-glycans resolved on Dionex HPAEC were desalted and redissolved in water. Aliquots (1 μl) were mixed with 1 μl 100% acetonitrile containing 2% formic acid and loaded into nanospray tips (Micromass type F) for ES-MS. Samples were analyzed in positive-ion mode with capillary and cone voltages of 0.9 kV and 30 V, respectively, using a Micromass Q-TOF2 orthogonal quadrupole-time of flight MS (Micromass, Manchester, United Kingdom). All spectra were collected and processed with Masslynx software. For the permethylated glycans, the spectra were processed using MaxEnt-2 to yield masses corresponding to [M+2Na].
Cultivation of trypanosomes.
Bloodstream form T. brucei isolates genetically modified to express T7 polymerase and the tetracycline repressor protein were cultivated in HMI-9 medium containing 2.5 μg/ml G418 at 37°C in a 5% CO2 incubator as described by Wirtz et al. (69).
DNA isolation and manipulation.
Plasmid DNA was purified from Escherichia coli (DH5α) using the Qiagen Miniprep or Maxiprep kit as appropriate. Gel extraction was performed using QIAquick kits. Custom oligonucleotides were obtained from Thermo Hybaid or the Dundee University oligonucleotide facility. T. brucei genomic DNA was isolated from ∼2 × 108 bloodstream form cells using DNAzol (Helena Biosciences).
Generation of constructs.
The 523-bp 5′ and 543-bp 3′ untranslated region (UTR) sequences next to the Tb927.3.4630 open reading frame (5) were a PCR amplified from genomic DNA using Pfu with 5′-tcaagtacGCGGCCGCccgtcgtgttgtacaaagc-3′ and 5′-tggacggtttaaacctaagcgaagctttggttctttgtgtaacttac-3′ and 5′-cgcttaggtttaaaccgtccaggatcctgcgagcttggggaatg-3′ and 5′-tcctcttaGCGGCCGCtcacaacatttgaattaatacg-3′ as forward and reverse primers, respectively. The two PCR products were used together in a further PCR analysis to yield a product containing the 5′ UTR linked to the 3′ UTR by short HindIII and BamHI cloning sites (underlined) and NotI restriction sites at each end (capitalized). The PCR product was cloned into the NotI site of the pGEM-5Zf(+) vector (Promega), and the HYG and PAC drug resistance genes were introduced into the targeting vector via the HindIII and BamHI cloning sites.
Transformation of bloodstream form T. brucei.
Constructs for gene replacement and ectopic expression were purified using the Qiagen Maxiprep kit, digested with NotI to linearize, precipitated, washed twice with 70% ethanol, and then redissolved in sterile water. The linearized DNA was electroporated into T. brucei bloodstream cells (strain 427, variant 221) that were stably transformed to express T7 RNA polymerase and the tetracycline repressor protein under G418 selection (69). Cell culture and transformation were carried out as previously described (44, 69).
Southern blotting.
Aliquots of genomic DNA isolated from 100 ml of bloodstream form T. brucei cultures (∼2 × 108 cells) were digested with various restriction enzymes. Fluorescein-labeled probes were generated using the CDP-Star random prime labeling kit (Gene Images); 250 ng of template was used in a reaction volume of 50 μl and incubated for 90 min. Aliquots of 5 μl were used for each Southern blot experiment.
Small-scale sVSG isolation.
Soluble-form VSG (sVSG) was isolated from 100-ml cultures containing ∼2 × 108 bloodstream form T. brucei cells. The cultures were chilled in ice water and centrifuged at 2,500 × g for 10 min. The pellet was washed twice in trypanosome dilution buffer (15) and transferred to a 1.5-ml Eppendorf tube. The pellet was resuspended in 300 μl of lysis buffer (10 mM NaH2PO4 buffer, pH 8.0, containing 0.1 mM 1-chloro-3-tosylamido-7-amino-2-heptanone, 1 μg/ml leupeptin, and 1 μg/ml aprotinin) prewarmed to 37°C and incubated for 5 min at the same temperature. The sample was centrifuged at 14,000 × g for 5 min, and the supernatant was applied to a 200-μl DE52 anion-exchange column preequilibrated in lysis buffer. Fresh lysis buffer (800 μl without protease inhibitors) was applied in four stages, and the pooled column eluate was concentrated and diafiltered with water on a YM-10 spin concentrator (Microcon). The final sample of 50 to 100 μg sVSG221 was recovered in a volume of 100 μl water.
ES-MS analysis of intact VSG.
Samples of the sVSG preparations were diluted to ∼0.07 μg/μl in 50% acetonitrile, 1% formic acid, loaded into nanotips (Micromass type F), and analyzed by positive-ion ES-MS on a Q-Star XL instrument (Applied Biosystems). Data were collected and processed using the Bayesian protein reconstruction algorithm of Analyst software.
SDS-PAGE and Western blotting.
T. brucei extracts, equivalent to 2 × 105 cells, were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% NuPAGE (Invitrogen) gels and transferred to polyvinylidene difluoride Hybond-P membranes (Amersham Biosciences) in a semidry transfer apparatus at 40 mA for 1 h. After blocking for 1 h with 5% bovine serum albumin in phosphate-buffered saline (PBS), the membranes were washed three times with PBS, incubated for 1 h with anti-VSG221 rabbit polyclonal antibody or anti-Grp78/BiP rabbit polyclonal antibody diluted 1:4,000 in PBS, washed three times with PBS, and incubated for 1 h with anti-rabbit antibody conjugated to horseradish peroxidase diluted 1:10,000 with the same buffer. The membranes were washed three times with PBS and developed with enhanced chemiluminescence (Amersham Biosciences) according to the manufacturer's instructions.
RESULTS
Analysis of the non-VSG N-glycans of wild-type and GII null mutant bloodstream form T. brucei.
