Abstract
Acinetobacter baumannii is an emerging bacterial pathogen that rapidly develops multiple-drug resistance and is responsible for many nosocomial pulmonary infections. This study investigated the role of the NADPH phagocyte oxidase (phox) and inducible nitric oxide synthase (NOS2) in the host defense against respiratory infection with A. baumannii in mouse models of intranasal A. baumannii infection. gp91phox−/− mice showed higher susceptibility to A. baumannii infection than wild-type (WT) C57BL/6 mice, with significantly greater bacterial counts in their lungs (1,000-fold) (P < 0.005) and spleens (10-fold) (P < 0.05). Moreover, all of the gp91phox−/− mice succumbed to infection within 48 h. In contrast, only a moderate increase in bacterial burdens was detected in the lungs of NOS2−/− mice, and all NOS2−/− mice survived infection. Compared to WT mice, the pulmonary influx of inflammatory cells and serum and local inflammatory cytokine/chemokine responses were not obviously impaired at 4 h and were significantly higher at 24 h (P < 0.05) in gp91phox−/− mice, but NADPH-deficient neutrophils were unable to control bacterial replication and extrapulmonary dissemination. Thus, NADPH phagocyte oxidase appears to play a crucial role in the neutrophil-mediated host defense against A. baumannii.
Acinetobacter baumannii infection has recently emerged as a major cause of both community-associated and nosocomial infections worldwide (6, 7). The overall 30-day mortality of Acinetobacter infection can be as high as 49%, with the respiratory tract being an important portal of entry (12). Moreover, A. baumannii infections are becoming increasingly difficult to treat because of its rapid development of resistance to multiple antibiotics (4). Despite its clinical importance, little is known regarding the host defense mechanisms against respiratory A. baumannii infection.
We and others have recently shown that C57BL/6 mice can effectively control and eliminate a sublethal A. baumannii infection within 72 h following intranasal inoculation, which requires an influx of neutrophils into the lung but does not require gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) (8, 10, 19, 22). The depletion of neutrophils with monoclonal antibody (22) or cyclophosphamide (8) significantly enhanced bacterial multiplication and converted an otherwise self-limiting infection into a fatal infection, suggesting that neutrophils are crucial in the control of local bacterial multiplication and subsequent extrapulmonary dissemination. However, the mechanisms by which neutrophils inhibit or kill A. baumannii remain unknown.
Neutrophils can contribute to host innate defense mechanisms through the production of reactive oxygen species (ROS) (11, 16, 17). Activated neutrophils produce ROS primarily through NADPH phagocyte oxidase catalyzation, which leads to the production of superoxide, which is the precursor to more toxic ROS including H2O2 and OH (14, 23). Also, stimulated neutrophils and macrophages release myeloperoxidase from cytoplasmic granules to interact with H2O2 to form hypohalous acids, which are very potent oxidants that can disrupt bacterial membrane integrity, normal metabolism, and replication (15). Neutrophils can also contribute to host innate defense mechanisms through the production of reactive nitrogen species (RNS) (11, 16, 17). Inducible nitric oxide synthase, also called NOS2, is an oxidant synthase found in the immune system that catalyzes the formation of NO and the subsequent auto-oxidation of a variety of RNS such as NO2 and N2O3, which have substantially more antimicrobial activity (14, 23). In addition, NOS2 and NADPH oxidase can act synergistically to form highly potent radicals, including ONOO−, which can damage lipids, proteins, and DNA (18).
In this study, we examined the roles of NADPH phagocyte oxidase and NOS2 in limiting bacterial replication during respiratory A. baumannii infection by using gp91phox−/− and NOS2−/− mice. We found that NADPH-dependent antimicrobial action is essential for an effective host defense against acute respiratory A. baumannii infection.
MATERIALS AND METHODS
Mice.
Eight- to twelve-week-old specific-pathogen-free female C57BL/6 mice were purchased from Charles River Laboratories. B6.129S-Cybbtm1 Din/J (NADPH oxidase-deficient [gp91phox−/−]) and B6.129P2-Nos2tm1 Lau/J (inducible nitric oxide synthase-deficient [NOS2−/−]) mice were purchased from Jackson Laboratories. The animals were maintained and used in accordance with the recommendations of the Canadian Council on Animal Care Guide to the Care and Use of Experimental Animals (http://www.ccac.ca), and experimental procedures were approved by the institutional animal care committee.
