Abstract
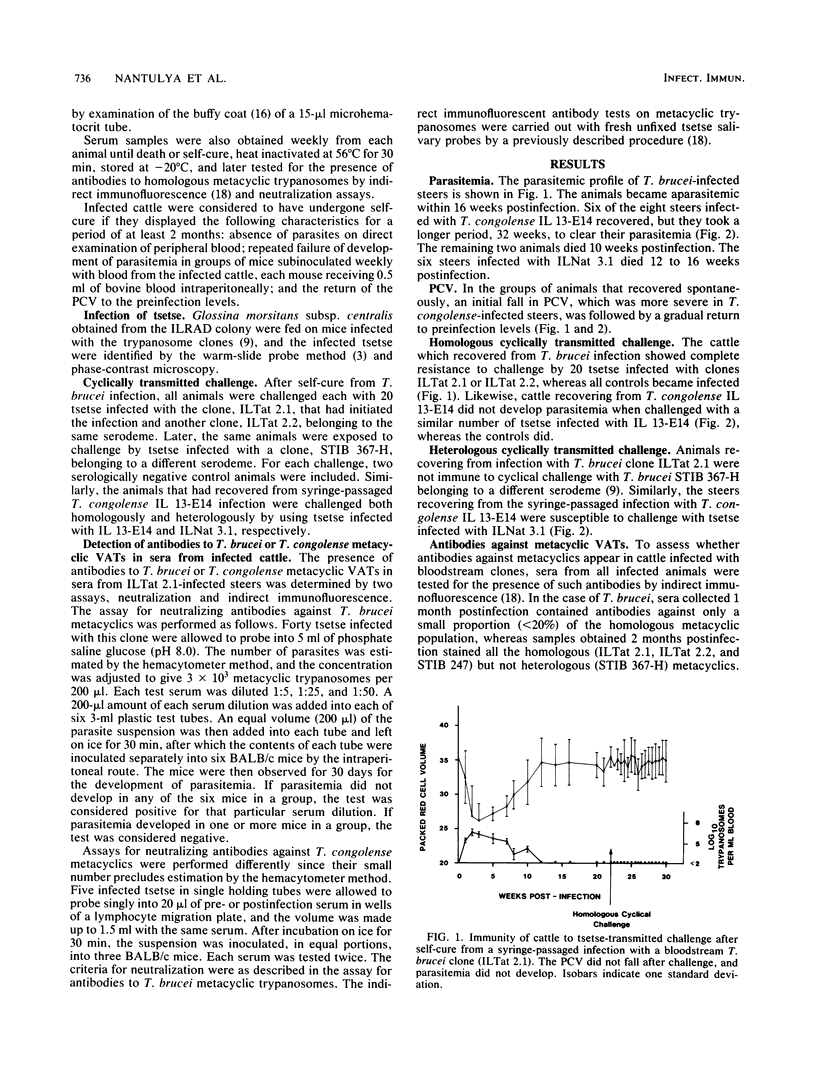
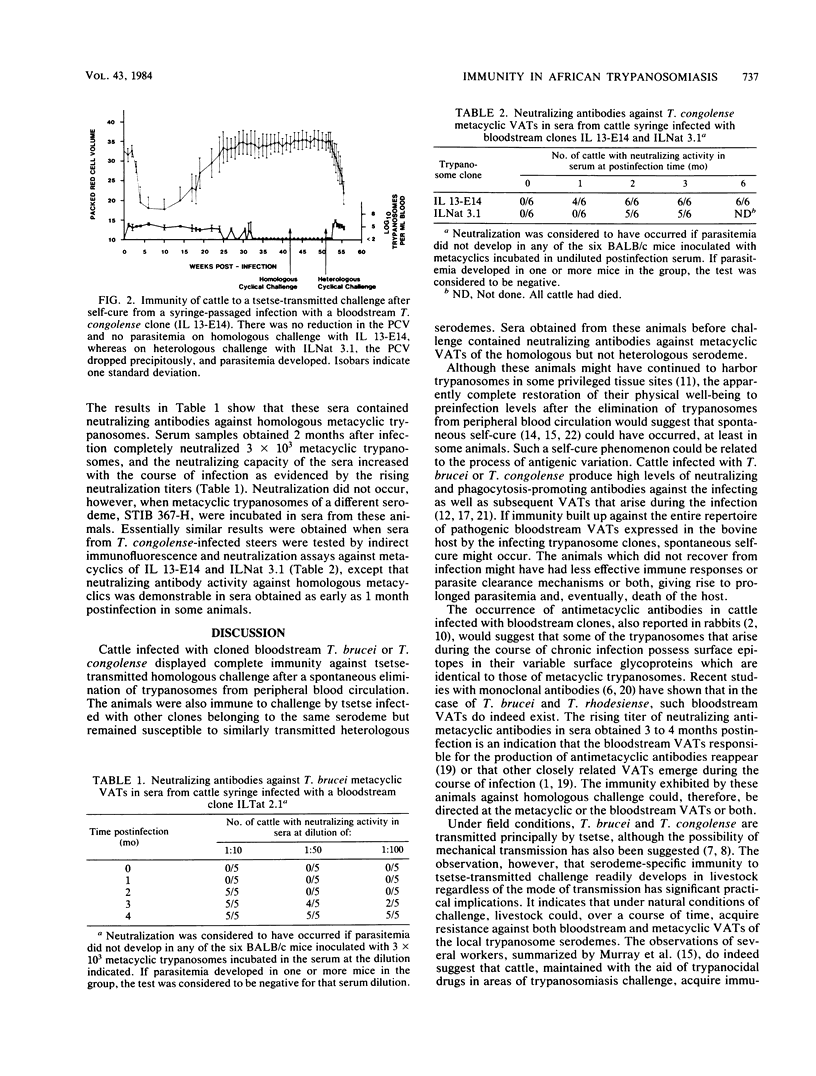
Groups of cattle were inoculated intravenously with cloned populations of bloodstream forms of Trypanosoma brucei or Trypanosoma congolense. All five steers infected with T. brucei ILTat 2.1 and six of the eight steers infected with T. congolense IL 13-E14 became aparasitemic within 16 and 32 weeks postinfection, respectively. Examination of sera from animals infected with T. brucei by indirect immunofluorescence and neutralization assays revealed the presence of antibodies against all the metacyclic variable antigen types (VATs) of the infecting clone. The neutralizing capacity of the sera increased with the course of infection from 1:10 at 2 months to 1:100 at 3 to 4 months postinfection. The recovered animals were completely immune to challenge by Glossina morsitans subsp. centralis infected with clone IL Tat 2.1, which had initiated the infection, as well as with another clone (IL Tat 2.2) belonging to the same serodeme, but they were susceptible to a tsetse-transmitted heterologous challenge with isolate STIB 367-H. Similar results were obtained with sera from T. congolense IL 13-E14-infected steers. The six steers infected with a different T. congolense ILNat 3.1 clone did not recover spontaneously; however, 2 months postinfection, sera from five of them also contained neutralizing antibodies against ILNat 3.1 metacyclic VATs. These results indicate that some of the bloodstream VATs that arise during the course of a chronic infection possess surface epitopes in their variable surface glycoproteins that are identical to those of the metacyclic VATs. It is suggested that in chronic infection, the infecting trypanosomes could exhaust their VAT repertoire, including those that cross-react with metacyclics, thereby leading to both "self-cure" and subsequent immunity to homologous cyclically transmitted challenge.