Abstract
Photorhabdus species are gram-negative entomopathogenic bacteria of the family Enterobacteriaceae. Among the different members of the genus, one species, Photorhabdus asymbiotica, is a pathogen of both insects and humans. The pathogenicity mechanisms of this bacterium are unknown. Here we show that P. asymbiotica is a facultative intracellular pathogen that is able to replicate inside human macrophage-like cells. Furthermore, P. asymbiotica was shown for the first time in an intracellular location after insect infection. We also demonstrated that among Australian and American clinical isolates, only the Australian strains were able to invade nonphagocytic human cells. In cell culture infection experiments, Australian clinical isolates as well as cell-free bacterial culture supernatant induced strong apoptosis of a macrophage cell line at 6 h postinfection. American isolates also induced cellular death, but much later than that induced by Australian ones. Mammalian cultured cells analyzed for key features of apoptosis displayed apoptotic nuclear morphology, activation of the initiator caspases 8 and 9 and the executioner caspases 3 and 7, and poly(ADP-ribose) polymerase proteolysis, suggesting activation of both the intrinsic and extrinsic apoptotic pathways.
Photorhabdus spp. are motile, bioluminescent, gram-negative bacteria belonging to the family Enterobacteriaceae. Three species belonging to the entomopathogenic genus Photorhabdus, namely, Photorhabdus luminescens, P. temperata, and P. asymbiotica (14), form a mutualistic association with specific nematodes of the Heterorhabditidae family. The nematobacterial complex is highly pathogenic for a broad range of insects and is therefore utilized worldwide as a biological agent in crop control (10). Besides the economic interest, the life cycle of such a nematobacterial complex could be a valuable tool to study virulence factors as well as the complex ecological and evolutionary aspects of pathogen transmission by invertebrates (31).
Photorhabdus organisms are transported by their invertebrate vectors into the insect host, which is killed within 48 h by a combination of toxin action and septicemia (15). The capacity of this pathogen to overcome the strong innate immune response is similar to that observed in the phylogenetically close bacterium Yersinia. The human pathogens Yersinia enterocolitica, Yersinia pseudotuberculosis, and Yersinia pestis possess type III secretion systems (T3SS) delivering effector molecules directly into the host cell, modulating key signaling pathways. This allows the bacteria to resist phagocytosis and to suppress proinflammatory molecule production as well as to induce apoptosis. Recently, the T3SS of Y. pestis was shown to transport the insecticidal toxin complex (16). These toxins were first isolated from P. luminescens culture supernatants (Sn) and showed oral activity against different insect orders (2). A T3SS is present in the genus Photorhabdus (9, 13), but the predicted effectors are different in P. luminescens and P. asymbiotica (4). This T3SS is functional in the TT01 strain of P. luminescens and plays a role in the extracellular localization of the bacterium in the phagocytic organs of Locusta migratoria (3).
Among Photorhabdus species, P. asymbiotica is unique because it is the only species which has been isolated from human clinical specimens in the United States and Australia (12, 17, 18, 23). The new clinical entity was named P. asymbiotica (1, 14) because at that time the nematode vector was unknown. Recently, it was shown that Heterorhabditis indica was present in the soil where a patient was infected with P. asymbiotica (17) and that this nematode naturally harbors P. asymbiotica (20), indicating the possible nematoid origin of the clinical strains. To date, 14 cases have been documented, including 6 in the United States and 8 in Australia. The infections caused by P. asymbiotica are characterized by local infection of soft tissue, with the formation of subcutaneous nodules (12, 18, 23, 32). P. asymbiotica seems to be mainly a primary pathogen rather than an opportunistic one and was qualified in Australia as an emergent pathogen. To date, no clear route of infection is known, but all patients worked outside and/or had lesions due to spider bites.
Nothing is known of the pathogenicity mechanisms of P. asymbiotica. In the present work, we study the interactions of P. asymbiotica with human and insect cells. Our data show a clear difference in pathogenicity between the American and Australian strains. Under our experimental conditions, strains of Photorhabdus were facultative intracellular bacteria and were able to trigger apoptosis of a human macrophage cell line in vitro. Australian strains were much more virulent than the American ones, in agreement with the observed differences in clinical symptoms.
MATERIALS AND METHODS
Bacterial strains, cell cultures, and insect hemocytes.
Bacterial strains used are listed in Table 1. P. luminescens TT01, Escherichia coli XL1-Blue, E. coli HB101 Inv+, and E. coli HB101 Inv− (27) were used as controls. For cell culture infection experiments, P. asymbiotica overnight cultures grown at 28°C were diluted (1/25) in fresh Luria-Bertani broth and grown for 3 h at 28°C. Bacteria were then washed twice and resuspended in phosphate-buffered saline (PBS) (Invitrogen). The bacterial concentration was adjusted by measuring the optical density at 540 nm and counting cells in a Thoma numeration cell. The concentration of viable bacteria was checked by plating appropriate serial dilutions on agar plates and counting CFU after incubation at 28°C for 48 h. For P. asymbiotica Sn preparation, overnight cultures from American and Australian strains were centrifuged at 12,000 × g for 20 min, and the Sn was filtered (0.22 μm; Millipore). The Lepidoptera Spodoptera littoralis and Mythimna unipuncta were reared on an artificial diet at 23°C with a photoperiod of 12 h. Fifth-instar larvae (caterpillars) were used throughout this study. Insects have an open circulatory system, and insect blood is called hemolymph with free cells, the hemocytes, in a liquid called plasma. For further information on insect hemocytes, see the work of Ribeiro and Brehélin (26). Insect hemocyte monolayer preparations were prepared as described elsewhere (7). Briefly, insects were surface sterilized in 70% ethanol, and after piercing of a proleg, hemolymph was collected in sterile anticoagulant buffer (62 mM NaCl, 100 mM glucose, 10 mM EDTA, 30 mM trisodium citrate, 26 mM citric acid) at 4°C. After centrifugation (800 × g for 15 s), the hemocyte pellet was resuspended in PBS, and this suspension was layered on 12-mm heat-sterilized glass coverslips in 24-well plates. Cells were allowed to adhere on glass for 15 min before being used as monolayers.
