Abstract
Pertussis is an acute respiratory disease caused by the bacterium Bordetella pertussis, for which humans are the only known reservoir. During infection, B. pertussis releases several toxins, including pertussis toxin (PT) and adenylate cyclase toxin (ACT), which have both been shown to play roles in promoting bacterial growth during early infection in a mouse model. Furthermore, in vitro and in vivo studies suggest that PT and ACT affect neutrophil chemotaxis and/or function, thereby altering the innate immune response. In this study we depleted animals of neutrophils to investigate whether neutrophils play a protective role during B. pertussis infection in mice. In addition, by infection with toxin-deficient strains, we investigated whether neutrophils are the main targets for PT and/or ACT activity in promoting bacterial growth. Surprisingly, we found no role for neutrophils during B. pertussis infection in naïve mice. However, in previously infected (immune) mice or in mice receiving immune serum, we observed a significant role for neutrophils during infection. Furthermore, in this immune mouse model our evidence indicates that neutrophils appear to be the main target cells for ACT, but not for PT.
Pertussis, also known as whooping cough, is an acute respiratory disease caused by the bacterium Bordetella pertussis. B. pertussis infects the human respiratory tract and binds to ciliated cells in the upper and lower airways (8, 15, 27, 38). B. pertussis releases several toxins, including pertussis toxin (PT) and adenylate cyclase toxin (ACT), which have both been shown to play a role in promoting bacterial growth in the airways in a mouse model (4, 6). Furthermore, evidence suggests that both toxins have effects on cells of the immune system, including neutrophils and macrophages, which are important effectors of the innate immune response (2, 7, 28, 34-36, 40).
Neutrophils play a protective role in response to infection by several different respiratory pathogens (23), including Pseudomonas aeruginosa (33), Legionella pneumophila (29), and Klebsiella pneumoniae (39). A study by Tsai et al. showed that inhibition of neutrophil recruitment in response to P. aeruginosa lung infection in mice resulted in increased bacterial loads and a decreased survival rate of the mice (33), and data reported by Ye et al. suggest that induction of neutrophil recruitment through a Th17 response is essential for protection against K. pneumoniae (39). Furthermore, neutrophils have been shown to be essential for effective clearance of lower respiratory tract Haemophilus influenzae infection in a mouse model (31).
PT, which is produced exclusively by B. pertussis, has been shown by us and others to suppress several aspects of the immune response, including macrophage function, serum antibody production, and neutrophil chemotaxis (5-7, 18, 30). Previous data from our lab demonstrated a role for PT in establishing B. pertussis infection in the mouse respiratory tract (6), and we recently showed that PT inhibits neutrophil recruitment to the airways in response to infection by suppressing the release of neutrophil-attracting chemokines by resident airway cells (2).
ACT, another toxin released by B. pertussis, has been described to have inhibitory effects on neutrophil function by decreasing phagocytosis and superoxide generation and inhibiting chemotaxis (10). Mobberley-Schuman et al. demonstrated that mutant strains of B. pertussis that do not produce ACT (ΔCYA) are readily ingested by human neutrophils, while wild-type (WT) strains are able to resist phagocytosis (25). ACT has been shown to inhibit both CD11b/CD18-mediated phagocytosis (25) as well as antibody-mediated phagocytosis via the Fc receptor by human neutrophils (24, 34). Opsonizing antibodies generally increase phagocytosis of pathogens by phagocytes (16), and a study by Weingart et al. showed that a ΔCYA strain of B. pertussis was efficiently phagocytosed by human neutrophils in the presence of immune serum (35). However, in the presence of ACT, opsonization of B. pertussis did not increase phagocytosis when compared to unopsonized controls (34). Evidence suggests that the increased levels of cyclic AMP in these cells lead to a decrease in phagocytosis and superoxide generation as well as inhibition of chemotaxis, thereby affecting a major part of the innate immune response (10).
Based on these observations, we hypothesized that neutrophils play a role in controlling and/or clearing B. pertussis infection in the airways and that PT and ACT play roles by inhibiting neutrophil chemotaxis and function. Furthermore, we hypothesized that the absence of neutrophils would increase bacterial loads as well as the duration of the infection and that the mutant strains, ΔPT and ΔCYA, would be able to grow to WT levels in a neutropenic mouse model. Surprisingly, we found that neutrophils apparently did not play a role in controlling or clearing B. pertussis infection in naïve mice. Furthermore, neutrophils did not appear to be the main targets of PT and ACT in this model. However, in previously infected (immune) mice, the absence of neutrophils led to a significant increase in bacterial loads, and the ΔCYA strain, but not the ΔPT strain, infected neutropenic mice at WT levels, suggesting that ACT targets neutrophils in this model and that neutrophils play a role in controlling the infection.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The B. pertussis strains used in this study were streptomycin- and nalidixic acid-resistant derivatives of Tohama I. The ΔPT and ΔCYA strains were constructed as previously described (4, 6). The ΔPT ΔCYA double mutant was derived by introducing the cyaA deletion into the ΔPT strain by conjugation and allelic exchange as previously described (4). B. pertussis strains were grown on Bordet-Gengou (BG) agar plates containing 10% defibrinated sheep blood and 400 μg/ml of streptomycin.
Mouse infection.