A. baumannii and experimental protocols.
Fresh inocula were prepared for each experiment from a frozen stock of A. baumannii (ATCC 17961; American Type Culture Collection, Manassas, VA) as previously described (22). Anesthetized mice were inoculated intranasally (i.n.) with ∼107 A. baumannii cells in 50 μl saline. This dosage induces a self-limiting bronchopneumonia without mortality in C57BL/6 mice (22). Actual inoculum concentrations in each experiment were determined by plating 10-fold serial dilutions onto brain heart infusion agar supplemented with 50 μg/ml streptomycin. Mouse clinical signs and survival were monitored and scored as described previously (22). Groups of five gp91phox−/−, NOS2−/−, or C57BL/6 mice were sacrificed 0, 4, 24, or 72 h postinoculation (p.i.). The lungs and spleens were aseptically removed and used for quantitative bacteriology or histopathology. In some experiments, blood samples were collected for serum separation, and lungs were lavaged.
Quantitative bacteriology and histopathology.
The lungs and spleen were homogenized in sterile saline using aerosol-proof homogenizers. Aliquots (100 μl) of 10-fold serial dilutions of the homogenates were cultured on plates of brain heart infusion agar supplemented with 50 μg/ml streptomycin to quantify the number of viable A. baumannii cells in the respective organs (22). For histopathology, lungs and spleens were fixed immediately in 10% neutral buffered formalin and processed by standard methods for paraffin embedding (22). Sections were cut 4 μm thick, stained with hematoxylin-eosin, and examined by light microscopy.
BAL.
The lungs were lavaged five times with 1.0 ml phosphate-buffered saline supplemented with 3 mM EDTA as previously described (3). The total number of bronchoalveolar lavage (BAL) cells was determined with a hemacytometer, and differential cell counts were determined by examining 200 cells on cytospin slides (Cytospin 3; Shandon, Pittsburgh, PA) stained with Hema-3 (Fisher Scientific, Kalamazoo, MI). The lavage fluid was centrifuged at 2,450 × g for 7 min, and the supernatant was collected, filter sterilized, and stored at −80°C.
Serum and BAL cytokine and chemokine assays.
Levels of cytokines and chemokines in the sera and BAL fluid were determined using the mouse panel of Fluorokine MAP Multiplex kits (R&D Systems, Inc., Minneapolis, MN) on a Luminex (Austin, TX) 100IS system as specified by the manufacturer. The analysis was done in duplicate, and cytokine and/or chemokine concentrations were calculated against standards using Beadview software (version 1.03; Upstate) (22).
Statistical analysis.
Data are presented as means ± standard deviations (SD) for each group unless otherwise specified. Differences in quantitative measurements were assessed by a Student's t test or two-way analysis of variance followed by Bonferroni's post hoc multiple-comparison tests, when appropriate. Differences were considered to be significant when the P value was <0.05.
RESULTS
gp91phox−/− mice, but not NOS2−/− mice, are highly susceptible to intranasal A. baumannii inoculation.
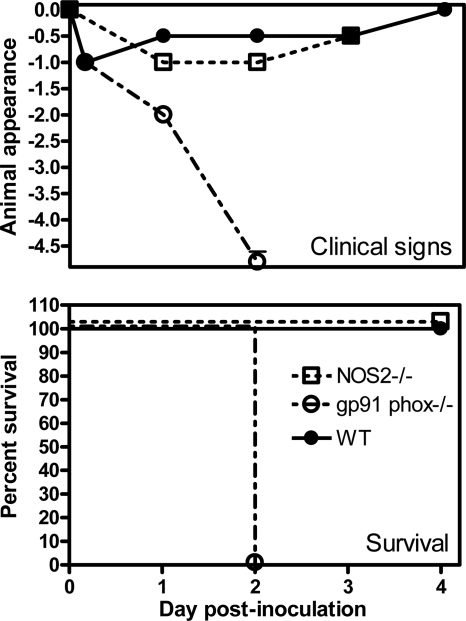
As the first step to examine the relative importance of NADPH phagocyte oxidase and inducible nitric oxide synthase in the host defense against respiratory infection with A. baumannii, gp91phox−/−, NOS2−/−, and C57BL/6 mice were inoculated i.n. with ∼107 CFU of A. baumannii. Their clinical signs, survival rates, and tissue bacterial burdens were determined. As expected (22), no overt clinical signs were observed in wild-type (WT) C57BL/6 mice at this modest challenge dose (Fig. 1), whereas gp91phox−/− mice showed moderate clinical signs at 24 h, and all mice died of infection by 48 h. In contrast, the clinical sign scores of NOS2−/− mice were similar to those of WT mice, and all NOS2−/− mice survived the infection (Fig. 1).