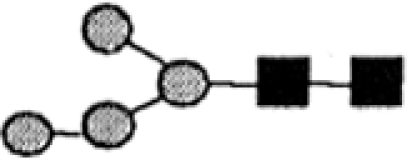
The majority of VSG was released from the plasma membrane by osmotic lysis (15), which causes the cleavage of the VSG GPI anchor by endogenous GPI-specific phospholipase C (10, 20). The remaining glycoproteins were solubilized in SDS-urea, purified by ricin affinity chromatography, and treated with peptide:N-glycosidase F, and the released N-glycans were separated into high- and low-molecular-weight glycan fractions by gel filtration (3). The wild-type low-molecular-weight fraction was shown to contain Man, Gal, and GlcNAc by GC-MS composition, while the GII null low-molecular-weight fraction also contained Glc. Methylation linkage analysis of the wild-type material by GC-MS revealed terminal Man, 2-O-substituted Man, 3,6-di-O-substituted Man, terminal Gal, and 4-O-substituted GlcNAc, consistent with a mixture of oligomannose and complex glycans (see Fig. S1A in the supplemental material). The same derivatives were observed in the GII null material except that terminal Glc and 3-O-substituted Man were also found (see Fig. S1B in the supplemental material). The last two derivatives are consistent with the presence of structures terminating in Glcα1-3Man, as would be expected in a GII null mutant. Aliquots of the low-molecular-weight fractions were also analyzed by ES-MS and ES-MS-MS following permethylation, and quite different profiles were obtained (Fig. 1A and B). Differences were also apparent upon chromatography of the native low-molecular-weight fractions by Dionex HPAEC using pulsed amperometric detection (Fig. 1C and D), and each of the labeled peaks was analyzed by ES-MS to deduce its Hex/HexNAc ratio. By combining these data, we were able to propose the major structures for each glycan composition in both samples (Table 1).
FIG. 1.
Profiles of wild-type and GII null mutant non-VSG N-glycans. ES-MS spectra of non-VSG-permethylated N-glycans from wild-type T. brucei (A) and the GII null mutant (B). Dionex HPAEC chromatograms of non-VSG native N-glycans from wild-type T. brucei (C) and the GII null mutant (D).
TABLE 1.
N-glycans expressed by wild-type and GII null mutant trypanosomes
The peak identifiers and retention times refer to the Dionex HPAEC chromatograms shown in Fig. 1C and D.
The positive-ion ES-MS analyses of the individual peaks revealed the main molecular species as being [M+H]+, [M+2H]2+, or [M+2Na]2+ ions from which the glycan compositions were deduced.
The proportions of each glycan are estimated from the areas of the peaks in Fig. 1C and D. WT, wild type. Underlined values indicate N-glycans retaining one terminal κ-Glc residue.
The proposed structures are based on the known compositions, the ES-MS-MS spectra of the principal ions shown in Fig. 1A and B, and literature precedent for T. brucei N-glycans with those compositions.
Likely origin of the N-glycan with respect to the OST (OST1 or OST2) (39) that will have transferred its Man5GlcNAc2 (OST1) or Man9GlcNAc2 (OST2) precursor.
As expected, there is a marked shift to structures containing terminal Glc residues in the GII mutant. However, whereas our analysis of the effects of the GII mutation specifically on the glycosylation of VSG221 showed that only the biantennary Man5GlcNAc2 and related structures at Asn263 (and not the Man9-7GlcNAc2 structures at Asn468) were glucosylated (29), this analysis of all the remaining T. brucei glycoproteins clearly shows that Man9-7GlcNAc2 structures are also efficiently glucosylated. In both the wild-type and GII mutant preparations, about 60% of the structures are biantennary paucimannose or complex structures, believed to originate from the transfer of Man5GlcNAc2 via T. brucei OST1 activity, and about 40% are triantennary oligomannose structures, believed to originate from the transfer of Man9GlcNAc2 via T. brucei OST2 activity (39). Of these, only small proportions (6% and 3%, respectively) are glucosyated in wild-type cells, whereas large proportions (69% and 82%, respectively) are glucosylated in the GII null mutant.
Generation of a bloodstream form T. brucei TbUGGT null mutant.
The DNA sequence of the putative T. brucei UGGT gene (TbUGGT), gene number Tb927.3.4630 (5), predicts a protein of 1,675 amino acids with a molecular mass of 187 kDa and seven potential N glycosylation sites. It shows a high degree of sequence similarity with other previously characterized UGGT sequences, like those of T. cruzi (63%), Drosophila melanogaster (59%), and Mus musculus (56%) (12, 33, 48). This similarity extends over the entire protein but is particularly high in a C-terminal region, from Val1075 to Lys1642, where the sequence identities between the T. brucei UGGT and the T. cruzi and Leishmania major UGGTs are 80% and 70%, respectively (28). This region contains the conserved glycosyltransferase family 8 domain typical of UGGTs (13).
The T. brucei genome (5) suggested that the gene TbUGGT was present in a single copy per haploid genome, and this was confirmed by Southern blot analysis (Fig. 2A, lanes 1, 3, and 5). The TbUGGT alleles were replaced sequentially with puromycin acetyltransferase (PAC) and hygromycin phosphotransferase (HYG) drug resistance cassettes by homologous recombination and selection on the relevant antibiotic(s), to generate a TbUGGT::PAC/TbUGG::HYG mutant (Fig. 2B). Southern blot analysis using a probe that hybridizes with the TbUGGT open reading frame indicated that both TbUGGT alleles had been replaced (Fig. 2A, lanes 2, 4, and 6).
FIG. 2.
Construction of the bloodstream form T. brucei TbUGGT null mutant. (A) Southern blot analysis of genomic DNA from the wild-type (lanes 1, 3, and 5) and UGGT::PAC/UGGT::HYG null mutant parasites (lanes 2, 4, and 6) digested with BamHI (lanes 1 and 2), EcoRV (lanes 3 and 4), and XcmI (lanes 5 and 6) and probed for the gene TbUGGT (upper panel) and Tb927.2.3370, a putative glycosyltransferase gene, as a loading control (lower panel). There is an XcmI site inside the gene TbUGGT. (B) Schematic representation showing the targeted replacement of the one TbUGGT allele with PAC and of the other TbUGGT allele with HYG.
TbUGGT null mutant and wild-type parasites show the same pattern of VSG glycosylation.