Six-week-old female BALB/c mice (Charles River Laboratories) were used in our studies. Inocula were prepared by plating bacterial strains from frozen culture on BG blood agar plates with streptomycin. After growth for 3 days at 37°C, bacteria were transferred to new plates and allowed to grow for an additional 2 days. Bacterial strains were resuspended and appropriate dilutions were prepared using sterile phosphate-buffered saline (PBS). Mice were anesthetized by inhalation of either Metofane (Medical Developments Australia) or isoflurane (Baxter) and inoculated intranasally with 50 μl of inoculum. In addition, the inoculum was diluted and plated on BG blood agar plates with streptomycin to determine viable counts. Mice were euthanized by carbon dioxide inhalation at specified time points and the lungs and trachea were removed and homogenized in 2 ml of sterile PBS. Appropriate dilutions were plated on BG blood agar plates with streptomycin and counted after 4 days of incubation at 37°C to determine CFU per respiratory tract. A minimum of three mice per group was used. A two-tailed t test was used for statistical analysis.
BAL.
Bronchoalveolar lavage (BAL) was performed by flushing the lungs two times with 0.7 ml sterile PBS as previously described (2). Aliquots of BAL fluid were used for cell counting on a hemocytometer and for cytospin centrifugation for 5 min at 600 rpm. The slides were stained with modified Wright's stain to identify different cell types. Approximately 100 cells from several microscope fields were counted and identified for each sample.
Preparation of lung tissues for gene expression studies.
Mice were euthanized by carbon dioxide inhalation. The thoracic cavity was exposed and the left lobes of the lung were removed and snap-frozen in a dry ice-ethanol bath and stored at −80°C until further use.
RNA preparation and cDNA synthesis.
RNA preparation from whole lung tissue was performed using the phenol-chloroform method as previously described (2). The RNA pellet was resuspended in 50 μl of nuclease-free H2O, and quantification of RNA was performed using an ND-1000 NanoDrop spectrophotometer. The synthesis of cDNA was performed with a reverse transcription system kit (Promega) according to the manufacturer's protocol using random primers. One μg of RNA was used for each sample.
Real-time PCR.
Real-time PCR was performed using the SYBR green system (ABI). Primer sequences for hypoxanthine phosphoribosyltransferase (HPRT) and chemokines (KC) were generously provided by Stefanie Vogel (University of Maryland, Baltimore). Primer sequences used were as follows: HPRT forward primer, 5′-GCTGACCTGCTGGATTACATTAA-3′, and HPRT reverse primer, 5′-TGATCATTACAGTAGCTCTTCAGTCTGA-3′; KC forward primer, 5′-GCTTGAAGGTGTTGCCCTCA-3′, and KC reverse primer, 5′-GTGGCTATGACTTCGGTTTGG-3′. Samples were prepared as previously described (2). Duplicate reactions were performed for each sample and all genes were normalized using the mouse housekeeping gene HPRT. Results were measured as the increase over a PBS control.
Neutrophil depletion and inhibition of chemotaxis.
Mouse neutrophils were depleted by intraperitoneal (i.p.) administration of 1 mg of a rat monoclonal antibody (RB6) against Ly-6G (Gr-1), which is expressed predominantly on neutrophils. The antibody was administered the day before intranasal inoculation with bacteria and every other day thereafter until mouse dissection. Control mice were treated with 1 mg of purified rat immunoglobulin G (IgG; Sigma). Inhibition of neutrophil chemotaxis was achieved by i.p. administration of 1 mg of a goat polyclonal anti-CXCR2 antibody (kindly provided by R. M. Strieter, University of Virginia), which binds to CXCR2 chemokine receptors on neutrophils and inhibits chemotactic responses to chemokines (20). The same regimen of administration was performed as described above for RB6. Control mice were treated with heat-inactivated goat serum (Sigma).
Processing of immune and naïve serum.
Blood used for serum transfer experiments was obtained from naïve or immunized mice by cardiac puncture. Blood was allowed to clot and centrifuged at 5,000 rpm for 10 min. Serum was transferred to a separate tube, heat inactivated to deplete complement, and stored at −20°C until use.
Statistical analysis.
Statistical significance in gene expression experiments was determined using a one-way analysis of variance, allowing for comparison of three sets of data per time point. A Tukey post hoc test was used for pairwise comparisons of data sets. All other data were analyzed using a two-tailed t test.
RESULTS
Efficacy of neutrophil depletion and inhibition of neutrophil chemotaxis.