TABLE 1.
Photorhabdus species from human clinical isolates
| Species, origin | Isolate | Strain designation | Patient sex/age (yr)b | Source of isolate | Reference |
|---|---|---|---|---|---|
| P. asymbiotica subsp. asymbiotica, United States | 3105/77 | US77 | F/80 | Blood and skin | 12 |
| 2407/88 | US88 | F/36 | Abdomen and submandible | 12 | |
| 3265/86 | US86 | M/78 | Pretibial wound | 12 | |
| 2617/87 | US87 | M/45 | Lesion cavity | 12 | |
| P. asymbiotica subsp. australis, Australia | 9802397a | AU97 | M/50 | Abscess | 24 |
| 9802336a | AU36 | M/50 | Abscess | 24 | |
| 9802892a | AU92 | M/50 | Abscess | 24 | |
| 9805888a | AU88 | M/50 | Abscess | 24 | |
| 9800946 | AU46 | M/90 | Blood | 24 | |
| SN98-1 | AU81 | M/55 | Blood, sputum, pus, and tissue | 24 |
Collected at different times and sites from the same patient.
F, female; M, male.
THP-1 and HeLa cells were cultured at 37°C in a 5% CO2 atmosphere in RPMI supplemented with 10% heat-inactivated fetal calf serum and antibiotic (100 U/ml penicillin-streptomycin) (all from Invitrogen). THP-1 cells were differentiated with 100 nM phorbol myristate acetate (PMA) (Sigma). Before experiments, antibiotic-free medium was used to replace the medium in culture plates.
In cell culture and hemocyte monolayers, cell viability was checked by light microscopy and trypan blue dye exclusion or lactate dehydrogenase (LDH) release (Promega).
Infection and intracellular survival assay.
We performed a gentamicin killing assay as described previously (11). Briefly, P. asymbiotica or E. coli was added to THP-1 cells (2.5 × 105), HeLa cells (1 × 105), or insect hemocyte monolayers in 24-well plates at a multiplicity of infection (MOI) of 25:1 and centrifuged at 300 × g for 5 min. After 30 min of incubation at 37°C (0 h time point), infected cell monolayers were washed three times with PBS, and then RPMI complete medium supplemented with 100 μg/ml of gentamicin was added in order to kill the remaining extracellular bacteria. After 2 h of further incubation at 37°C, the medium was replaced again by antibiotic-free complete RPMI. At various times postinfection, eukaryotic cells were lysed (0.5% Triton X-100), and intracellular bacteria were recovered and counted (CFU). The assays were conducted in triplicate and repeated at least three times. For insect infections, each larva was injected with 2 × 103 bacteria, and at different times postinfection, hemocytes were collected as described above and treated for CFU determination.
DNA fragmentation detection.
Hemocytes or HeLa or THP-1 cells (1 × 105) were either left untreated or treated with E. coli XL1-Blue, P. asymbiotica, or Sn. Infections were performed at an MOI of 100 bacteria per eukaryotic cell. At various times postinfection, cells were observed by inverted light microscopy. We assessed apoptosis in target cells by the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) method, implemented with an in situ cell death detection kit (Roche Applied Science), after incubating eukaryotic cells for 6 h with bacteria or Sn. We also performed DAPI (4′,6-diamidino-2-phenylindole) (Sigma) labeling. The percentage of cells presenting nuclear fragmentation and TUNEL-positive staining (apoptotic cells) was determined under fluorescence microscopic observation. For apoptosis inhibition experiments, THP-1 monolayers were preincubated for 20 min with the pan-caspase inhibitor Z-Val-Ala-Asp-fluoromethyl ketone (Z-VAD-FMK; 20 μM), the caspase-8 inhibitor Z-Ile-Glu-Thr-Asp-fluoromethyl ketone (Z-IETD-FMK; 10 μM), the caspase-9 inhibitor Z-Leu-Glu-His-Asp-fluoromethyl ketone (Z-LEHD-FMK; 10 μM) (all from Calbiochem), or the phagocytosis inhibitors wortmannin (100 nM) and cytochalasin D (5 μg/ml). Staurosporine (2.5 μM) (Sigma) was used as an apoptosis positive control.
Caspase activity assay.
The enzymatic activities of caspase-3/7, caspase-8, and caspase-9 were assayed using the Caspase-Glo assay (Promega) according to the manufacturer's instructions. THP-1 cells (4 × 104) were treated with staurosporine as a control, with strain AU92, and with AU92 Sn as well as with strain US77 and US77 Sn for 6 h. At that time, caspase activities were determined in a microplate reader (Tecan).
Measurement of procaspase and PARP cleavage.
We performed a caspase-3/7 and poly(ADP-ribose) polymerase (PARP) cleavage study by using Western blotting. At 1 h and 4 h postinfection, cells (6 × 106) were recovered and washed three times with PBS. Cell extracts were prepared by lysing the cells in a buffer containing 50 mM Tris, pH 8, 150 mM NaCl, 1% Ipegal CA 630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mM sodium orthovanadate, 5 mM NaF, 1 mM phenylmethylsulfonyl fluoride, and a 1/100 dilution of protease inhibitor cocktail (Sigma). Subsequently, 50 μg of protein was loaded into an SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gel, transferred to a polyvinylidene difluoride membrane (Sigma), and immunoblotted using specific antibodies (apoptosis sampler kit; Cell Signaling) followed by secondary antibodies conjugated with horseradish peroxidase. Signals were generated by the enhanced chemiluminescence reaction (GE Healthcare) and detected using X-ray film.
TEM.
Cell morphology following infection with P. asymbiotica AU92 and P. luminescens TT01 was examined by transmission electron microscopy (TEM). At different infection times, cells were fixed in 5% glutaraldehyde in phosphate buffer (pH 7.2), postfixed in 1% osmic acid in the same buffer, dehydrated in a graded series of ethanol, and embedded in Epon 812. Ultrathin sections were stained according to the method of Reynolds (25) and examined with a JEOL 200-CX transmission electron microscope at 70 kV.