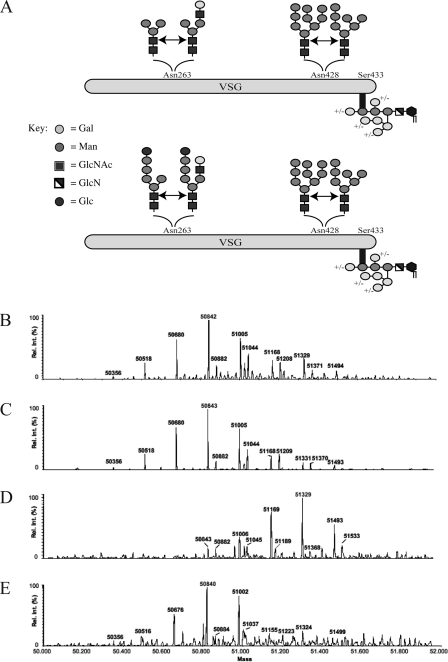
The cell line used in this study was bloodstream form T. brucei strain 427 expressing VSG221 (also known as MITat1.2). VSG221 has two occupied N glycosylation sites, the glycan structures of which have been fully characterized (70). The Asn428 site, five residues from the GPI attachment site, is occupied mostly by oligomannose structures (Man7-9GlNAc2), whereas the Asn263 site is occupied by small biantennary structures, ranging from Man3GlcNAc2 to Gal1GlcNAc1Man3GlcNAc2 (Fig. 3A).
FIG. 3.
Mass spectrometric analysis of intact sVSG221 from wild-type and TbUGGT null mutant trypanosomes treated with and without 1-deoxynojirimycin. (A) Scheme of the primary structure of wild-type VSG221 used as a reporter of protein N glycosylation in this study (top) and wild-type VSG made in the presence of 1-deoxynojirimycin (bottom). Samples of whole sVSG were analyzed by ES-MS, and the spectra were deconvolved. The sVSG samples were from wild-type cells (B), TbUGGT null mutant cells (C), wild-type cells grown in the presence of 1-deoxynojirimycin (D), and TbUGGT null mutant cells grown in the presence of 1-deoxynojirimycin (E). Rel. int., relative intensity.
The wild-type and TbUGGT null mutant cell lines were grown in vitro, and samples of sVSG221 were purified from them. Aliquots were analyzed by positive-ion ES-MS, and the deconvolved mass spectra of the intact glycoproteins are shown in Fig. 3. The wild-type (Fig. 3B) and TbUGGT null mutant (Fig. 3C) profiles both show the same range of glycoforms that arise from the known heterogeneity in the GPI anchor (42) and N-glycans (70) of this particular VSG (29, 39, 62, 67) (Table 2). Thus, we may conclude that there is no alteration in the glycosylation pattern of mature VSG expressed in the TbUGGT null mutant. However, this result could have arisen if the gene TbUGGT we removed simply did not encode a functional UGGT enzyme. To test this, we analyzed VSG isolated from wild-type and TbUGGT null mutant cells grown for 48 h in the presence of 6 mM 1-deoxynojirimycin, an α-glucosidase inhibitor (2) commonly used to inhibit GII of the UGGT/GII cycle of the ER quality control system (61). As expected, the VSG glycoform pattern observed for the glucosidase inhibitor-treated wild-type cells (Fig. 3D) was identical to that previously described for the T. brucei GII null mutant (29); i.e., both show a shift of glycoforms to a higher mass equivalent to approximately 3 hexose units (486 Da). This is due to the retention of two αMan residues and one αGlc residue on the 3-arm of the N-glycan at Asn263 such that the major structures at this site are now Glc1Man5GlcNAc2 and its products from the processing of its 6-arm (Fig. 3A). In contrast, 1-deoxynojirimycin had no effect on the glycoform pattern of VSG isolated from TbUGGT null parasites (Fig. 3E). This is consistent with TbUGGT encoding a functional TbUGGT enzyme, such that its removal abrogates the transfer of the terminal αGlc residue to Man5GlcNAc2 which, in the presence of the glucosidase inhibitor, would otherwise protect the two underlying α1-2-linked αMan residues of the 3-arm from removal by ER and/or Golgi α-mannosidases (29).
TABLE 2.
Isobaric glycoforms of sVSG detected by ES-MSa
| Measured (and theoretical) mass (Da) of WT/uggt KO/WT + dNJ/uggt KO + dNJb | Proteinc | GlcN-Ino-cPd | EtNPd | HexNAc | Hexose | WT | UGGT KO | WT + dNJ | UGGT KO + dNJ |
|---|---|---|---|---|---|---|---|---|---|
| —/—/51,533/— (51,531) | 1 | 1 | 1 | 5 | 23 | + | |||
| 51,494/51,493/51,493/51,499 (51,490) | 1 | 1 | 1 | 4 | 24 | + | + | ++ | + |
| 51,371/51,370/51,368/— (51,369) | 1 | 1 | 1 | 5 | 22 | + | + | + | |
| 51,329/51,331/51,329/51,324 (51,328) | 1 | 1 | 1 | 4 | 23 | + | + | +++ | + |
| 51,208/51,209/51,189/51,223 (51,207) | 1 | 1 | 1 | 5 | 21 | + | + | + | + |
| 51,168/51,168/51,169/51,155 (51,166) | 1 | 1 | 1 | 4 | 22 | + | + | +++ | + |
| 51,044/51,044/51,045/51,037 (51,045) | 1 | 1 | 1 | 5 | 20 | ++ | + | + | + |
| 51,005/51,005/51,006/51,002 (51,004) | 1 | 1 | 1 | 4 | 21 | ++ | ++ | + | +++ |
| 50,882/50,882/50,882/50,884 (50,883) | 1 | 1 | 1 | 5 | 19 | + | + | + | + |
| 50,842/50,843/50,843/50,840 (50,842) | 1 | 1 | 1 | 4 | 20 | +++ | +++ | + | +++ |
| 50,680/50,680/—/50,676 (50,680) | 1 | 1 | 1 | 4 | 19 | ++ | ++ | ++ | |
| 50,518/50,518/—/50,516 (50,518) | 1 | 1 | 1 | 4 | 18 | + | + | + | |
| 50,356/50,356/—/50,356 (50,356) | 1 | 1 | 1 | 4 | 17 | + | + | + |
The abundance of each isobaric group of VSG molecules is indicated as follows: +++, mass peaks >70% of the biggest species; ++, mass peaks >40% of the biggest species; +, mass peaks <40% of the biggest species. WT, wild type.
The measured masses (from Fig. 3) for sVSG221 samples from wild-type cells, TbUGGT null mutant cells (UGGT KO), wild-type cells grown with dNJ (WT + dNJ), and TbUGGT null mutant cells grown with dNJ (UGGT KO + dNJ) are tabulated. The theoretical mass of the assigned VSG composition is shown in parentheses. —, not measured.