FIG. 1.
Clinical scores (top) and rates of survival (bottom) of gp91phox−/−, NOS2−/−, and C57BL/6 mice after i.n. inoculation with A. baumannii. Groups of 5 to 10 gp91phox−/− (open circles), NOS2−/− (open squares), or C57BL/6 (closed circles) mice were i.n. inoculated with ∼107 CFU of A. baumannii on day 0, and their clinical scores and rates of survival were monitored for 4 days. Clinical signs of the mice were scored as follows: 0, no abnormal clinical signs; −1, ruffled fur but lively; −2, ruffled fur, activity level slowing, sick; −3, ruffled fur, eyes squeezed shut, hunched, hardly moving, very sick; −4, moribund; −5, dead. The data are compiled from data for two to three independent experiments with similar results.
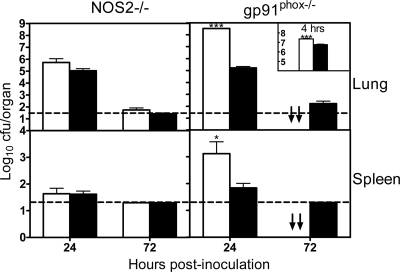
Quantitative bacteriology was performed for the lungs and spleens of NOS2−/−, gp91phox−/−, and WT mice. There were no statistically significant differences in bacterial burdens in either the lungs or spleens of NOS2−/− and WT mice at either 24 or 72 h (Fig. 2). In contrast, gp91phox−/− mice had significantly higher bacterial burdens in both the lungs (∼1,000-fold; P < 0.005) and spleens (∼10-fold; P < 0.05) than WT mice at 24 h (P < 0.001), and the differences in lung bacterial burdens were significant even at 4 h (P < 0.005) (Fig. 2, inset). These data suggest that NADPH phagocyte oxidase-dependent antimicrobial mechanisms play a crucial role in the host defense against respiratory A. baumannii infection in mice.
FIG. 2.
Bacterial burdens in the lung and spleen of gp91phox−/−, NOS2−/−, and WT mice following i.n. inoculation with A. baumannii cells. NOS2−/− (open bars) and WT C57BL/6 (solid bars) mice (left) or gp91phox−/− (open bars) and C57BL/6 control (solid bars) mice (right) were infected intranasally with ∼107 CFU of A. baumannii at 0 h. Bacterial burdens in the lung and spleen were determined by quantitative bacteriology at 4 h (inset), 24 h, and 72 h after inoculation. All A. baumannii-infected gp91phox−/− mice were dead by 72 h. The data are presented as means ± SD (n = 5 to 10) and represent one of at least two experiments with similar results. The detection limit (dotted lines) for bacterial burdens was 1.3 log10 CFU/organ. ↓↓, all mice were dead by this time point. *, P < 0.05 versus C57BL/6 mice; ***, P < 0.005 versus C57BL/6 mice.