Statistical methods.
All results are expressed as means ± standard deviations (SD) for three independent experiments. Statistical significance was assessed using the Student t test. Differences were considered statistically significant for P values of <0.05.
RESULTS
P. asymbiotica is a facultative intracellular bacterium.
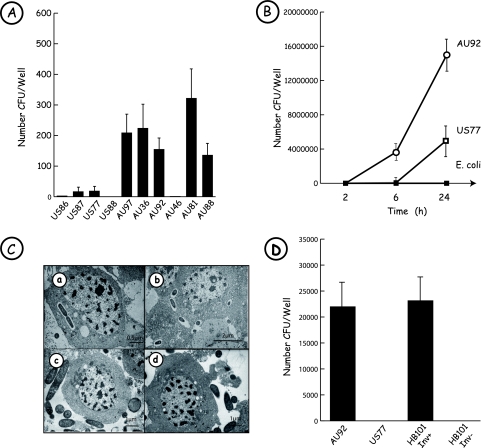
One of the first immune defense reactions that pathogenic bacteria must avoid in order to survive is phagocytosis. Thus, we first studied whether P. asymbiotica cells are engulfed by macrophages or whether they are able to escape phagocytosis. To address this issue, we used PMA-differentiated THP-1 macrophage-like cells and performed a gentamicin killing assay as described in Materials and Methods. Almost all clinical strains of Photorhabdus were engulfed by THP-1 cells within 2 h of infection, but in contrast to Australian isolates, the American ones were weakly phagocytosed (Fig. 1A). To determine if clinical strains of Photorhabdus were able to replicate during infection, we carried out an AU92 and US77 chase over 24 h (Fig. 1B). Bacteria were recovered from macrophages infected with P. asymbiotica at 2 h postinfection, with intracellular uptake rates similar to those obtained with the nonpathogenic organism E. coli XL1-Blue. Despite their low uptake rates, AU92 and US77 survived and multiplied all along the experiment, reaching an accentuated increase between 6 and 24 h postinfection. As expected, E. coli was unable to multiply during the infection experiment. Photorhabdus is also an insect-pathogenic bacterium, and to further characterize phagocytosis, we were interested in comparing the possible internalization into insect cells of the strictly entomopathogenic TT01 strain of P. luminescens and the clinical strains AU92 and US77 of P. asymbiotica (Fig. 1C). Studies achieved with TEM on hemocytes of the insect M. unipuncta showed that TT01 was found only outside the insect macrophage (Fig. 1C, panel c). Like the TT01 strain of P. luminescens, the P. asymbiotica clinical strain US77 was found mainly with an extracellular localization (Fig. 1C, panel d). In contrast, the P. asymbiotica clinical strain AU92 was clearly observed inside the cells (Fig. 1C, panels a and b), confirming the studies carried out on mammalian macrophages. We also performed a gentamicin killing assay on insect hemocytes, with strain AU92 compared to strain US77. The ranges of uptake by hemocytes were different between the two strains. At 2 h postinfection, the number of intracellular AU92 cells was 30 times higher than that of US77 cells (P < 0.01) (data not shown). The gentamicin killing assay was also performed on hemocytes removed from infected insect larvae. At 3 h postinjection, around 25 times more CFU per insect were recovered for the Australian strain than for the American one. At 24 h postinjection, the number of CFU per insect recovered for the American strain was 50 times higher than that at 3 h postinjection, but almost all insects infected with the Australian strain were dead (data not shown).
FIG. 1.
Photorhabdus asymbiotica is a facultative intracellular bacterium. (A) Comparison of Photorhabdus clinical strain uptake by human THP-1 cells. Infection experiments were performed at an MOI of 25:1 with PMA-differentiated THP-1 cells and with the different bacterial strains listed in Table 1. After 2 h of gentamicin treatment (100 μg/ml), macrophage-like cells were lysed, intracellular bacteria were recovered, and the number of CFU/well was determined. All Australian clinical isolates were engulfed by macrophages, except for strain AU46. The American isolates were weakly phagocytosed. Results are representative of three independent experiments. (B) Survival and growth of P. asymbiotica AU92 and US77 in human THP-1 cells. THP-1 cells were challenged with P. asymbiotica strains at an MOI of 25:1 and treated for 2 h with 100 μg/ml gentamicin. At different times postinfection, cells were lysed and bacteria plated. The American (open squares) and Australian (circles) clinical isolates studied were able to survive in THP-1 cells, in contrast to the nonpathogenic E. coli XL1-Blue strain (filled squares), which was eliminated by macrophage-like cells. Results are representative of three independent experiments. (C) Bacteria of clinical strains are present inside insect hemocytes. Hemocytes of M. unipuncta were incubated with P. asymbiotica AU92 (a and b), P. luminescens TT01 (c), or P. asymbiotica US77 (d). TEM observations show that the human clinical isolate P. asymbiotica AU92 is phagocytosed by hemocytes. In contrast, the strictly entomopathogenic organism P. luminescens, as well as P. asymbiotica US77, present an extracellular location. (D) Invasion of epithelial cells by P. asymbiotica. HeLa cells were infected for 1 h with the P. asymbiotica strains AU92 and US77 and with E. coli HB101 Inv+ (carrying the inv gene of Yersinia) and E. coli HB101 Inv− as negative controls (MOI, 25:1). After 2 h of treatment with 100 μg/ml gentamicin, cells were lysed and intracellular bacteria plated. The Australian strain of P. asymbiotica was able to invade HeLa cells, with an intracellular uptake rate similar to that of E. coli HB101 harboring the Yersinia inv gene. Results are representative of three independent experiments.
To determine whether P. asymbiotica was able to promote entry into nonphagocytic cells, we used epithelial HeLa cells and performed a gentamicin killing assay as described above. HeLa cells were incubated with strains AU92 and US77 for 1 h. CFU counts (Fig. 1D) revealed that AU92 was able to invade HeLa cells, with rates similar to those obtained with the positive control E. coli strain carrying the inv gene of Yersinia. On the other hand, US77 was unable to invade HeLa cells.