The average molecular weight of sVSG221 polypeptide (46,284 Da) minus amino acids 1 to 27 (signal peptide) and residues 460 to 476 (GPI attachment signal sequence) with four disulfide bonds (29).
Components of GPI common to all glycoforms of sVSG221: GlcN-Ino-cP, glucosamine-α1-6-myo-inositol-1,2-cyclic phosphate; EtNP, ethanolamine phosphate.
In summary, from these data, we may conclude that the gene TbUGGT is required for the addition of αGlc to the 3-arm of Man5GlcNAc2 at Asn263 and, given its sequence similarity to known UGGT enzymes from other eukaryotes, it is reasonable to conclude that TbUGGT encodes a functional UGGT enzyme in the ER of T. brucei.
The TbUGGT null mutant is more sensitive to ER stress conditions than wild-type T. brucei.
The normal growth kinetics in vitro at 37°C (Fig. 4) and the ability of the null mutant to infect mice (data not shown) allow us to conclude that TbUGGT is a nonessential gene in bloodstream form T. brucei under normal conditions, as described previously for T. cruzi and Schizosaccharomyces pombe (12, 23). However, since UGGT is generally involved in the quality control of glycoprotein folding in the ER (64), we decided to check the ability of the TbUGGT null mutant to cope with conditions likely to lead to the accumulation of misfolded proteins in the ER.
FIG. 4.
Growth at 37°C and 40°C of wild-type T. brucei and the TbUGGT null mutant. Wild-type cells (diamonds) and TbUGGT null mutant cells (circles) were grown in HMI-9 medium at 37°C (closed diamonds and circles) and 40°C (open diamonds and circles). Cells were counted in triplicate, and mean values ± standard deviations are shown.
The parasites were grown in vitro at 40°C to induce heat shock. Both wild-type and TbUGGT null mutant cells grew more slowly at the elevated temperature, but whereas the wild-type cells survived and proliferated in culture, the TbUGGT null mutant cells started to die after 3 days (Fig. 4). This sensitivity to elevated temperature suggests that TbUGGT does indeed play a role in protecting the parasite from the stress of heat shock. Typical ER UPR in other eukaryotes includes an upregulation in the ER chaperone Grp78/BiP (57, 60), and deletion of UGGT in T. cruzi has been reported to lead to increased BiP expression (12). Therefore, the levels of Grp78/BiP and in wild type and TbUGGT null cells were analyzed by SDS-PAGE and Western blotting at 0, 24, 48, and 72 h after the temperature shift from 37°C to 40°C (Fig. 5A). However, there were no obvious changes in BiP levels in either cell line, relative to α-tubulin controls, suggesting that unlike other organisms (30, 46, 68), T. brucei does not upregulate Grp78/BiP expression in response to the loss of UGGT or to heat shock.
FIG. 5.
The levels of Grp78/BiP protein are not increased in the T. brucei TbUGGT null mutant or in response to heat shock or tunicamycin. (A) Total cell lysates of the wild type (lanes 1, 3, 5, and 7) and TbUGGT null mutants (lanes 2, 4, 6, and 8) were subjected to SDS-PAGE and Western blotting with anti-BiP and anti-α-tubulin antibodies as indicated. Samples were taken at 0, 24, 48, and 72 h after shifting the cell culture temperature from 37°C to 40°C as indicated. (B) Total cell extracts of wild-type (lanes 1 and 3) and TbUGGT null mutant (lanes 2 and 4) cells incubated with and without 0.1 μg/ml tunicamycin were subjected SDS-PAGE and Western blotting with anti-BiP and anti-VSG221 antibodies as indicated.
An alternative stress expected to lead to UPR is glycoprotein underglycosylation (7, 36). We therefore analyzed the sensitivity of wild-type and TbUGGT null mutant parasites to tunicamycin, a compound that inhibits protein N glycosylation. The cells were treated overnight with 0.1 μg/ml tunicamycin (a submaximal dose with respect to the inhibition of protein N glycosylation in T. brucei (19), and their lysates were analyzed by Western blotting using anti-BiP and anti-VSG221 antibodies. However, despite underglycosylation of VSG221 in wild-type and TbUGGT null mutant cells, there was no obvious increase in Grp78/BiP (Fig. 5B). On the other hand, underglycosylation of VSG was more apparent in the TbUGGT null mutant cells, suggesting that they are hypersensitive to the drug.
DISCUSSION
In eukaryotes, UGGT is one of the key enzymes in the quality control system of glycoprotein folding where it acts as a folding sensor, selectively glucosylating incompletely folded glycoproteins and thus promoting binding of its substrates to calnexin and/or calreticulin chaperones, which are in turn associated with Erp57 oxidoreductase (8, 49, 63). As in the case of the analysis of the VSG from the GII null mutant (29), the data obtained in this study using the α-glucosidase inhibitor 1-deoxynojiromycin suggest that the T. brucei UGGT enzyme operates on the biantennary Man5GlcNAc2 glycan added to Asn263 of VSG221 in preference to the more conventional Man9GlcNAc2 glycan at Asn428. This unusual ability of T. brucei UGGT to glucosylate biantennary Man5GlcNAc2 has also been recently noted for the UGGTs of other protozoan parasites, namely Entamoeba histolytica and Trichomonas vaginalis (4, 38). However, for T. brucei, it was unclear whether the selective glucosylation of Man5GlcNAc2 at Asn263 of VSG221 was due to a strict preference of TbUGGT for Man5GlcNAc2 or whether TbUGGT is relatively nonspecific for the acceptor glycan structure and simply selective for certain glycosylation sites, e.g., those in more disordered domains immediately following protein synthesis. Analysis of the non-VSG N-glycans from the GII null mutant strongly suggests the latter model (Fig. 6B). Indeed, over 80% of the triantennary oligomannose structures were found to be glucosylated in the low-molecular-weight glycan fraction of the glucosidase null mutant. Thus, the oligomannose-containing C-terminal Asn428 site of VSG221 may be in a domain that does not need help with folding, whereas the Man5GlcNAc2-containing N-terminal Asn263 site is reversibly glucosylated in this glycoprotein. Interestingly, under UDP-GlcNAc starvation conditions, T. brucei produces two major species of VSG221; one form contains both C-terminal and N-terminal N-linked glycans, whereas the other form contains only the N-terminal glycan, whose precursor is Man5GlcNAc2 (29, 62). Underglycosylation of the Asn486 site, but not of the Asn263 site, is also seen in the T. brucei ALG3 mutant (39). This highlights the apparent importance of the Asn263 glycan for the correct folding of the T. brucei VSG coat. The crystal structure of VSG221 shows that the core of the Asn263 glycan replaces a short peptide α helix in other VSG variants (6), suggesting why this particular glycan may be relatively important.