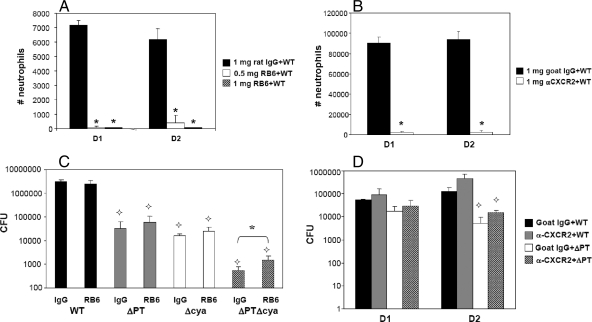
In order to assess the importance of neutrophils in the early immune response to B. pertussis infection in mice, we depleted neutrophils using a rat monoclonal antibody, RB6. This antibody binds to Ly-6G (Gr-1), which is present on the surface of granulocytes, and depletes neutrophils in a complement-dependent manner (33). Administration of doses as low as 50 to 150 μg of RB6 has been shown to cause blood neutropenia in mice for at least 48 h after i.p. injection (21, 22). Groups of mice were injected i.p. with 0.5 mg or 1 mg of RB6 (or 1 mg of anti-rat IgG as a control) 1 day before inoculation with 5 × 106 CFU of WT B. pertussis, a bacterial dose that induces a rapid and large neutrophil recruitment to the airways. On days 1 and 2 postinfection, the number of neutrophils in the BAL fluid of mice treated with 0.5 mg of RB6 was decreased to approximately 1% and 8%, respectively, of that of the control group (Fig. 1A). No neutrophils were observed in the BAL fluid on day 1 or 2 postinfection with WT B. pertussis in mice treated with 1 mg of RB6 (Fig. 1A). Extension of the time course indicated that the longevity of the depleting effect of RB6 at this dose was 3 to 4 days (data not shown). Analysis of blood of mice treated with 1 mg of RB6 also showed a nearly complete neutropenia (data not shown). As an alternative treatment to ensure the results obtained using RB6 were due to neutropenia, and not some unexpected side effect caused by complement-mediated depletion of neutrophils, we also used a goat polyclonal antibody against CXCR2 (anti-CXCR2). This antibody binds the CXCR2 chemokine receptor (the receptor for the neutrophil-attracting chemokines KC, lipopolysaccharide-induced chemokine, and macrophage inflammatory protein 2 in mice) on neutrophils and inhibits neutrophil chemotaxis without depleting peripheral neutrophils (1, 17). The number of neutrophils in the airways of mice treated with anti-CXCR2 and infected with 5 × 106 CFU of WT B. pertussis was decreased to approximately 2 to 3% of that of the control group (treated with heat-inactivated goat serum) on days 1 and 2 postinfection (Fig. 1B). These data indicate that i.p. administration of 1 mg of RB6 or anti-CXCR2 the day before infection effectively inhibits neutrophil recruitment to the airways (either by depletion or inhibition of chemotaxis) in response to B. pertussis for at least 2 days postinfection.
FIG. 1.
Effects of neutrophil depletion and inhibition of neutrophil chemotaxis on bacterial load. (A and B) Numbers of neutrophils in the airways on days 1 and 2 after B. pertussis WT infection (5 × 106 CFU) in mice treated with the indicated dose of RB6 or control rat IgG (complete inhibition of neutrophil recruitment to the airways was observed at both time points when using 1 mg of RB6) (A) or with the indicated dose of anti-CXCR2 polyclonal antibody or control goat serum. (C and D) Numbers (CFU) of bacteria recovered from the lower respiratory tract (trachea plus lungs) are shown for mice treated with either RB6 or control rat IgG and infected with B. pertussis WT or the indicated mutant strains on day 4 postinoculation (C) or for mice treated with either anti-CXCR2 polyclonal antibody or control goat serum and infected with B. pertussis WT or ΔPT on days 1 and 2 postinoculation (D). n = three to four mice/treatment group. The results represent one of three separate experiments. *, P < 0.05 versus control;  , significantly different from the WT strain.
, significantly different from the WT strain.
Growth and clearance of B. pertussis in the respiratory tract of naïve neutropenic mice.
In order to examine the role of neutrophils in protection against B. pertussis infection and as possible targets for PT and ACT, we inoculated groups of mice with 5 × 105 CFU of the B. pertussis strain WT, ΔPT, ΔCYA, or ΔPT ΔCYA (a double mutant strain) and examined the effect of neutrophil depletion on bacterial loads. RB6 (1 mg) was administered the day before bacterial inoculation and every other day thereafter to ensure complete neutrophil depletion in the airways throughout the experiment. Neutrophil depletion was verified at the time of dissection by differential staining of BAL cells and microscopy (data not shown). Surprisingly, no significant differences in bacterial loads were observed between neutrophil-depleted versus control mice on day 4 after infection with WT B. pertussis (Fig. 1C). There were small but nonsignificant increases in bacterial loads of the ΔPT and ΔCYA strains in neutrophil-depleted versus control mice (Fig. 1C). Furthermore, in neutrophil-depleted mice bacterial loads of the WT strain were still significantly higher than those of ΔPT and ΔCYA, indicating that neutrophils are not the only targets of PT and ACT during infection. However, we did see a significant increase in bacterial loads in neutrophil-depleted mice (compared to controls) infected with the ΔPT ΔCYA strain (Fig. 1C), and although this difference was relatively small (threefold), these results indicate that PT and ACT may have overlapping inhibitory effects on neutrophils.
The experiment was repeated for B. pertussis WT versus ΔPT infection in mice treated with the anti-CXCR2 antibody. In order to achieve long-term inhibition of neutrophil recruitment in the airways using anti-CXCR2, reinjection every other day is necessary (M. Burdick, Strieter Laboratory, University of Virginia, personal communication). Due to limited amounts of anti-CXCR2, bacterial loads were examined on days 1 and 2 postinfection rather than on day 4 as in the previous experiment. In control mice infected with B. pertussis WT, bacterial loads were significantly higher than in those infected with the ΔPT strain on days 1 (P < 0.01) and 2 (P < 0.04) postinfection (Fig. 1D), as we observed previously (5). However, although there were slight increases in bacterial loads of both strains in anti-CXCR2-treated mice versus control mice (Fig. 1D), these were not significant differences. These data are similar to the results obtained using the RB6 antibody and suggest that neutrophils do not play an important role in controlling bacterial loads early during B. pertussis infection in a naïve BALB/c mouse model.