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbet A. F., Davis W. C., McGuire T. C. Cross-neutralization of two different trypanosome populations derived from a single organism. Nature. 1982 Dec 2;300(5891):453–456. doi: 10.1038/300453a0. [DOI] [PubMed] [Google Scholar]
- Barry J. D., Hajduk S. L., Vickerman K., Le Ray D. Detection of multiple variable antigen types in metacyclic populations of Trypanosoma brucei. Trans R Soc Trop Med Hyg. 1979;73(2):205–208. doi: 10.1016/0035-9203(79)90213-x. [DOI] [PubMed] [Google Scholar]
- Capbern A., Giroud C., Baltz T., Mattern P. Trypanosoma equiperdum: etude des variations antigéniques au cours de la trypanosomose experimentale du lapin. Exp Parasitol. 1977 Jun;42(1):6–13. doi: 10.1016/0014-4894(77)90055-8. [DOI] [PubMed] [Google Scholar]
- Doyle J. J. Antigenic variation in the salivarian trypanosomes. Adv Exp Med Biol. 1977;93:31–63. doi: 10.1007/978-1-4615-8855-9_4. [DOI] [PubMed] [Google Scholar]
- Esser K. M., Schoenbechler M. J., Gingrich J. B. Trypanosoma rhodesiense blood forms express all antigen specificities relevant to protection against metacyclic (insect form) challenge. J Immunol. 1982 Oct;129(4):1715–1718. [PubMed] [Google Scholar]
- Geigy R., Kauffmann M. Sleeping sickness survey in the Serengeti area (Tanzania) 1971. I. Examination of large mammals for trypanosomes. Acta Trop. 1973;30(1):12–23. [PubMed] [Google Scholar]
- Jenni L. Comparisons of antigenic types of Trypanosoma (T)brucei strains transmitted by Glossina m. morsitans. Acta Trop. 1977 Mar;34(1):35–41. [PubMed] [Google Scholar]
- Le Ray D., Barry J. D., Vickerman K. Antigenic heterogeneity of metacyclic forms of Trypanosoma brucei. Nature. 1978 May 25;273(5660):300–302. doi: 10.1038/273300a0. [DOI] [PubMed] [Google Scholar]