FIG. 6.
Summary of the similarities and differences between general eukaryote N-glycan-dependent endoplasmic reticulum quality control factors (A) and those of bloodstream form T. brucei (B). CNX, calnexin; CRT, calreticulin; ERAD, ER-associated degradation.
In other species, such as S. pombe, the accumulation of misfolded proteins in the ER triggers the induction of Grp78/BiP mRNA synthesis (22, 55). Similarly, in T. cruzi UGGT null mutants, the Grp78/BiP levels are increased in response to the lack of calreticulin-glycoprotein interaction (12). This general induction of the synthesis of Grp78/BiP and other proteins that facilitate the proper folding of newly synthesized species is part of the so-called UPR (53, 57, 60). We did not detect any upregulation of the Grp78/BiP mRNA or protein levels in the TbUGGT null mutant. Similarly, Grp78/BiP protein levels in wild-type and TbUGGT null mutant cells did not increase under tunicamycin or heat shock stress conditions. Nevertheless, under sustained elevated temperatures, TbUGGT was essential for T. brucei growth in culture. This is reminiscent of the essentiality of the UGGT enzyme for the growth of S. pombe under ER stress conditions induced by underglycosylation and high temperatures (18).
According to the VSG221 glycoforms from wild-type T. brucei cells grown in the presence of the α-glucosidase inhibitor 1-deoxynojirimycin (2) (Fig. 4C), every Asn236 N-glycan is glucosylated, suggesting that the T. brucei enzyme constitutively glucosylates the N-terminal N-glycans in the biosynthesis of VSG221. This is in contrast to the situation in S. pombe, where even misfolded proteins are not quantitatively glucosylated by UGGT (22). Similarly, in the case of T. cruzi, the oligosaccharides present at the N glycosylation site of the COOH-terminal domain of cruzipain, a lysosomal glycoprotein, are glucosylated in some molecules and not in others (35).
The constitutive glucosylation of the VSG221 Asn263 glycan and constitutive expression of Grp78/BiP in bloodstream form T. brucei suggest that although the UGGT/GII cycle clearly operates in this parasite and that without it the cells are more susceptible to elevated temperatures, the parasite does not regulate its protein folding/quality control pathway through a classical UPR (Fig. 6). Consistent with this view, searches for components of the UPR pathways, like IRE-1, ATF-6, and PERK (60), or for their ER luminal unfolded protein-sensing domains (14), failed to identify candidate T. brucei genes. Thus, we suggest that T. brucei has evolved an unregulated, classical UPR-less ER protein folding/quality control system that is constitutively active to cope with the extremely high flux of glycoprotein synthesis and export (>107 glycoprotein molecules per cell division) that is required to create a VSG surface coat, upon which the organism depends for survival. Transcriptional profiling also suggests that a classical UPR does not exist in T. brucei (34). However, a nonclassical UPR-like response, linking stress to the silencing of spliced leader RNA synthesis and consequent arrest of mRNA production, was described recently (37). The latter decouples the necessity for a stress response from ER unfolded protein sensing, and this may better suit an organism with such a high constitutive flux of glycoprotein biosynthesis.
Supplementary Material
Acknowledgments
This work was supported by a Wellcome Trust program grant (085622). J.R. was supported by an MRC program grant. L.I. was supported in part by a Marie Curie fellowship.
We thank Dan Turnock, M. Lucia Güther, Angela Mehlert, Isabelle Nett, and Jim Procter for hints and helpful discussions and J. Bangs for the BiP antibody.
Footnotes
Published ahead of print on 29 December 2008.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Acosta-Serrano, A., J. O'Rear, G. Quellhorst, S. H. Lee, K. Y. Hwa, S. S. Krag, and P. T. Englund. 2004. Defects in the N-linked oligosaccharide biosynthetic pathway in a Trypanosoma brucei glycosylation mutant. Eukaryot. Cell 3255-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asano, N. 2003. Glycosidase inhibitors: update and perspectives on practical use. Glycobiology 1393R-104R. [DOI] [PubMed] [Google Scholar]