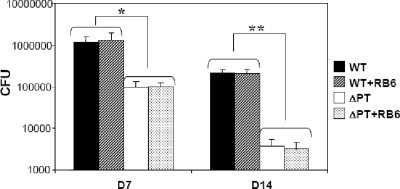
The mechanism(s) of clearance of B. pertussis infection is still quite obscure. In order to determine if neutrophils are important for clearance of B. pertussis infection in naïve mice, we inoculated groups of mice with 5 × 105 CFU of the WT or ΔPT strains. The mice were injected i.p. with 1 mg of RB6 (or rat IgG to control mice) on the day before and every other day following infection to maintain neutrophil depletion throughout the time course. BAL samples were analyzed for cell content by differential staining and microscopy to confirm neutrophil depletion at the time of dissection (data not shown). No significant differences in bacterial loads were observed in neutrophil-depleted mice versus controls at 7 days (late in the peak phase) or 14 days (during clearance) after infection with either strain, suggesting that neutrophils do not play an important role in clearing B. pertussis WT or ΔPT infection from the airways (Fig. 2). As observed previously in our laboratory, there was still a 10- to 100-fold difference in bacterial loads of WT versus ΔPT strains at these time points (5).
FIG. 2.
Clearance of B. pertussis infection in neutropenic mice. Numbers (CFU) of bacteria recovered from the lower respiratory tract of mice treated with either RB6 or control rat IgG and infected with B. pertussis WT or ΔPT on days 7 and 14 postinoculation are shown. n = four mice/treatment group. *, P < 0.005; **, P < 0.001.
Neutrophil recruitment in response to B. pertussis infection in immune mice.
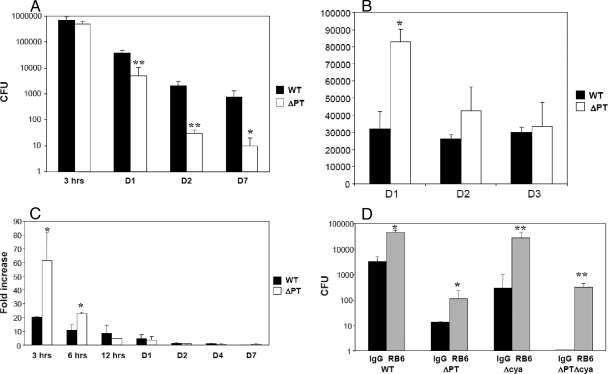
We hypothesized that, despite the results observed in naïve mice, neutrophils may be important in controlling B. pertussis infection in an immune host and that PT and ACT may play roles during infection in immune individuals by targeting neutrophils. First, we wanted to demonstrate that PT plays a role in inhibiting neutrophil recruitment to the airways in immune mice, as it does in naïve mice (2, 3, 5, 15). To obtain immune hosts, we inoculated groups of mice with a low dose (5 × 104 CFU) of B. pertussis ΔPT and allowed the infection to clear over 30 days (data not shown). The ΔPT strain was used to immunize mice in order to avoid the generation of anti-PT antibodies that would only affect the WT and not the ΔPT strain during the secondary infection and might obscure the effects of PT. Studies from our lab using mice previously infected with B. pertussis ΔPT have shown that bacterial loads on day 4 after challenge with the WT strain are relatively low compared to those in naïve mice, due to efficient immune clearance (5); therefore, immune mice were challenged with a high dose (5 × 106 CFU) of the WT or ΔPT strain to avoid immediate clearance of the infection. As previously observed, bacterial loads of WT or ΔPT did not increase after infection in these immune mice, and both strains were nearly cleared from the respiratory tract by day 7 postinoculation (800 and 10 CFU, respectively) (Fig. 3A). Bacterial loads of the WT strain were significantly higher than those of the ΔPT strain by day 1 postinfection (approximately 10-fold), and ΔPT numbers remained significantly lower throughout the time course (Fig. 3A), indicating that, as observed in naïve mice, PT is an important virulence factor for B. pertussis in previously infected mice.
FIG. 3.
Bacterial loads, neutrophil recruitment, and KC gene expression during B. pertussis WT and ΔPT infection in immune mice. (A) Numbers (CFU) of bacteria recovered from the lower respiratory tract of immune mice (previously infected with ΔPT) that were challenged with 5 × 106 CFU of WT or ΔPT at the indicated times postchallenge. (B) Numbers of neutrophils in the airways of immune mice challenged with 5 × 106 CFU of WT or ΔPT at the indicated time postchallenge. (C) Kinetics of KC gene expression (measured by real-time RT-PCR) over 7 days after challenge of immune mice with 5 × 106 CFU of WT or ΔPT. Data are mean fold increases relative to samples from PBS control-treated mice. (D) Numbers (CFU) of bacteria recovered from the lower respiratory tract of immune mice treated with either RB6 or control rat IgG and challenged with B. pertussis WT or the indicated mutant strains (5 × 106 CFU) on day 4 postchallenge. n = three to four mice/treatment group. The results represent one of two separate experiments. *, P < 0.05; **, P < 0.02.