P. asymbiotica induces apoptosis of host cells.
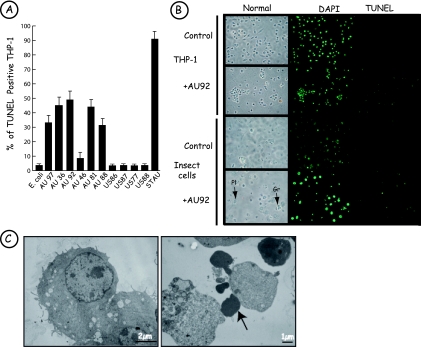
During infection experiments, THP-1 cells in contact with AU92 were visualized under an inverted microscope. After 4 h of interaction, cells started to round, and at 6 h postinfection, most of them became unstuck from the plates. These nonadherent cells presented large cytoplasmic protrusions but were not able to absorb trypan blue dye or to release LDH, demonstrating membrane integrity. Moreover, numerous cells infected with AU92 were TUNEL positive, and DAPI labeling revealed nuclear fragmentation (Fig. 2B). These morphological observations were confirmed by TEM: THP-1 cells infected with AU92 presented cellular shrinkage, membrane blebbing, and chromatin condensation (Fig. 2C). We also assessed if all Photorhabdus clinical strains induced THP-1 cell apoptosis. Nearly all Australian Photorhabdus strains induced strong programmed cell death within 6 h of interaction, with an average of 41% apoptotic cells. Only the Australian AU46 isolates induced weak apoptosis, with 8% TUNEL-positive cells at 6 h (Fig. 2A). In contrast, American strains did not induce apoptosis of THP-1 cells at 6 h postinfection (Fig. 2A). However, in a long-term incubation (up to 24 h), apoptosis was observed with the American clinical strains, but at a lower level than that for the Australian ones, with 46% and 70% apoptotic host cells, respectively (data not shown). Under our experimental conditions, THP-1 cells infected with E. coli XL1-Blue were TUNEL negative and cells treated with staurosporine were TUNEL positive. We were interested in studying the effect of the P. asymbiotica AU92 strain on different cell lines. At 6 h postinfection, human macrophage-like THP-1 cells (Fig. 2B), but also human primary macrophages, mouse J774 macrophage-like cells, and human Jurkat lymphocyte-like cells (data not shown), were TUNEL positive. In contrast, HeLa cells presented no nuclear fragmentation and were TUNEL negative (data not shown). Furthermore, when insect cells were used as a target, only granulocytes, which are the functional equivalent of mammalian macrophages, showed apoptotic features, in contrast to plasmatocytes, which were TUNEL negative (Fig. 2B). In 6-h infection experiments, whatever the nature of the bacteria or the target cell, no necrotic cell death with loss in membrane integrity and release of LDH was observed. But after 24 h of incubation of THP-1 cells with strain AU92, a low level of LDH was recorded in the medium.
FIG. 2.
Photorhabdus asymbiotica induces apoptosis of host cells. (A) Australian strains of P. asymbiotica induce apoptosis of THP-1 cells upon 6 h of infection. THP-1 cells were infected with the American and Australian Photorhabdus clinical strains at an MOI of 100:1 (E. coli XL1-Blue was used as a negative control, and staurosporine [STAU] was used as a positive control). TUNEL-positive cells were determined by fluorescence microscopy. At 6 h postinfection, all Australian strains induced apoptosis of THP-1 cells. Almost no TUNEL-positive THP-1 cells were found at 6 h postinfection when infection was done with the American strains. Results are representative of three independent experiments. (B) Apoptosis induced by P. asymbiotica strain AU92 is specific for macrophage-like cells. THP-1 cells and insect hemocytes were incubated for 6 h with P. asymbiotica at an MOI of 100:1. After DAPI staining, some THP-1 cells presented nuclear fragmentation, and these cells were TUNEL positive. Insect granulocytes (Gr) were TUNEL positive, in contrast to plasmatocytes (Pl). (C) Ultrastructure of THP-1 cells following infection with P. asymbiotica (by TEM). Control cells (left) and THP-1 cells (right) are shown after 6 h of incubation with AU92 (MOI, 100:1). Note the presence of typical apoptotic bodies (arrow).
P. asymbiotica triggers caspase activation.
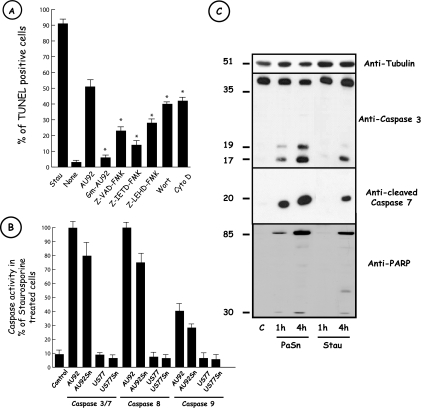
In order to better characterize apoptosis induced by the Photorhabdus clinical strain AU92, we first used gentamicin-inactivated bacteria for THP-1 cell infection experiments. We observed a loss of apoptosis. Moreover, treatment of THP-1 cells with cytochalasin D or wortmannin prior to bacterial infection significantly lowered the level of apoptosis (Fig. 3A). To further investigate apoptosis induced by AU92, we performed apoptosis inhibition experiments with the broad-spectrum caspase inhibitor Z-VAD-FMK and specific caspase-8 and -9 inhibitors, i.e., Z-IETD-FMK and Z-LEHD-FMK, respectively (Fig. 3A). Cells pretreated with Z-VAD-FMK showed a significant reduction in the percentage of THP-1 cell death (23% versus 52% in the absence of inhibitor). Both the Z-IETD-FMK and Z-LEHD-FMK inhibitors presented a significant effect on the apoptosis rate in THP-1 cells (15% and 28%, respectively, versus 52%) incubated with bacteria alone. Similar results were obtained with AU92 Sn. Under our experimental conditions, >90% of staurosporine-treated cells were apoptotic. In order to confirm the involvement of caspases suggested by the use of the broad-spectrum or specific caspase-8/9 inhibitors, we also examined the in vitro activities of caspases in THP-1 cells by using fluorometric assay kits. As shown in Fig. 3B, exposure of THP-1 cells to AU92 or AU92 Sn induced strong caspase-3/7, caspase-8, and caspase-9 activities. These activities were comparable to those in a control cell background when THP-1 cells were treated with the American strain US77 or its Sn. The involvement of caspases in P. asymbiotica-associated apoptosis was further investigated by measuring the cleavage of known caspase substrates. Protein extracts from AU92-, AU92 Sn-, or staurosporine-treated cells or from control cells were prepared at various time points and subjected to SDS-PAGE followed by Western blotting. A rapid and time-dependent proteolysis of the caspase-specific substrate PARP was detected (Fig. 3C). The characteristic catalytic 85-kDa and regulatory 30-kDa fragments were observed for both bacterium- and Sn-treated cells. In accordance with this result, detection of executioner caspase-3 and -7 with specific antibodies showed that as soon as 1 h after treatment with AU92 or AU92 Sn, both caspases were cleaved into the active form. When THP-1 cells were cultured with gentamicin-inactivated AU92, no caspase cleavage was observed (data not shown).