- 3.Atrih, A., J. M. Richardson, A. R. Prescott, and M. A. Ferguson. 2005. Trypanosoma brucei glycoproteins contain novel giant poly-N-acetyllactosamine carbohydrate chains. J. Biol. Chem. 280865-871. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee, S., P. Vishwanath, J. Cui, D. J. Kelleher, R. Gilmore, P. W. Robbins, and J. Samuelson. 2007. The evolution of N-glycan-dependent endoplasmic reticulum quality control factors for glycoprotein folding and degradation. Proc. Natl. Acad. Sci. USA 10411676-11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berriman, M., E. Ghedin, C. Hertz-Fowler, G. Blandin, H. Renauld, D. C. Bartholomeu, N. J. Lennard, E. Caler, N. E. Hamlin, B. Haas, U. Bohme, L. Hannick, M. A. Aslett, J. Shallom, L. Marcello, L. Hou, B. Wickstead, U. C. Alsmark, C. Arrowsmith, R. J. Atkin, A. J. Barron, F. Bringaud, K. Brooks, M. Carrington, I. Cherevach, T. J. Chillingworth, C. Churcher, L. N. Clark, C. H. Corton, A. Cronin, R. M. Davies, J. Doggett, A. Djikeng, T. Feldblyum, M. C. Field, A. Fraser, I. Goodhead, Z. Hance, D. Harper, B. R. Harris, H. Hauser, J. Hostetler, A. Ivens, K. Jagels, D. Johnson, J. Johnson, K. Jones, A. X. Kerhornou, H. Koo, N. Larke, S. Landfear, C. Larkin, V. Leech, A. Line, A. Lord, A. Macleod, P. J. Mooney, S. Moule, D. M. Martin, G. W. Morgan, K. Mungall, H. Norbertczak, D. Ormond, G. Pai, C. S. Peacock, J. Peterson, M. A. Quail, E. Rabbinowitsch, M. A. Rajandream, C. Reitter, S. L. Salzberg, M. Sanders, S. Schobel, S. Sharp, M. Simmonds, A. J. Simpson, L. Tallon, C. M. Turner, A. Tait, A. R. Tivey, S. Van Aken, D. Walker, D. Wanless, S. Wang, B. White, O. White, S. Whitehead, J. Woodward, J. Wortman, M. D. Adams, T. M. Embley, K. Gull, E. Ullu, J. D. Barry, A. H. Fairlamb, F. Opperdoes, B. G. Barrell, J. E. Donelson, N. Hall, C. M. Fraser, S. E. Melville, and N. M. El-Sayed. 2005. The genome of the African trypanosome Trypanosoma brucei. Science 309416-422. [DOI] [PubMed] [Google Scholar]
- 6.Blum, M. L., J. A. Down, A. M. Gurnett, M. Carrington, M. J. Turner, and D. C. Wiley. 1993. A structural motif in the variant surface glycoproteins of Trypanosoma brucei. Nature 362603-609. [DOI] [PubMed] [Google Scholar]
- 7.Brewer, J. W., J. L. Cleveland, and L. M. Hendershot. 1997. A pathway distinct from the mammalian unfolded protein response regulates expression of endoplasmic reticulum chaperones in non-stressed cells. EMBO J. 167207-7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caramelo, J. J., O. A. Castro, L. G. Alonso, G. De Prat-Gay, and A. J. Parodi. 2003. UDP-Glc:glycoprotein glucosyltransferase recognizes structured and solvent accessible hydrophobic patches in molten globule-like folding intermediates. Proc. Natl. Acad. Sci. USA 10086-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caramelo, J. J., O. A. Castro, G. de Prat-Gay, and A. J. Parodi. 2004. The endoplasmic reticulum glucosyltransferase recognizes nearly native glycoprotein folding intermediates. J. Biol. Chem. 27946280-46285. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso de Almeida, M. L., and M. J. Turner. 1983. The membrane form of variant surface glycoproteins of Trypanosoma brucei. Nature 302349-352. [DOI] [PubMed] [Google Scholar]
- 11.Castro, O., F. Movsichoff, and A. J. Parodi. 2006. Preferential transfer of the complete glycan is determined by the oligosaccharyltransferase complex and not by the catalytic subunit. Proc. Natl. Acad. Sci. USA 10314756-14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conte, I., C. Labriola, J. J. Cazzulo, R. Docampo, and A. J. Parodi. 2003. The interplay between folding-facilitating mechanisms in Trypanosoma cruzi endoplasmic reticulum. Mol. Biol. Cell 143529-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coutinho, P. M., E. Deleury, G. J. Davies, and B. Henrissat. 2003. An evolving hierarchical family classification for glycosyltransferases. J. Mol. Biol. 328307-317. [DOI] [PubMed] [Google Scholar]
- 14.Credle, J. J., J. S. Finer-Moore, F. R. Papa, R. M. Stroud, and P. Walter. 2005. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 10218773-18784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross, G. A. 1975. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology 71393-417. [DOI] [PubMed] [Google Scholar]
- 16.Cross, G. A. 1996. Antigenic variation in trypanosomes: secrets surface slowly. Bioessays 18283-291. [DOI] [PubMed] [Google Scholar]
- 17.de la Canal, L., and A. J. Parodi. 1987. Synthesis of dolichol derivatives in trypanosomatids. Characterization of enzymatic patterns. J. Biol. Chem. 26211128-11133. [PubMed] [Google Scholar]
- 18.Fanchiotti, S., F. Fernandez, C. D'Alessio, and A. J. Parodi. 1998. The UDP-Glc:glycoprotein glucosyltransferase is essential for Schizosaccharomyces pombe viability under conditions of extreme endoplasmic reticulum stress. J. Cell Biol. 143625-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson, M. A., M. Duszenko, G. S. Lamont, P. Overath, and G. A. Cross. 1986. Biosynthesis of Trypanosoma brucei variant surface glycoproteins. N-glycosylation and addition of a phosphatidylinositol membrane anchor. J. Biol. Chem. 261356-362. [PubMed] [Google Scholar]
- 20.Ferguson, M. A., K. Haldar, and G. A. Cross. 1985. Trypanosoma brucei variant surface glycoprotein has a sn-1,2-dimyristyl glycerol membrane anchor at its COOH terminus. J. Biol. Chem. 2604963-4968. [PubMed] [Google Scholar]
- 21.Ferguson, M. A. J. 1994. Glycobiology: a practical approach. IRL Press at Oxford University Press, Oxford, United Kingdom.