Neutrophil recruitment to the airways in response to WT and ΔPT challenge in immune mice was examined on days 1, 2, and 3 postinfection. The number of neutrophils in the airways on day 1 postinfection was significantly higher (2.5-fold) in response to ΔPT than to WT (Fig. 3B). On days 2 and 3 postinfection, there was no significant difference in the number of neutrophils in the airways in response to infection with the two strains. This indicates that PT inhibits early neutrophil influx in response to infection in an immune host, similar to what is observed after WT and ΔPT infection in naïve mice (2, 6, 18). To determine whether the inhibition of neutrophil recruitment in response to WT infection in immune mice is due to the inhibitory effect of PT on early chemokine gene expression, KC gene expression in whole lung tissue in response to infection with 5 × 106 CFU of the WT or ΔPT strain was examined. There was an early (3 and 6 h postinfection) inhibition of KC gene expression by PT in response to B. pertussis infection in immune mice (Fig. 3C). At the 3-h time point, KC gene expression was induced approximately 60-fold above control levels in response to ΔPT infection and only 20-fold in response to WT infection (P = 0.03). While lower expression in response to both strains was observed at 6 h postinfection, the level of KC gene expression in response to WT was still lower than that in response to ΔPT (10-fold versus 20-fold increased above control; P = 0.04). By day 2 postinfection, KC gene expression in response to WT and ΔPT infection was decreased to a level not significantly different from that of the PBS control group. These results indicate that PT inhibits early production of neutrophil-attracting chemokines in response to B. pertussis infection in immune mice, thereby inhibiting early neutrophil recruitment in response to infection. These observations are similar to the early responses observed in naïve mice infected with WT or ΔPT (2). However, KC gene expression was induced earlier in this experiment in the immune mice than in previous experiments with naïve mice (2), possibly due to the larger dose of bacteria used here.
Bacterial loads in neutrophil-depleted immune mice infected with B. pertussis.
To examine if neutrophils play a role in protection against B. pertussis infection in an immune host and to determine if neutrophils are important targets for PT and ACT, previously infected immune mice were inoculated with 5 × 106 CFU of B. pertussis WT, ΔPT, ΔCYA, or ΔPT ΔCYA, and bacterial loads in the airways were determined 4 days postinfection. There was a significant increase in bacterial loads of all four strains of B. pertussis in neutropenic mice compared to control groups (Fig. 3D). Bacterial loads of the WT and ΔPT strains were increased approximately 10-fold in neutrophil-depleted groups, while the bacterial loads of the ΔCYA strain were 100-fold higher in neutropenic mice compared to control mice. Furthermore, the bacterial loads of the ΔCYA strain were not significantly different from those of the WT strain in the absence of neutrophils (P = 0.26), indicating that neutrophils are the primary targets for ACT during infection in an immune host. The double mutant strain (ΔPT ΔCYA) was cleared by day 4 postinfection in control mice. However, in neutropenic mice, bacterial loads of the ΔPT ΔCYA strain were similar to those in neutrophil-depleted mice infected with ΔPT (P = 0.11). This suggests that neutrophils are not the only targets for PT activity in immune mice, which is consistent with data from our lab indicating that airway macrophages may be the main target cells for PT during B. pertussis infection (7).
Effect of immune serum transfer on bacterial loads in mice infected with B. pertussis.
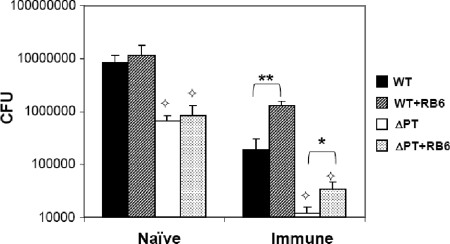
To further evaluate the mechanism by which neutrophils are able to control B. pertussis infection in immune (but not naïve) mice, we examined whether neutrophils play a role in controlling WT and ΔPT infection in naïve BALB/c mice receiving immune serum at the time of infection. Opsonizing antibodies present in immune serum play an important role in response to several respiratory infections caused by other human pathogens (9, 13, 14, 32). Naïve and immune sera were obtained from BALB/c mice 35 days after inoculation with PBS or ΔPT (1 × 106 CFU), respectively. The presence of polyclonal anti-B. pertussis antibodies 28 to 35 days postinoculation with WT or ΔPT has been described previously by us (5) and others (18, 19). Naïve mice were injected i.p. with 125 μl of immune serum (or naïve serum as a control) at the time of intranasal inoculation with 5 × 106 CFU of the WT or ΔPT strain. As expected, no significant differences in bacterial loads (of either strain) were observed in neutrophil-depleted versus control mice that received naïve serum (Fig. 4). Furthermore, the defect of the ΔPT strain compared to the WT strain was still significant on day 4 postinfection, consistent with our results in naïve mice. In mice receiving immune serum, there was a significant increase (5- to 10-fold) in bacterial loads in response to both strains of B. pertussis in neutropenic mice (compared to control mice), suggesting that factors present in immune serum, likely opsonizing antibodies, are important for the ability of neutrophils to control B. pertussis infection. This may explain the lack of an effect of neutrophil depletion in naïve mice. However, bacterial loads of the ΔPT strain in neutropenic mice receiving immune serum were still significantly lower than those of the WT strain in control mice receiving immune serum, again indicating that PT has important targets other than neutrophils in promoting B. pertussis infection of the airways.
FIG. 4.
Bacterial loads after B. pertussis infection in mice after transfer of naïve or immune serum. Numbers (CFU) of bacteria recovered from the lower respiratory tract of mice that received either immune or naïve serum and treated with either RB6 or control rat IgG and infected with B. pertussis WT or ΔPT (5 × 105 CFU) on day 4 postinfection are shown. n = four mice/treatment group. *, P < 0.02; **, P < 0.002;  , significantly different from the WT strain.