FIG. 3.
Apoptosis induced by AU92 requires caspase activity. (A) Effects of various inhibitors on AU92-induced THP-1 cell apoptosis. THP-1 cells were incubated with 20 μM Z-VAD-FMK, 10 μM Z-IETD-FMK, 10 μM Z-LEHD-FMK, 100 nM wortmannin (Wort), or 5 μg/ml cytochalasin D (Cyto D) 20 min before infection. Cells were infected with P. asymbiotica AU92 at an MOI of 100 for 6 h, and TUNEL labeling was performed. The different caspase and phagocytosis inhibitors significantly reduced the number of TUNEL-positive cells after P. asymbiotica AU92 treatment. Staurosporine (2.5 μM) (Stau) was used as an apoptosis positive control. None, no treatment. Results are means ± SD for three independent experiments. *, P < 0.01. (B) P. asymbiotica AU92 and P. asymbiotica AU92 Sn activated caspases in THP-1 cells. THP-1 cells were incubated with P. asymbiotica AU92, AU92 Sn, US77, US77 Sn, or staurosporine. Caspase activities were determined by the released of luminescent products following the manufacturer's protocol. Caspase activities are expressed as percentages of those in staurosporine-treated cells. Results are means ± SD for three independent experiments. (C) P. asymbiotica induces caspase-3/7 and PARP cleavage. After 1 or 4 h of incubation of THP-1 cells with P. asymbiotica AU92 or bacterial supernatant (PaSn), total protein extracts were separated by 15% SDS-PAGE, followed by Western blotting with anti-caspase-3, anti-cleaved caspase-7, and anti-PARP antibodies. Native (around 35 kDa) and cleaved (around 20 kDa) forms of caspase-3, the cleaved form of caspase-7 (around 20 kDa), and PARP (around 85 kDa and 30 kDa) were already present after 1 h of incubation of THP-1 cells with PaSn. The same results were obtained with living but not with dead bacteria (data not shown). Staurosporine (2.5 μM) (Stau) was used as an apoptosis positive control. c, no treatment. Data are representative of three independent experiments.
DISCUSSION
Gram-negative bacteria have evolved different strategies to survive and disseminate inside the host. Little is known about the virulence factors used by members of the genus Photorhabdus in order to escape insect immune responses. Human-pathogenic P. asymbiotica escapes from human immune defense reactions and provokes several injuries in patients. Under laboratory conditions, P. asymbiotica is highly virulent for insects such as Galleria mellonella and Spodoptera littoralis (Pages et al., unpublished data). To better understand the adaptation of Photorhabdus to a new ecological niche—humans—we were interested in investigating the interactions between P. asymbiotica and eukaryotic cells, especially macrophage-like cells. Monocytes/macrophages play a central role in inflammatory reactions caused by pathogenic bacteria. Many pathogens have evolved specific mechanisms for avoiding, altering, or disabling the antimicrobial effects of these cells.
We have previously shown that in vivo, P. luminescens is found in an extracellular location, a feature due to the T3SS (3). Our present data demonstrate that P. asymbiotica is a facultative intracellular pathogen. Despite an initial low intracellular uptake rate, P. asymbiotica was able to avoid destruction in human macrophage-like cells and to replicate throughout the experiment. Here again, the parallel between the behaviors of Photorhabdus and Yersinia is noteworthy. In vivo, yersiniae are mainly extracellular pathogens, and it was shown only recently that they are able to survive and replicate inside macrophages during the early stages of infection. Thus, we can hypothesize that during evolution, Photorhabdus clinical strains may have acquired virulence factors that allowed this insect-pathogenic bacterium to colonize a wider eukaryote niche. The ability of Yersinia to replicate inside macrophages was assumed to be a strategy to avoid destruction upon infecting a host. This could also be a skillful technique that uses macrophages as a vehicle for bacterial transport from the initial site of infection to lymph nodes (24). Interestingly, several patients infected with P. asymbiotica presented first with a single lesion on the hands or leg skin. But a few days later, multiple lesions were found in other parts of the body. Bacteria were found in these new lesions, and some patients presented lymph node inflammation. Later still, bacteremia was reported for some patients (12, 23). From these clinical observations and from our results, we can hypothesize that like Yersinia, P. asymbiotica was internalized by resident macrophages very soon after entering the host, avoided destruction, and then used macrophages for dissemination.