- 22.Fernández, F., C. D'Alessio, S. Fanchiotti, and A. J. Parodi. 1998. A misfolded protein conformation is not a sufficient condition for in vivo glucosylation by the UDP-Glc:glycoprotein glucosyltransferase. EMBO J. 175877-5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez, F., M. Jannatipour, U. Hellman, L. A. Rokeach, and A. J. Parodi. 1996. A new stress protein: synthesis of Schizosaccharomyces pombe UDP-Glc:glycoprotein glucosyltransferase mRNA is induced by stress conditions but the enzyme is not essential for cell viability. EMBO J. 15705-713. [PMC free article] [PubMed] [Google Scholar]
- 24.Hebert, D. N., and M. Molinari. 2007. In and out of the ER: protein folding, quality control, degradation, and related human diseases. Physiol. Rev. 871377-1408. [DOI] [PubMed] [Google Scholar]
- 25.Helenius, A., and M. Aebi. 2004. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 731019-1049. [DOI] [PubMed] [Google Scholar]
- 26.Hese, K., C. Otto, F. H. Routier, and L. Lehle. 2008. The yeast oligosaccharyltransferase complex can be replaced by STT3 from Leishmania major. Glycobiology 19160-171. [DOI] [PubMed] [Google Scholar]
- 27.Hutchinson, O. C., W. Smith, N. G. Jones, A. Chattopadhyay, S. C. Welburn, and M. Carrington. 2003. VSG structure: similar N-terminal domains can form functional VSGs with different types of C-terminal domain. Mol. Biochem. Parasitol. 130127-131. [DOI] [PubMed] [Google Scholar]
- 28.Ivens, A. C., C. S. Peacock, E. A. Worthey, L. Murphy, G. Aggarwal, M. Berriman, E. Sisk, M. A. Rajandream, E. Adlem, R. Aert, A. Anupama, Z. Apostolou, P. Attipoe, N. Bason, C. Bauser, A. Beck, S. M. Beverley, G. Bianchettin, K. Borzym, G. Bothe, C. V. Bruschi, M. Collins, E. Cadag, L. Ciarloni, C. Clayton, R. M. Coulson, A. Cronin, A. K. Cruz, R. M. Davies, J. De Gaudenzi, D. E. Dobson, A. Duesterhoeft, G. Fazelina, N. Fosker, A. C. Frasch, A. Fraser, M. Fuchs, C. Gabel, A. Goble, A. Goffeau, D. Harris, C. Hertz-Fowler, H. Hilbert, D. Horn, Y. Huang, S. Klages, A. Knights, M. Kube, N. Larke, L. Litvin, A. Lord, T. Louie, M. Marra, D. Masuy, K. Matthews, S. Michaeli, J. C. Mottram, S. Muller-Auer, H. Munden, S. Nelson, H. Norbertczak, K. Oliver, S. O'Neil, M. Pentony, T. M. Pohl, C. Price, B. Purnelle, M. A. Quail, E. Rabbinowitsch, R. Reinhardt, M. Rieger, J. Rinta, J. Robben, L. Robertson, J. C. Ruiz, S. Rutter, D. Saunders, M. Schafer, J. Schein, D. C. Schwartz, K. Seeger, A. Seyler, S. Sharp, H. Shin, D. Sivam, R. Squares, S. Squares, V. Tosato, C. Vogt, G. Volckaert, R. Wambutt, T. Warren, H. Wedler, J. Woodward, S. Zhou, W. Zimmermann, D. F. Smith, J. M. Blackwell, K. D. Stuart, B. Barrell, and P. J. Myler. 2005. The genome of the kinetoplastid parasite, Leishmania major. Science 309436-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones, D. C., A. Mehlert, M. L. Guther, and M. A. Ferguson. 2005. Deletion of the glucosidase II gene in Trypanosoma brucei reveals novel N-glycosylation mechanisms in the biosynthesis of variant surface glycoprotein. J. Biol. Chem. 28035929-35942. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman, R. J. 1999. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 131211-1233. [DOI] [PubMed] [Google Scholar]
- 31.Kelleher, D. J., S. Banerjee, A. J. Cura, J. Samuelson, and R. Gilmore. 2007. Dolichol-linked oligosaccharide selection by the oligosaccharyltransferase in protist and fungal organisms. J. Cell Biol. 17729-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelleher, D. J., and R. Gilmore. 2006. An evolving view of the eukaryotic oligosaccharyltransferase. Glycobiology 1647R-62R. [DOI] [PubMed] [Google Scholar]
- 33.Korotkov, K. V., E. Kumaraswamy, Y. Zhou, D. L. Hatfield, and V. N. Gladyshev. 2001. Association between the 15-kDa selenoprotein and UDP-glucose:glycoprotein glucosyltransferase in the endoplasmic reticulum of mammalian cells. J. Biol. Chem. 27615330-15336. [DOI] [PubMed] [Google Scholar]
- 34.Koumandou, V. L., S. K. Natesan, T. Sergeenko, and M. C. Field. 2008. The trypanosome transcriptome is remodelled during differentiation but displays limited responsiveness within life stages. BMC Genomics 9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labriola, C., J. J. Cazzulo, and A. J. Parodi. 1995. Retention of glucose units added by the UDP-GLC:glycoprotein glucosyltransferase delays exit of glycoproteins from the endoplasmic reticulum. J. Cell Biol. 130771-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, A. S. 1992. Mammalian stress response: induction of the glucose-regulated protein family. Curr. Opin. Cell Biol. 4267-273. [DOI] [PubMed] [Google Scholar]
- 37.Lustig, Y., L. Sheiner, Y. Vagima, H. Goldshmidt, A. Das, V. Bellofatto, and S. Michaeli. 2007. Spliced-leader RNA silencing: a novel stress-induced mechanism in Trypanosoma brucei. EMBO Rep. 8408-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magnelli, P., J. F. Cipollo, D. M. Ratner, J. Cui, D. Kelleher, R. Gilmore, C. E. Costello, P. W. Robbins, and J. Samuelson. 2008. Unique Asn-linked oligosaccharides of the human pathogen Entamoeba histolytica. J. Biol. Chem. 28318355-18364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manthri, S., M. L. Guther, L. Izquierdo, A. Acosta-Serrano, and M. A. Ferguson. 2008. Deletion of the TbALG3 gene demonstrates site-specific N-glycosylation and N-glycan processing in Trypanosoma brucei. Glycobiology 18367-383. [DOI] [PubMed] [Google Scholar]
- 40.Marcello, L., and J. D. Barry. 2007. From silent genes to noisy populations—dialogue between the genotype and phenotypes of antigenic variation. J. Eukaryot. Microbiol. 5414-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McConville, M. J., K. A. Mullin, S. C. Ilgoutz, and R. D. Teasdale. 2002. Secretory pathway of trypanosomatid parasites. Microbiol. Mol. Biol. Rev. 66122-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehlert, A., J. M. Richardson, and M. A. Ferguson. 1998. Structure of the glycosylphosphatidylinositol membrane anchor glycan of a class-2 variant surface glycoprotein from Trypanosoma brucei. J. Mol. Biol. 277379-392. [DOI] [PubMed] [Google Scholar]