, significantly different from the WT strain.
DISCUSSION
Neutrophils are important cells of the innate immune system and have been shown to play a crucial role in protection against several bacterial pathogens of the respiratory tract (26, 29, 31, 33). Previously we and others have shown that PT modulates the innate immune response to B. pertussis infection by delaying early (day 1 to 2) neutrophil recruitment in response to infection (2, 18). One of the questions we set out to answer in this study was whether this activity of PT is its major role in promoting B. pertussis growth in the airways in a mouse model. Our data indicate that this is not the case.
For this study, inhibition of neutrophil recruitment to the airways of mice was successfully accomplished using a neutrophil-depleting antibody (RB6) or by inhibition of neutrophil chemotaxis by administration of an anti-CXCR2 antibody. Surprisingly, neutrophil depletion did not significantly increase the bacterial loads on day 4 postinfection with WT, ΔPT, or ΔCYA strains of B. pertussis. Bacterial loads of a double mutant strain of B. pertussis lacking both toxins, ΔPT ΔCYA, were modestly but significantly higher in neutrophil-depleted mice than in control mice. While ACT has been shown to inhibit phagocytosis and killing by human neutrophils (24, 25, 35), an inhibitory effect of PT on neutrophil function (other than chemotaxis) has not been reported. However, our data indicate that PT and ACT may have overlapping effects on neutrophils during B. pertussis infection. However, neutrophil depletion did not eliminate the need for either of the toxins to achieve WT levels of bacterial growth in the airways, suggesting that neutrophils are not the only targets of PT or ACT action during B. pertussis infection in naïve mice.
Interestingly, the kinetics of bacterial clearance in naïve mice infected with either WT or ΔPT were unaffected by neutrophil depletion, suggesting that neutrophils also do not play a role in clearing the infection in naïve mice. The mechanisms of clearance of B. pertussis infection from the airways are still rather obscure, but mouse models indicate that adaptive immunity is involved, since SCID mice (which lack adaptive immunity) fail to clear the infection (3, 6, 11).
Pertussis is endemic in humans and is recurrent every 3 to 5 years, even in vaccinated populations (37), which results in the presence of many immune and partially immune individuals in the population at any time. A recent review of epidemiologic studies suggested that immunity lasts 4 to 12 years after receiving a pertussis vaccine, while infection-acquired immunity lasts 4 to 20 years (37), making it likely that B. pertussis has evolved to evade immune responses in human hosts with partial immunity to pertussis. Our data demonstrate that neutrophils do play a protective role in immune mice, since bacterial loads after challenge of immune mice with WT, ΔPT, ΔCYA, or ΔPT ΔCYA strains of B. pertussis were significantly higher in neutrophil-depleted mice than in controls. In addition, our serum transfer study showed that immune serum is important for the ability of neutrophils to control B. pertussis infection, probably due to the presence of opsonizing antibodies, which bind to surfaces of pathogens and increase phagocytosis and killing by neutrophils (16). This is consistent with a previous study in C57BL/6 mice where immune serum transfer enhanced B. pertussis clearance from the lungs of mice in a neutrophil-dependent manner (15). Interestingly, ΔCYA bacterial numbers were equal to WT levels in neutropenic immune mice, suggesting that neutrophils are the main targets for ACT during B. pertussis infection in an immune individual. This is similar to findings with Bordetella bronchiseptica, for which the need for ACT for bacterial growth and disease in mice was obviated by neutropenia (12). In contrast, our data show that even in immune mice the absence of neutrophils does not eliminate the need for PT during infection. We showed that PT inhibits early KC gene expression and neutrophil recruitment to the airways in response to B. pertussis infection in immune mice, just as it does in naïve mice (2), but apparently this is not the only role for PT in promoting bacterial survival in the airways in an immune individual. This is consistent with other data from our lab showing that the ΔPT strain grows to WT levels in airway macrophage-depleted mice (7), suggesting that macrophages are the main target cells for PT during B. pertussis infection.
Interestingly, at the challenge dose (5 × 106 CFU) we detected no induction of KC gene expression after the initial early (3 and 6 h) induction postinoculation in immune mice. Also, no induction of Th1- or Th17-associated cytokine gene expression responses was observed in response to WT or ΔPT infection in immune mice (data not shown). This is in contrast to findings in naïve mice, in which evidence from our lab suggests that PT and ACT induce a proinflammatory Th1/Th17 response in the lungs at the peak of B. pertussis infection which is associated with a substantial neutrophil recruitment to the airways (C. Andreasen and N. H. Carbonetti, unpublished results). It remains possible that neutrophils recruited to the airways are associated with local pathology that leads to cough responses and transmission of the pathogen.
Overall, we conclude that neutrophils play only a minor role in response to B. pertussis infection in naïve mice, and PT and ACT may have overlapping inhibitory effects on neutrophil function in this model. However, in immune mice, neutrophils play an important role in controlling the infection, likely due to the presence of opsonizing antibodies that enhance phagocytosis. Furthermore, neutrophils were found to be the primary targets for ACT, but not for PT, during B. pertussis infection in immune mice. Whether PT, ACT, and neutrophils play the same roles in human infection is still unclear, since we know nothing about the early events after B. pertussis acquisition in humans. However, studies with human neutrophils in vitro have shown that B. pertussis possesses factors, including ACT, that inhibit its phagocytosis by neutrophils, primarily in the presence of opsonizing antibodies (24, 34, 35), and these data are consistent with the results from this study.