In agreement with the results for human cells, our assays achieved with insect cells display for the first time that a bacterium from the genus Photorhabdus can be found inside eukaryotic cells, in contrast to strictly entomopathogenic strains. Recently, Tounsi et al. reported differences between P. luminescens and P. asymbiotica during a comparative genomic analysis. Interestingly, an open reading frame homologous to the sopB gene is present only in the human pathogen (29). SopB is a Salmonella T3SS effector with phosphoinositide phosphatidase activity and is implicated in cell invasion and actin rearrangements during the infectious process (21). We show here that the Australian clinical strain of Photorhabdus is also invasive and able to enter a nonmacrophagic cell line and that the American strain is not. The reason for this difference is unknown but can be linked to the previous observations of Farmer et al., who tested the association of an American clinical isolate with Heterorhabditis nematodes and failed to detect it (12). In contrast, P. asymbiotica Australian isolates are retained by infective juvenile nematodes (17, 20). Thus, the American strains may have a lowered ability to invade cells, a useful property during the natural life cycle of Photorhabdus when bacteria are maternally transmitted to infective juveniles (6).
Apoptosis plays an important role in metazoan development, homeostasis, and immune responses, and host cell apoptosis may represent a host defense reaction. Apoptosis leads to the elimination of injured or infected cells without the release of harmful substances or activation of the inflammatory response. Bacteria, viruses, and parasites can either induce or prevent apoptosis to augment infection. Yersinia selectively causes the apoptosis of macrophages and silences inflammatory reactions. This phenomenon allows bacterial dissemination in a lymphatic way, causing a systemic infection (22). On other hand, Salmonella as well as Shigella activates an apoptosis cascade with the release of cytokines, inducing an acute inflammatory response (19, 33).
We show here that P. asymbiotica induces apoptosis of human cell lines. In human macrophage-like cells (but also in human primary macrophages and murine J774 macrophages), up to 40% of apoptotic cells were detected with Australian clinical isolates at 6 h postinfection. Gentamicin-killed P. asymbiotica could not induce cell death. In addition, after 6 h of incubation of THP-1 cells with US77, the number of intracellular bacteria was very low and the number of TUNEL-positive cells was comparable to that for untreated cells. At 24 h postinfection, TUNEL-positive cells were present, and the number of intracellular US77 cells was comparable to that of AU92 cells at 6 h. These observations suggest that apoptosis is due to an active process which requires live bacteria, and for the first time, it could be reasonable to link the differences in the rates of apoptosis observed between American and Australian strains to the initial ability of the bacteria to invade macrophages. On the other hand, inhibition of phagocytosis by cytochalasin D or wortmannin (Fig. 3A) did not suppress but only slightly reduced P. asymbiotica-induced THP-1 cell apoptosis. Thus, phagocytosis is not a prerequisite for Photorhabdus-induced macrophage-like cell death but rather suggests a qualitative difference between American and Australian strains. In agreement with these observations, P. asymbiotica AU92 Sn triggered apoptosis and caspase activation, whereas US77 Sn did not, even after 24 h of incubation. LDH begins to be detectable in the culture medium at 24 h postinfection. This is probably the consequence of a secondary necrosis due to the absence of phagocytosis of all apoptotic cells. In vitro, it is the standard fate of apoptotic cells that were not phagocytosed by neighboring live cells.
At this time, we do not know if apoptosis induction by bacteria or by Sn is due to the same molecule. The simplest hypothesis would be to suppose that the same factor was involved in bacteria and in Sn. On the other hand, since US77 triggered apoptosis after 24 h of incubation with eukaryotic cells, whereas US77 Sn did not, the factors involved with bacteria and Sn could be different. Experiments are under way in order to clarify these points. What is sure is that it was not necessary for P. asymbiotica AU92 to be present inside eukaryotic cells to trigger apoptosis. We do not know if the Sn factor that triggered apoptosis can be secreted specifically by bacteria. In fact, the effect of this factor was detected only in the Sn of overnight cultures, which are in the stationary phase of bacterial culture. At that stage, many bacteria begin to lyse and, in this way, could release the apoptotic factor(s) into the culture medium.
The rapidity with which the induction of programmed cell death takes place is specific to the origin of the target cell line, since 6 h after infection with P. asymbiotica AU92, all HeLa cells were TUNEL negative. Remarkably, in our insect model, only granulocytes, the functional equivalent of mammalian macrophages, were subjected to apoptosis upon infection with P. asymbiotica, and no TUNEL-positive plasmatocytes were ever observed.
The caspase activities and cleavage pattern were analyzed in macrophage cultures after the addition of P. asymbiotica or the bacterial culture Sn. Caspases are intracellular cysteine proteases involved in apoptosis. Procaspases can be activated by two pathways, called the intrinsic and extrinsic pathways. These proteases are divided into two groups, the initiator caspases (caspases 8 and 9) and the executioner caspases (caspases 3, 6, and 7). The extrinsic pathway involves the engagement of death receptors and the activation of procaspase-8. The intrinsic pathway is initiated in the mitochondrion, with the release of cytochrome c and the activation of procaspase-9. Activation of both pathways results in the proteolytic activation of downstream executioner caspases (for a review, see reference 5). We clearly demonstrated, using specific inhibitors and measuring caspase activities, that both bacterium- and Sn-induced apoptosis involves the activation of both the intrinsic and extrinsic pathways. Activation of the initiator caspases leads to cleavage of caspases 3 and 7. Caspase-3 represents one of the key proteases responsible for cleavage of PARP and subsequent apoptosis. Apoptosis can be due to translocated T3SS effectors. Within the P. asymbiotica T3SS locus, the only effector found is a homologue of the ExoU phospholipase of Pseudomonas aeruginosa. P. aeruginosa induces apoptosis in a T3SS-dependent manner, but the effector involved has been identified as the bifunctional protein ExoT (28). In P. aeruginosa, ExoU is involved in necrotic cell death and can most likely be excluded from the proapoptotic factor which we seek. Recently, it was shown that the XaxAB binary toxin from Xenorhabdus nematophila (30) and the Mcf1 toxin of P. luminescens (8) induce cell apoptosis. Both toxins are present in the P. asymbiotica genome. Experiments are in progress to test if one of these toxins could be involved in apoptosis triggered by P. asymbiotica.