- 43.Mehlert, A., N. Zitzmann, J. M. Richardson, A. Treumann, and M. A. Ferguson. 1998. The glycosylation of the variant surface glycoproteins and procyclic acidic repetitive proteins of Trypanosoma brucei. Mol. Biochem. Parasitol. 91145-152. [DOI] [PubMed] [Google Scholar]
- 44.Milne, K. G., M. L. Guther, and M. A. Ferguson. 2001. Acyl-CoA binding protein is essential in bloodstream form Trypanosoma brucei. Mol. Biochem. Parasitol. 112301-304. [DOI] [PubMed] [Google Scholar]
- 45.Nasab, F. P., B. L. Schulz, F. Gamarro, A. J. Parodi, and M. Aebi. 2008. All in one: Leishmania major STT3 proteins substitute for the whole oligosaccharyltransferase complex in Saccharomyces cerevisiae. Mol. Biol. Cell 193758-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okamura, K., Y. Kimata, H. Higashio, A. Tsuru, and K. Kohno. 2000. Dissociation of Kar2p/BiP from an ER sensory molecule, Ire1p, triggers the unfolded protein response in yeast. Biochem. Biophys. Res. Commun. 279445-450. [DOI] [PubMed] [Google Scholar]
- 47.Olivari, S., and M. Molinari. 2007. Glycoprotein folding and the role of EDEM1, EDEM2 and EDEM3 in degradation of folding-defective glycoproteins. FEBS Lett. 5813658-3664. [DOI] [PubMed] [Google Scholar]
- 48.Parker, C. G., L. I. Fessler, R. E. Nelson, and J. H. Fessler. 1995. Drosophila UDP-glucose:glycoprotein glucosyltransferase: sequence and characterization of an enzyme that distinguishes between denatured and native proteins. EMBO J. 141294-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parodi, A. J. 2000. Protein glucosylation and its role in protein folding. Annu. Rev. Biochem. 6969-93. [DOI] [PubMed] [Google Scholar]
- 50.Parodi, A. J., D. H. Mendelzon, and G. Z. Lederkremer. 1983. Transient glucosylation of protein-bound Man9GlcNAc2, Man8GlcNAc2, and Man7GlcNAc2 in calf thyroid cells. A possible recognition signal in the processing of glycoproteins. J. Biol. Chem. 2588260-8265. [PubMed] [Google Scholar]
- 51.Parodi, A. J., L. A. Quesada Allue, and J. J. Cazzulo. 1981. Pathway of protein glycosylation in the trypanosomatid Crithidia fasciculata. Proc. Natl. Acad. Sci. USA 786201-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parodi, A. J., and L. A. Quesada-Allue. 1982. Protein glycosylation in Trypanosoma cruzi. I. Characterization of dolichol-bound monosaccharides and oligosaccharides synthesized “in vivo.” J. Biol. Chem. 2577637-7640. [PubMed] [Google Scholar]
- 53.Patil, C., and P. Walter. 2001. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr. Opin. Cell Biol. 13349-355. [DOI] [PubMed] [Google Scholar]
- 54.Pays, E., and D. P. Nolan. 1998. Expression and function of surface proteins in Trypanosoma brucei. Mol. Biochem. Parasitol. 913-36. [DOI] [PubMed] [Google Scholar]
- 55.Pidoux, A. L., and J. Armstrong. 1992. Analysis of the BiP gene and identification of an ER retention signal in Schizosaccharomyces pombe. EMBO J. 111583-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Previato, J. O., D. H. Mendelzon, and A. J. Parodi. 1986. Characterization of dolichol monophosphate- and dolichol diphosphate-linked saccharides in trypanosomatid flagellates. Mol. Biochem. Parasitol. 18343-353. [DOI] [PubMed] [Google Scholar]
- 57.Ron, D., and P. Walter. 2007. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Rev. Mol. Cell Biol. 8519-529. [DOI] [PubMed] [Google Scholar]
- 58.Ruddock, L. W., and M. Molinari. 2006. N-glycan processing in ER quality control. J. Cell Sci. 1194373-4380. [DOI] [PubMed] [Google Scholar]
- 59.Samuelson, J., S. Banerjee, P. Magnelli, J. Cui, D. J. Kelleher, R. Gilmore, and P. W. Robbins. 2005. The diversity of dolichol-linked precursors to Asn-linked glycans likely results from secondary loss of sets of glycosyltransferases. Proc. Natl. Acad. Sci. USA 1021548-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schröder, M., and R. J. Kaufman. 2005. The mammalian unfolded protein response. Annu. Rev. Biochem. 74739-789. [DOI] [PubMed] [Google Scholar]
- 61.Sousa, M. C., M. A. Ferrero-Garcia, and A. J. Parodi. 1992. Recognition of the oligosaccharide and protein moieties of glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. Biochemistry 3197-105. [DOI] [PubMed] [Google Scholar]
- 62.Stokes, M. J., M. L. Guther, D. C. Turnock, A. R. Prescott, K. L. Martin, M. S. Alphey, and M. A. Ferguson. 2008. The synthesis of UDP-N-acetylglucosamine is essential for bloodstream form Trypanosoma brucei in vitro and in vivo and UDP-N-acetylglucosamine starvation reveals a hierarchy in parasite protein glycosylation. J. Biol. Chem. 28316147-16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trombetta, E. S., and A. Helenius. 2000. Conformational requirements for glycoprotein reglucosylation in the endoplasmic reticulum. J. Cell Biol. 1481123-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trombetta, E. S., and A. J. Parodi. 2003. Quality control and protein folding in the secretory pathway. Annu. Rev. Cell Dev. Biol. 19649-676. [DOI] [PubMed] [Google Scholar]
- 65.Trombetta, E. S., and A. J. Parodi. 2005. Glycoprotein reglucosylation. Methods 35328-337. [DOI] [PubMed] [Google Scholar]
- 66.Trombetta, S. E., M. Bosch, and A. J. Parodi. 1989. Glucosylation of glycoproteins by mammalian, plant, fungal, and trypanosomatid protozoa microsomal membranes. Biochemistry 288108-8116. [DOI] [PubMed] [Google Scholar]
- 67.Urbaniak, M. D., D. C. Turnock, and M. A. Ferguson. 2006. Galactose starvation in a bloodstream form Trypanosoma brucei UDP-glucose 4′-epimerase conditional null mutant. Eukaryot. Cell 51906-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watowich, S. S., and R. I. Morimoto. 1988. Complex regulation of heat shock- and glucose-responsive genes in human cells. Mol. Cell. Biol. 8393-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wirtz, E., S. Leal, C. Ochatt, and G. A. Cross. 1999. A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol. Biochem. Parasitol. 9989-101. [DOI] [PubMed] [Google Scholar]
- 70.Zamze, S. E., D. A. Ashford, E. W. Wooten, T. W. Rademacher, and R. A. Dwek. 1991. Structural characterization of the asparagine-linked oligosaccharides from Trypanosoma brucei type II and type III variant surface glycoproteins. J. Biol. Chem. 26620244-20261. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.