- Masake R. A., Musoke A. J., Nantulya V. M. Specific antibody responses to the variable surface glycoproteins of Trypanosoma congolense in infected cattle. Parasite Immunol. 1983 Jul;5(4):345–355. doi: 10.1111/j.1365-3024.1983.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Morrison W. I., Roelants G. E., Mayor-Withey K. S., Murray M. Susceptibility of inbred strains of mice to Trypanosoma congolense: correlation with changes in spleen lymphocyte populations. Clin Exp Immunol. 1978 Apr;32(1):25–40. [PMC free article] [PubMed] [Google Scholar]
- Murray M., Morrison W. I., Whitelaw D. D. Host susceptibility to African trypanosomiasis: trypanotolerance. Adv Parasitol. 1982;21:1–68. doi: 10.1016/s0065-308x(08)60274-2. [DOI] [PubMed] [Google Scholar]
- Musoke A. J., Nantulya V. M., Barbet A. F., Kironde F., McGuire T. C. Bovine immune response to African ;trypanosomes: specific antibodies to variable surface glycoproteins of Trypanosoma brucei. Parasite Immunol. 1981 Summer;3(2):97–106. doi: 10.1111/j.1365-3024.1981.tb00388.x. [DOI] [PubMed] [Google Scholar]
- Nantulya V. M., Doyle J. J., Jenni L. Studies on Trypanosoma (nannomonas) congolense III. Antigenic variation in three cyclically transmitted stocks. Parasitology. 1980 Feb;80(1):123–131. doi: 10.1017/s0031182000000573. [DOI] [PubMed] [Google Scholar]
- Nantulya V. M., Musoke A. J., Barbet A. F., Roelants G. E. Evidence for reappearance of Trypanosoma brucei variable antigen types in relapse populations. J Parasitol. 1979 Oct;65(5):673–679. [PubMed] [Google Scholar]
- Nantulya V. M., Musoke A. J., Moloo S. K., Ngaira J. M. Analysis of the variable antigen composition of Trypanosoma brucei brucei metacyclic trypanosomes using monoclonal antibodies. Acta Trop. 1983 Mar;40(1):19–24. [PubMed] [Google Scholar]
- Ngaira J. M., Nantulya V. M., Musoke A. J., Hirumi K. Phagocytosis of antibody-sensitized Trypanosoma brucei in vitro by bovine peripheral blood monocytes. Immunology. 1983 Jun;49(2):393–400. [PMC free article] [PubMed] [Google Scholar]