Taken together, our results show that there are considerable differences among clinical isolates of P. asymbiotica. Indeed, American clinical strains were weakly phagocytosed by THP-1 cells and were not able to invade HeLa cells, whereas the Australian strain seemed to penetrate easily into THP-1 cells as well as the nonphagocytic HeLa cells. Another difference between the American and the Australian isolates is related to apoptosis. Australian clinical strains triggered the apoptosis of mammalian cells much more rapidly (6 h versus 24 h) and to a larger extent than the American isolates did. Moreover, AU92 Sn triggered caspase activation, whereas US77 Sn did not. Interestingly, tests carried out in our laboratory revealed that when P. asymbiotica was injected into insect larvae of the lepidopteran Spodoptera littoralis, the median lethal time was much longer for the American strain than for Australian ones (120 h versus 38 h). Thus, the difference in median lethal time can be correlated with the numbers of bacteria recovered from hemocytes of insects infected with the two strains. Moreover, most of the American patients infected with P. asymbiotica were immunocompromised people, whereas Australian patients were not. Finally, Australian patients took longer than the American patients to recover from infection caused by P. asymbiotica. The differences between Australian and American clinical isolates described in the present paper could account, at least in part, for the difference in virulence observed in Australia and in the United States.
In summary, our data support a model in which the new emergent pathogen P. asymbiotica is able to survive inside macrophages and to manipulate apoptotic pathways. Intracellular survival and macrophage apoptosis appear to be key elements for pathogenicity, and P. asymbiotica may act like its close relative Yersinia, using macrophages as an efficient vehicle for dissemination upon entering the host. Moreover, apoptosis seems to be an evolved strategy to counterbalance the powerful inflammatory reactions developed by mammals. The identification of effectors responsible for uptake by macrophages and for apoptosis induction is important to further elucidate the mechanisms of pathology developed by P. asymbiotica and is currently in progress.
Acknowledgments
This work received financial support from INRA. S.C.P.C. was funded by grant SFRH/BD/18730/2004 from Fundação para a Ciência e a Tecnologia-Operational Programs Science Technology and Innovation (POCTI), Portugal.
We thank Carlos Ribeiro from Universidade dos Açores.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 15 December 2008.
REFERENCES
- 1.Akhurst, R. J., N. E. Boemare, P. H. Janssen, M. M. Peel, D. A. Alfredson, and C. E. Beard. 2004. Taxonomy of Australian clinical isolates of the genus Photorhabdus and proposal of Photorhabdus asymbiotica subsp. asymbiotica subsp. nov. and P. asymbiotica subsp. australis subsp. nov. Int. J. Syst. Evol. Microbiol. 541301-1310. [DOI] [PubMed] [Google Scholar]
- 2.Bowen, D., T. A. Rocheleau, M. Blackburn, O. Andreev, E. Golubeva, R. Bhartia, and R. H. Ffrench-Constant. 1998. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science 2802129-2132. [DOI] [PubMed] [Google Scholar]
- 3.Brugirard-Ricaud, K., E. Duchaud, A. Givaudan, P. A. Girard, F. Kunst, N. Boemare, M. Brehélin, and R. Zumbihl. 2005. Site-specific antiphagocytic function of the Photorhabdus luminescens type III secretion system during insect colonization. Cell. Microbiol. 7363-371. [DOI] [PubMed] [Google Scholar]
- 4.Brugirard-Ricaud, K., A. Givaudan, J. Parkhill, N. Boemare, F. Kunst, R. Zumbihl, and E. Duchaud. 2004. Variation in the effectors of the type III secretion system among Photorhabdus species as revealed by genomic analysis. J. Bacteriol. 1864376-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowdhury, I., B. Tharakan, and G. K. Bhat. 2006. Current concepts in apoptosis: the physiological suicide program revisited. Cell. Mol. Biol. Lett. 11506-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciche, T. A., K. S. Kim, B. Kaufmann-Daszczuk, K. C. Nguyen, and D. H. Hall. 2008. Cell invasion and matricide during Photorhabdus luminescens transmission by Heterorhabditis bacteriophora nematodes. Appl. Environ. Microbiol. 742275-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa, S. C. P., C. Ribeiro, P. A. Girard, R. Zumbihl, and M. Brehélin. 2005. Modes of phagocytosis of gram-positive and gram-negative bacteria by Spodoptera littoralis granular haemocytes. J. Insect Physiol. 5139-46. [DOI] [PubMed] [Google Scholar]
- 8.Dowling, A. J., N. R. Waterfield, M. C. Hares, G. Le Goff, C. H. Streuli, and R. H. Ffrench-Constant. 2007. The Mcf1 toxin induces apoptosis via the mitochondrial pathway and apoptosis is attenuated by mutation of the BH3-like domain. Cell. Microbiol. 92470-2484. [DOI] [PubMed] [Google Scholar]
- 9.Duchaud, E., C. Rusniok, L. Frangeul, C. Buchrieser, A. Givaudan, S. Taourit, S. Bocs, C. Boursaux-Eude, M. Chandler, J. F. Charles, et al. 2003. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat. Biotechnol. 211307-1313. [DOI] [PubMed] [Google Scholar]
- 10.Ehlers, R. U. 2003. Entomopathogenic nematodes in the European biocontrol market. Commun. Agric. Appl. Biol. Sci. 683-16. [PubMed] [Google Scholar]
- 11.Elsinghorst, E. A. 1994. Measurement of invasion by gentamicin resistance. Methods Enzymol. 236405-420. [DOI] [PubMed] [Google Scholar]