Acknowledgments
These studies were supported by Public Health Service grant R01 AI063080 from the National Institute of Allergy and Infectious Diseases.
We are grateful to Paul Mann, Eric Harvill, and Bill Scheuchenzuber (Penn State University) for providing RB6 antibody, to Marie Burdick and Robert Strieter (University of Virginia) for the CXCR2 antibody, to Stefanie Vogel for providing primers for gene expression experiments, and to Carbonetti lab members for many helpful discussions.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 22 December 2008.
REFERENCES
- 1.Addison, C. L., T. O. Daniel, M. D. Burdick, H. Liu, J. E. Ehlert, Y. Y. Xue, L. Buechi, A. Walz, A. Richmond, and R. M. Strieter. 2000. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J. Immunol. 1655269-5277. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen, C., and N. H. Carbonetti. 2008. Pertussis toxin inhibits early chemokine production to delay neutrophil recruitment in response to Bordetella pertussis respiratory tract infection in mice. Infect. Immun. 765139-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbic, J., M. F. Leef, D. L. Burns, and R. D. Shahin. 1997. Role of gamma interferon in natural clearance of Bordetella pertussis infection. Infect. Immun. 654904-4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbonetti, N. H., G. V. Artamonova, C. Andreasen, and N. Bushar. 2005. Pertussis toxin and adenylate cyclase toxin provide a one-two punch for establishment of Bordetella pertussis infection of the respiratory tract. Infect. Immun. 732698-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbonetti, N. H., G. V. Artamonova, C. Andreasen, E. Dudley, R. M. Mays, and Z. E. Worthington. 2004. Suppression of serum antibody responses by pertussis toxin after respiratory tract colonization by Bordetella pertussis and identification of an immunodominant lipoprotein. Infect. Immun. 723350-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbonetti, N. H., G. V. Artamonova, R. M. Mays, and Z. E. Worthington. 2003. Pertussis toxin plays an early role in respiratory tract colonization by Bordetella pertussis. Infect. Immun. 716358-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbonetti, N. H., G. V. Artamonova, N. Van Rooijen, and V. I. Ayala. 2007. Pertussis toxin targets airway macrophages to promote Bordetella pertussis infection of the respiratory tract. Infect. Immun. 751713-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collier, A. M., L. P. Peterson, and J. B. Baseman. 1977. Pathogenesis of infection with Bordetella pertussis in hamster tracheal organ culture. J. Infect. Dis. 136 (Suppl.)S196-S203. [DOI] [PubMed] [Google Scholar]
- 9.Collin, M., M. D. Svensson, A. G. Sjoholm, J. C. Jensenius, U. Sjobring, and A. Olsen. 2002. EndoS and SpeB from Streptococcus pyogenes inhibit immunoglobulin-mediated opsonophagocytosis. Infect. Immun. 706646-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Confer, D. L., and J. W. Eaton. 1982. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science 217948-950. [DOI] [PubMed] [Google Scholar]
- 11.Harvill, E. T., P. A. Cotter, and J. F. Miller. 1999. Pregenomic comparative analysis between Bordetella bronchiseptica RB50 and Bordetella pertussis Tohama I in murine models of respiratory tract infection. Infect. Immun. 676109-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvill, E. T., P. A. Cotter, M. H. Yuk, and J. F. Miller. 1999. Probing the function of Bordetella bronchiseptica adenylate cyclase toxin by manipulating host immunity. Infect. Immun. 671493-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Held, T. K., N. R. Jendrike, T. Rukavina, R. Podschun, and M. Trautmann. 2000. Binding to and opsonophagocytic activity of O-antigen-specific monoclonal antibodies against encapsulated and nonencapsulated Klebsiella pneumoniae serotype O1 strains. Infect. Immun. 682402-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herting, E., X. Gan, P. Rauprich, C. Jarstrand, and B. Robertson. 1999. Combined treatment with surfactant and specific immunoglobulin reduces bacterial proliferation in experimental neonatal group B streptococcal pneumonia. Am. J. Respir. Crit. Care Med. 1591862-1867. [DOI] [PubMed] [Google Scholar]
- 15.Hewlett, E. L. 1997. Pertussis: current concepts of pathogenesis and prevention. Pediatr. Infect. Dis. J. 16S78-S84. [DOI] [PubMed] [Google Scholar]
- 16.Janeway, C. A. 2001. Immunobiology: the immune system in health and disease, 5th ed. Garland Science, New York, NY.