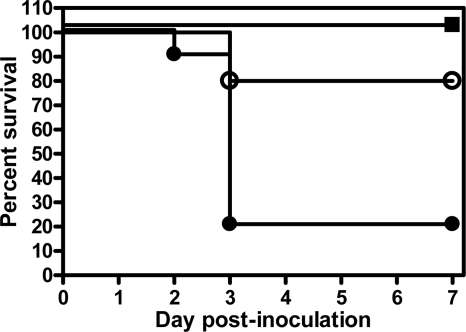
Given that gp91phox−/− mice succumbed to respiratory infection with a relatively modest challenge dose, we decided to quantify the magnitude of the difference in survival rates between gp91phox−/− and WT mice by administering higher doses of A. baumannii to WT mice. While gp91phox−/− mice all succumbed to infection after i.n. inoculation with ∼107 CFU of A. baumannii (Fig. 1), all WT mice survived i.n. inoculation with ∼108 CFU, and 8 of 10 WT mice survived i.n. inoculation with ∼5 × 108 CFU (Fig. 3). Once the dose reached ∼109 CFU, only 2 of 10 WT mice survived the infection (Fig. 3). Thus, gp91phox−/− mice are at least 100 times more susceptible to respiratory A. baumannii infection than WT mice. These results underline the critical role of NADPH oxidase in the host defense against respiratory A. baumannii infection in mice.
FIG. 3.
Survival of C57BL/6 mice after i.n. inoculation with A. baumannii cells. Groups of 10 C57BL/6 mice were i.n. inoculated with ∼108 CFU (closed squares), 5 × 108 CFU (open circles), or 109 CFU (closed circles) of A. baumannii on day 0, and their rate of survival was monitored for 7 days.
Inflammatory cell responses to i.n. A. baumannii inoculation in the lungs of gp91phox−/− and WT mice.
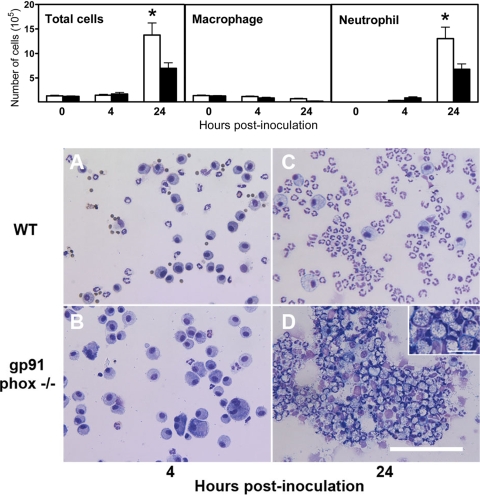
To further elucidate the role of NADPH phagocyte oxidase in the host defense against i.n. A. baumannii infection, we next determined the kinetics of the pulmonary recruitment of inflammatory cells in response to infection. The total numbers of BAL cells in gp91phox−/− and C57BL/6 mice were comparable at 0 h, with 99% being alveolar macrophages (Fig. 4, top). Compared to WT mice, the number of total and differential BAL cells was not otherwise significantly affected in gp91phox−/− mice at 4 h p.i., although the percentage of BAL neutrophils in gp91phox−/− mice was slightly, but not significantly, lower than that in WT mice (21.8% ± 6.4% in gp91phox−/− mice versus 49.2% ± 13.6% in WT mice) (Fig. 4, top). In addition, many BAL macrophages in gp91phox−/− mice were unusually large, with a dense, basophilic cytoplasm at this time point (Fig. 4B). At 24 h, gp91phox−/− mice had significantly more total BAL cells and neutrophils (both P < 0.05) than WT mice (Fig. 4, top). Moreover, the neutrophils in gp91phox−/− mice (Fig. 4D) were usually aggregated, with a large, foamy cytoplasm in which large numbers of bacterial rods were apparent (Fig. 4D, inset).
FIG. 4.
(Top) Cellular composition in BAL fluid from gp91phox−/− and C57BL/6 mice following i.n. inoculation with A. baumannii cells. Groups of five gp91phox−/− (open bars) or WT C57BL/6 (solid bars) mice were i.n. inoculated with ∼107 CFU of A. baumannii. At the indicated times, mice were exsanguinated, their lungs were lavaged, and total and differential cell counts were determined. *, P < 0.05 versus WT mice. (Bottom) Photomicrographs of BAL cells from WT (top) and gp91phox−/− (bottom) mice killed at the indicated times following i.n. inoculation with ∼107 CFU A. baumannii (Hema-3 staining). (A) BAL cells from a WT mouse killed at 4 h p.i. showing the presence of alveolar macrophages admixed with moderate numbers of neutrophils. (B) Composition of BAL cells from a gp91phox−/− mouse killed at 4 h illustrating largely alveolar macrophages with small numbers of neutrophils. Note that some macrophages are very large with a dense and basophilic cytoplasm. (C) BAL cells from a WT mouse killed at 24 h showing predominantly neutrophils of normal appearance with very few alveolar macrophages. (D) BAL cells from a gp91phox−/− mouse killed at 24 h showing the presence of large numbers of aggregated neutrophils with foamy cytoplasm and many bacterium-like materials (see inset). Bar, 100 μm (20 μm for the inset).