- 12.Farmer, J. J., III, J. H. Jorgensen, P. A. Grimont, R. J. Akhurst, G. O. Poinar, E. Ageron, G. V. Pierce, J. A. Smith, G. P. Carter, K. L. Wilson, et al. 1989. Xenorhabdus luminescens (DNA hybridization group 5) from human clinical specimens. J. Clin. Microbiol. 271594-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ffrench-Constant, R. H., N. R. Waterfield, V. Burland, N. T. Perna, P. J. Daborn, D. Bowen, and F. R. Blattner. 2000. A genomic sample sequence of the entomopathogenic bacterium Photorhabdus luminescens W14: potential implications for virulence. Appl. Environ. Microbiol. 663310-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer-Le Saux, M., V. Viallard, B. Brunel, P. Normand, and N. E. Boemare. 1999. Polyphasic classification of the genus Photorhabdus and proposal of new taxa: P. luminescens subsp. luminescens subsp. nov., P. luminescens subsp. akhurstii subsp. nov., P. luminescens subsp. laumondii subsp. nov., P. temperata sp. nov., P. temperata subsp. temperata subsp. nov. and P. asymbiotica sp. nov. Int. J. Syst. Bacteriol. 491645-1656. [DOI] [PubMed] [Google Scholar]
- 15.Forst, S., B. Dowds, N. E. Boemare, and E. Stackebrandt. 1997. Xenorhabdus and Photorhabdus spp.: bugs that kill bugs. Annu. Rev. Microbiol. 5147-72. [DOI] [PubMed] [Google Scholar]
- 16.Gendlina, I., K. G. Held, S. S. Bartra, B. M. Gallis, C. E. Doneanu, D. R. Goodlett, G. V. Plano, and C. M. Collins. 2007. Identification and type III-dependent secretion of the Yersinia pestis insecticidal-like proteins. Mol. Microbiol. 641214-1227. [DOI] [PubMed] [Google Scholar]
- 17.Gerrard, J. G., S. A. Joyce, D. J. Clarke, R. H. Ffrench-Constant, G. R. Nimmo, D. F. Looke, E. L. Feil, L. Pearce, and N. R. Waterfield. 2006. Nematode symbiont for Photorhabdus asymbiotica. Emerg. Infect. Dis. 121562-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerrard, J. G., R. Vohra, and G. R. Nimmo. 2003. Identification of Photorhabdus asymbiotica in cases of human infection. Commun. Dis. Intell. 27540-541. [DOI] [PubMed] [Google Scholar]
- 19.Hersh, D., D. M. Monack, M. R. Smith, N. Ghori, S. Falkow, and A. Zychlinsky. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 962396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuwata, R., T. Yoshiga, M. Yoshida, and E. Kondo. 2008. Mutualistic association of Photorhabdus asymbiotica with Japanese Heterorhabditid entomopathogenic nematodes. Microbes Infect. 10734-741. [DOI] [PubMed] [Google Scholar]
- 21.Marcus, S. L., L. A. Knodler, and B. B. Finlay. 2002. Salmonella enterica serovar Typhimurium effector SigD/SopB is membrane-associated and ubiquitinated inside host cells. Cell. Microbiol. 4435-446. [DOI] [PubMed] [Google Scholar]
- 22.Monack, D. M., J. Mecsas, D. Bouley, and S. Falkow. 1998. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J. Exp. Med. 1882116-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peel, M. M., D. A. Alfredson, J. G. Gerrard, J. M. Davis, J. M. Robson, R. J. McDougall, B. L. Sculli, and R. J. Akhurst. 1999. Isolation, identification, and molecular characterization of strains of Photorhabdus luminescens from infected humans in Australia. J. Clin. Microbiol. 373647-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pujol, C., and J. B. Bliska. 2005. Turning Yersinia pathogenesis outside in: subversion of macrophage function by intracellular yersiniae. Clin. Immunol. 114216-226. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds, E. S. 1963. The use of lead citrate at high pH as an electron opaque staining in electron microscopy. J. Cell Biol. 17208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribeiro, C., and M. Brehélin. 2006. Insect haemocytes: what type of cell is that? J. Insect Physiol. 52417-429. [DOI] [PubMed] [Google Scholar]
- 27.Schulte, R., R. Zumbihl, D. Kampik, A. Fauconnier, and I. B. Autenrieth. 1998. Wortmannin blocks Yersinia invasin-triggered internalization, but not interleukin-8 production by epithelial cells. Med. Microbiol. Immunol. 18753-60. [DOI] [PubMed] [Google Scholar]
- 28.Shafikhani, S. H., C. Morales, and J. Engel. 2008. The Pseudomonas aeruginosa type III secreted toxin ExoT is necessary and sufficient to induce apoptosis in epithelial cells. Cell. Microbiol. 10994-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tounsi, S., M. Blight, S. Jaoua, and A. de Lima Pimenta. 2006. From insects to human hosts: identification of major genomic differences between entomopathogenic strains of Photorhabdus and the emerging human pathogen Photorhabdus asymbiotica. Int. J. Med. Microbiol. 296521. [DOI] [PubMed] [Google Scholar]
- 30.Vigneux, F., R. Zumbihl, G. Jubelin, C. Ribeiro, J. Poncet, S. Baghdiguian, A. Givaudan, and M. Brehélin. 2007. The xaxAB genes encoding a new apoptotic toxin from the insect pathogen Xenorhabdus nematophila are present in plant and human pathogens. J. Biol. Chem. 2829571-9580. [DOI] [PubMed] [Google Scholar]
- 31.Waterfield, N. R., B. W. Wren, and R. H. Ffrench-Constant. 2004. Invertebrates as a source of emerging human pathogens. Nat. Rev. Microbiol. 2833-841. [DOI] [PubMed] [Google Scholar]
- 32.Weissfeld, A. S., R. J. Halliday, D. E. Simmons, E. A. Trevino, P. H. Vance, C. M. O'Hara, E. G. Sowers, R. Kern, R. D. Koy, K. Hodde, et al. 2005. Photorhabdus asymbiotica, a pathogen emerging on two continents that proves that there is no substitute for a well-trained clinical microbiologist. J. Clin. Microbiol. 434152-4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zychlinsky, A., M. C. Prevost, and P. J. Sansonetti. 1992. Shigella flexneri induces apoptosis in infected macrophages. Nature 358167-169. [DOI] [PubMed] [Google Scholar]