- 17.Keane, M. P., J. A. Belperio, Y. Y. Xue, M. D. Burdick, and R. M. Strieter. 2004. Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. J. Immunol. 1722853-2860. [DOI] [PubMed] [Google Scholar]
- 18.Kirimanjeswara, G. S., L. M. Agosto, M. J. Kennett, O. N. Bjornstad, and E. T. Harvill. 2005. Pertussis toxin inhibits neutrophil recruitment to delay antibody-mediated clearance of Bordetella pertussis. J. Clin. Investig. 1153594-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirimanjeswara, G. S., P. B. Mann, and E. T. Harvill. 2003. Role of antibodies in immunity to Bordetella infections. Infect. Immun. 711719-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Londhe, V. A., J. A. Belperio, M. P. Keane, M. D. Burdick, Y. Y. Xue, and R. M. Strieter. 2005. CXCR2 is critical for dsRNA-induced lung injury: relevance to viral lung infection. J. Inflamm. (London) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lysenko, E. S., A. J. Ratner, A. L. Nelson, and J. N. Weiser. 2005. The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces. PLoS Pathog. 1e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marquis, M., D. Lewandowski, V. Dugas, F. Aumont, S. Senechal, P. Jolicoeur, Z. Hanna, and L. de Repentigny. 2006. CD8+ T cells but not polymorphonuclear leukocytes are required to limit chronic oral carriage of Candida albicans in transgenic mice expressing human immunodeficiency virus type 1. Infect. Immun. 742382-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuzaki, G., and M. Umemura. 2007. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol. Immunol. 511139-1147. [DOI] [PubMed] [Google Scholar]
- 24.Mobberley-Schuman, P. S., B. Connelly, and A. A. Weiss. 2003. Phagocytosis of Bordetella pertussis incubated with convalescent serum. J. Infect. Dis. 1871646-1653. [DOI] [PubMed] [Google Scholar]
- 25.Mobberley-Schuman, P. S., and A. A. Weiss. 2005. Influence of CR3 (CD11b/CD18) expression on phagocytosis of Bordetella pertussis by human neutrophils. Infect. Immun. 737317-7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore, T. A., M. W. Newstead, R. M. Strieter, B. Mehrad, B. L. Beaman, and T. J. Standiford. 2000. Bacterial clearance and survival are dependent on CXC chemokine receptor-2 ligands in a murine model of pulmonary Nocardia asteroides infection. J. Immunol. 164908-915. [DOI] [PubMed] [Google Scholar]
- 27.Opremcak, L. B., and M. S. Rheins. 1983. Scanning electron microscopy of mouse ciliated oviduct and tracheal epithelium infected in vitro with Bordetella pertussis. Can. J. Microbiol. 29415-420. [DOI] [PubMed] [Google Scholar]
- 28.Spangrude, G. J., F. Sacchi, H. R. Hill, D. E. Van Epps, and R. A. Daynes. 1985. Inhibition of lymphocyte and neutrophil chemotaxis by pertussis toxin. J. Immunol. 1354135-4143. [PubMed] [Google Scholar]
- 29.Tateda, K., T. A. Moore, M. W. Newstead, W. C. Tsai, X. Zeng, J. C. Deng, G. Chen, R. Reddy, K. Yamaguchi, and T. J. Standiford. 2001. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect. Immun. 692017-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomazzi, S. M., M. H. Souza, A. A. Melo-Filho, E. L. Hewlett, A. A. Lima, and R. A. Ribeiro. 1995. Pertussis toxin from Bordetella pertussis blocks neutrophil migration and neutrophil-dependent edema in response to inflammation. Braz. J. Med. Biol. Res. 28120-124. [PubMed] [Google Scholar]
- 31.Toews, G. B., W. C. Vial, and E. J. Hansen. 1985. Role of C5 and recruited neutrophils in early clearance of nontypable Haemophilus influenzae from murine lungs. Infect. Immun. 50207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trautmann, M., K. Triest, T. Hofstaetter, F. R. Seiler, and H. Hahn. 1985. Influence of different immunoglobulin G preparations on phagocytosis of Pseudomonas aeruginosa by polymorphonuclear granulocytes. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 259104-117. [DOI] [PubMed] [Google Scholar]
- 33.Tsai, W. C., R. M. Strieter, B. Mehrad, M. W. Newstead, X. Zeng, and T. J. Standiford. 2000. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect. Immun. 684289-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weingart, C. L., P. S. Mobberley-Schuman, E. L. Hewlett, M. C. Gray, and A. A. Weiss. 2000. Neutralizing antibodies to adenylate cyclase toxin promote phagocytosis of Bordetella pertussis by human neutrophils. Infect. Immun. 687152-7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weingart, C. L., and A. A. Weiss. 2000. Bordetella pertussis virulence factors affect phagocytosis by human neutrophils. Infect. Immun. 681735-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss, A. A., E. L. Hewlett, G. A. Myers, and S. Falkow. 1984. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J. Infect. Dis. 150219-222. [DOI] [PubMed] [Google Scholar]
- 37.Wendelboe, A. M., A. Van Rie, S. Salmaso, and J. A. Englund. 2005. Duration of immunity against pertussis after natural infection or vaccination. Pediatr. Infect. Dis. J. 24S58-S61. [DOI] [PubMed] [Google Scholar]
- 38.Wilson, R., R. Read, M. Thomas, A. Rutman, K. Harrison, V. Lund, B. Cookson, W. Goldman, H. Lambert, and P. Cole. 1991. Effects of Bordetella pertussis infection on human respiratory epithelium in vivo and in vitro. Infect. Immun. 59337-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye, P., F. H. Rodriguez, S. Kanaly, K. L. Stocking, J. Schurr, P. Schwarzenberger, P. Oliver, W. Huang, P. Zhang, J. Zhang, J. E. Shellito, G. J. Bagby, S. Nelson, K. Charrier, J. J. Peschon, and J. K. Kolls. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194519-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, X., and D. C. Morrison. 1993. Pertussis toxin-sensitive factor differentially regulates lipopolysaccharide-induced tumor necrosis factor-alpha and nitric oxide production in mouse peritoneal macrophages. J. Immunol. 1501011-1018. [PubMed] [Google Scholar]