Histopathology of i.n. A. baumannii infection in gp91phox−/− and WT mice.
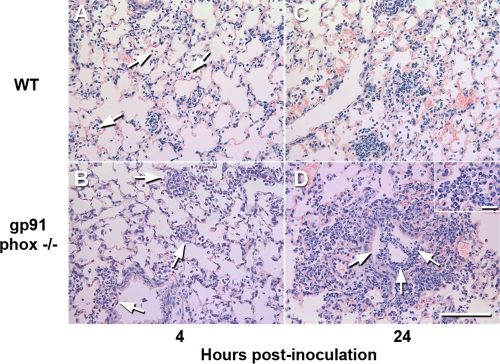
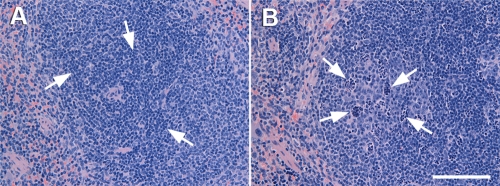
Histopathological examination of the lungs and spleens collected from gp91phox−/− or WT mice at various time points after i.n. challenge showed no remarkable changes in the lung or spleen at 0 h (data not shown). At 4 h, both WT and gp91phox−/− mice showed mild bronchopneumonia consisting of few neutrophil infiltrates in the alveolar spaces and the perivascular and peribronchial areas (Fig. 5A and B) and dilatation of perivascular and peribronchial spaces. However, pneumonia was slightly more severe and widespread in gp91phox−/− mice than in WT mice. By 24 h, gp91phox−/− mice (Fig. 5D) showed much more severe and extended lung lesions than WT mice (Fig. 5C), with moderately severe infiltration of mixed neutrophils and mononuclear cells in the airway and alveolar lumens. Many neutrophils in the alveoli contained extended foamy cytoplasm under high magnification (Fig. 5D, inset). In addition, at 24 h of infection, the spleens from gp91phox−/− mice showed the presence of moderate numbers of clusters of degenerating cells and cellular debris with a “starry” appearance in the lymphoid follicles (Fig. 6) and the infiltration of small numbers of neutrophils in the red pulps and interfollicular areas of the spleen. However, there was no obvious lymphocyte destruction.
FIG. 5.
Histopathological findings for lungs from WT and gp91phox−/− mice killed at 4 h (A and B) and 24 h (C and D) after i.n. inoculation with 107 CFU of A. baumannii. (A and B) The lung from a WT mouse killed at 4 h showed mild neutrophil infiltration in the alveolar spaces (arrows) (A), whereas the pneumonic lesions in a gp91phox−/− mouse killed at the same time were slightly more severe and involved larger areas (arrows) (B). (C) Lung from a WT mouse killed at 24 h showing moderate bronchopneumonia with the presence of large numbers of neutrophils in some alveolar spaces. (D) Lung from a gp91phox−/− mouse killed at 24 h showing severe bronchopneumonia with the presence of large numbers of neutrophils in the alveolar spaces and bronchial lumina (arrows). (Inset) Higher magnification showing the presence of large numbers of neutrophils with a foamy cytoplasm. Shown is staining with hematoxylin and eosin. Bar, 100 μm (20 μm for the inset).
FIG. 6.
Histopathological findings for spleens from WT and gp91phox−/− mice killed at 24 h after i.n. inoculation with ∼107 CFU A. baumannii. (A) Spleen from a WT mouse showing mild infiltration of small numbers of neutrophils in the red pulp and interfollicular areas (arrows) and very occasionally in the lymphoid follicles (arrows). (B) Spleen from a gp91phox−/− mouse showing the presence of moderate numbers of clusters of degenerating cells and cellular debris with a “starry” appearance in the lymphoid follicles (arrows). Shown is staining with hematoxylin and eosin. Bar, 100 μm.
Systemic and pulmonary cytokine and chemokine levels in A. baumannii-infected gp91phox−/− and WT mice.
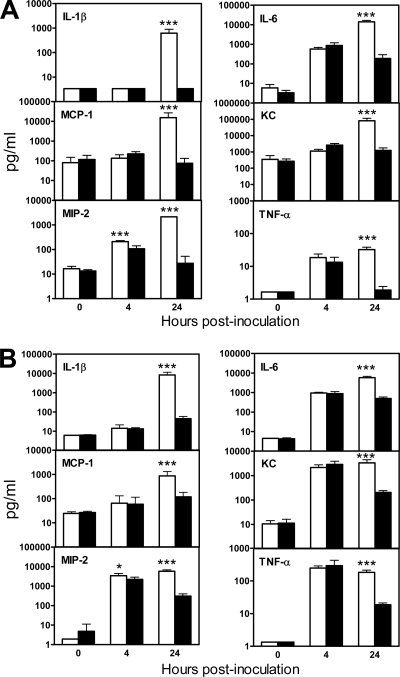
Serum and BAL levels of a panel of six proinflammatory cytokines and chemokines that were previously implicated in the pathogenesis of respiratory A. baumannii infection were determined at 0, 4, and 24 h after inoculation (10, 19, 22). There was no significant difference in the serum levels of any cytokines and/or chemokines between gp91phox−/− and WT mice at 4 h except for macrophage inflammatory protein 2 (MIP-2), the level of which was significantly higher in gp91phox−/− mice (P < 0.005) (Fig. 7A). However, the levels all the cytokines and/or chemokines examined (interleukin-1β [IL-1β], IL-6, monocyte chemoattractant protein 1 [MCP-1], keratinocyte-derived chemokine [KC], MIP-2, and TNF-α) were significantly higher in gp91phox−/− mice than in WT mice at 24 h (P < 0.005) (Fig. 7A), which corresponded to the extrapulmonary bacterial dissemination. In contrast, the BAL cytokine and/or chemokine levels in both gp91phox−/− and WT mice increased moderately (IL-1β and MCP-1) to substantially (IL-6, KC, MIP-2, and TNF-α) following i.n. A. baumannii challenge, with no statistically significant differences between gp91phox−/− and WT mice at 4 h (P > 0.05) (Fig. 7B), with the exception of MIP-2, the level of which was significantly higher in gp91phox−/− mice (P < 0.05). At 24 h, the levels of all cytokines and/or chemokines assayed in the BAL fluid of gp91phox−/− mice were significantly higher than those in WT mice (P < 0.005) (Fig. 7B).
FIG. 7.
Cytokine and chemokine levels in serum (A) and BAL fluid (B) of gp91phox−/− and C57BL/6 mice following i.n. inoculation of A. baumannii cells. Groups of gp91phox−/− or C57BL/6 mice were i.n. inoculated with ∼107 CFU of A. baumannii. Serum and BAL samples were collected at 0, 4, and 24 h, and cytokine and chemokine levels were determined using the mouse panel of Fluorokine MAP Multiplex kits (R&D Systems, Inc. Minneapolis, MN) on a Luminex 100 IS instrument. Data are expressed as means ± SD of data from five mice at each time point. The detection limits of the assays were 2.5 to 15 pg/ml. *, P < 0.05 versus C57BL/6 mice; ***, P < 0.005 versus C57BL/6 mice.
DISCUSSION
Interactions between A. baumannii and the host innate immune system likely govern the extent of bacterial replication and local inflammatory responses following i.n. challenge with the pathogen. We and others have recently demonstrated that neutrophils are normally recruited rapidly to the lungs after i.n. A. baumannii challenge in C57BL/6 mice (8, 10, 19, 22) and that C57BL/6 mice were capable of clearing A. baumannii cells within 72 h after i.n. inoculation with a sublethal dose (107 CFU) of the pathogen, whereas the same dosage was lethal to neutropenic mice, which failed to control bacterial replication at the primary site of implantation and the subsequent, although mild, extrapulmonary dissemination of the pathogen to the spleen (8, 10, 19, 22). This suggests that neutrophils play a crucial role in the effective control of A. baumannii infection and the prevention of the development of severe disease. Neutrophils contribute to host innate defense mechanisms through both oxidative (the production of superoxide, primarily by the multisubunit enzyme NADPH oxidase) and nonoxidative mechanisms (11, 16, 17). In this study, we examined the relative contribution of ROS and RNS in murine resistance to a sublethal A. baumannii infection. Our results show that gp91phox−/−, but not NOS2−/−, mice are highly susceptible to intranasal A. baumannii infection in that enhanced bacterial growth was observed in gp91phox−/− mice as early as 4 h after challenge, and all mice died of infection within 48 h. In contrast, NOS2−/− mice controlled bacterial growth in the tissues well, with only a slight increase in lung bacterial burdens but with no extrapulmonary dissemination or death. Effective control of A. baumannii replication and dissemination is important for the clinical outcome of Acinetobacter-associated pneumonia. Our study indicates that the early killing of A. baumannii by resident phagocytes and acute inflammatory cells appears to be heavily dependent on the presence of an intact NADPH phagocyte oxidase, suggesting a crucial role for the oxidative burst in the rapid killing of ingested A. baumannii by phagocytes.
It is probably not entirely surprising that NOS2 does not play a crucial role in the host defense against acute respiratory infection with A. baumannii. In this regard, the role of macrophages in host defense in this model remains unknown, but circumstantial evidence suggests that macrophages play only a minor, if any, role, as there is very little change in the macrophage numbers in the lungs following bacterial inoculation (Fig. 4) (10, 19, 22), and A. baumannii cells are rarely seen within macrophages compared to neutrophils (our unpublished observations). Moreover, it is well established that IFN-γ and TNF-α upregulate NOS2 production and enhance host defense against microbes via NO-dependent mechanisms. However, we previously showed that mice treated with anti-IFN-γ or anti-TNF-α neutralizing antibodies are no more susceptible to A. baumannii infection than control immunoglobulin G-treated mice (22). Furthermore, RNS can exercise synergistic antimicrobial interactions with ROS (11, 16, 17). Although we have not directly examined this possibility in this study, such interactions are unlikely to be crucial for the early killing of A. baumannii because gp91phox−/− mice succumb very rapidly to infection (within 48 h), and NOS2 plays a limited role in the control of A. baumannii replication (Fig. 1 and 2).
It is interesting that the susceptibility and bacterial burdens in gp91phox−/− mice were substantially higher than those in neutropenic mice (22). This differential susceptibility may reflect that (i) NADPH from other cell sources (such as macrophages) plays a partial or compensatory role in the control of infection in neutropenic mice; (ii) neutrophils may not be completely depleted by monoclonal antibodies, and the remaining neutrophils, although few, are able to kill A. baumannii via NADPH mechanisms; and (iii) NADPH oxidase has been shown to exhibit immune regulatory functions and accentuate the inflammatory response (1, 16, 20), which might also contribute to its role in resistance against A. baumannii in vivo.
Similar to a number of other bacterial and viral infections (1, 2, 5, 14, 20, 21), the local inflammatory response to A. baumannii, as assessed by the pulmonary recruitment of inflammatory cells and BAL cytokine and/or chemokine levels, was not significantly impaired in gp91phox−/− mice at an early stage of infection (4 h) and was even enhanced at 24 h (Fig. 4 and 7). The enhanced inflammatory response observed in gp91phox−/− mice at 24 h is probably due to increased bacterial burdens, which were previously suggested to be a critical factor in activating the release of chemokines and thereby augmenting neutrophil and macrophage recruitment into the lung tissues (5, 14). Indeed, BAL levels of several cytokines and chemokines, including the neutrophil-inducing chemokines MIP-2 and KC and the macrophage-inducing and -activating chemokine MCP-1, were significantly increased in gp91phox−/− mice at 24 h p.i. compared to levels in infected WT mice (Fig. 7B). Furthermore, the elevation of serum levels of selected cytokines and/or chemokines following i.n. A. baumannii infection was closely associated with extrapulmonary bacterial dissemination, which occurred mainly in gp91phox−/− mice (Fig. 2). Thus, it appears that the gp91phox−/− deficiency does not directly impair circulating or local levels of these cytokine and chemokine responses during A. baumannii infection.
The innate immune system can completely control an infection by mobilizing phagocytes (neutrophils and macrophages) to sites of infection, where they ingest and destroy the pathogens. Neutrophils and other phagocytes such as monocytes and macrophages are considered to be the first line of host defense against pathogens since these cells phagocytose and kill invading bacteria via the elaboration of toxic ROS and RNS products. Indeed, a substantial portion of the bactericidal activity of neutrophils is mediated by NADPH oxidase (17). However, despite the increased infiltration of neutrophils and the important bactericidal activity of neutrophils, gp91phox−/− mice failed to control bacterial growth and rapidly succumbed to the infection in this study. This suggests that NADPH-deficient neutrophils were incapable of killing A. baumannii cells. Indeed, cytological examination of BAL cytospins showed the presence of a substantial proportion of large alveolar macrophages with a dense, basophilic cytoplasm at 4 h p.i. and the presence of numerous bacterial rods in the cytoplasm of infiltrating neutrophils in both BAL cytospins and lung sections at 24 h p.i., indicating that neutrophils from gp91phox−/− mice were capable of phagocytizing A. baumannii cells but were incapable of suppressing and killing the bacteria.
In conclusion, our study has convincingly demonstrated the crucial role of NADPH phagocyte oxidase in the host defense against acute respiratory A. baumannii infection and the products of the NADPH phagocyte oxidase as the potential effector molecules for the neutrophil-mediated killing of A. baumannii. If the results from this study can be extrapolated to human situations, special attention should be exercised in the clinical management of A. baumannii-associated pneumonia in patients with chronic granulomatous disease, who are deficient in NADPH phagocyte oxidase and are susceptible to recurrent microbial infections including some gram-negative bacterial pneumonias (13). Currently, it is unknown if patients with chronic granulomatous disease are more susceptible to A. baumannii infection, but it was previously reported that Acinetobacter is frequently isolated from neutropenic, febrile patients in nosocomial settings (9). In addition, future studies to examine the role of NADPH phagocyte oxidase in the host defense against different clinical isolates of A. baumannii may generate further insights into the immunopathogenesis of clinical A. baumannii infections.
Acknowledgments
We thank Tom Devecseri for assistance in the preparation of photomicrography.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 22 December 2008.
REFERENCES
- 1.Blanchard, T. G., F. Yu, C. L. Hsieh, and R. W. Redline. 2003. Severe inflammation and reduced bacteria load in murine Helicobacter infection caused by lack of phagocyte oxidase activity. J. Infect. Dis. 1871609-1615. [DOI] [PubMed] [Google Scholar]
- 2.Breitbach, K., S. Klocke, T. Tschernig, N. van Rooijen, U. Baumann, and I. Steinmetz. 2006. Role of inducible nitric oxide synthase and NADPH oxidase in early control of Burkholderia pseudomallei infection in mice. Infect. Immun. 746300-6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, W., E. A. Havell, L. L. Moldawer, K. W. McIntyre, R. A. Chizzonite, and A. G. Harmsen. 1992. Interleukin 1: an important mediator of host resistance against Pneumocystis carinii. J. Exp. Med. 176713-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fournier, P. E., and H. Richet. 2006. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin. Infect. Dis. 42692-699. [DOI] [PubMed] [Google Scholar]
- 5.Gao, X. P., T. J. Standiford, A. Rahman, M. Newstead, S. M. Holland, M. C. Dinauer, Q. H. Liu, and A. B. Malik. 2002. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by gram-negative sepsis: studies in p47phox−/− and gp91phox−/− mice. J. Immunol. 1683974-3982. [DOI] [PubMed] [Google Scholar]
- 6.Gaynes, R., and J. R. Edwards. 2005. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41848-854. [DOI] [PubMed] [Google Scholar]
- 7.Joly-Guillou, M. L. 2005. Clinical impact and pathogenicity of Acinetobacter. Clin. Microbiol. Infect. 11868-873. [DOI] [PubMed] [Google Scholar]
- 8.Joly-Guillou, M. L., M. Wolff, J. J. Pocidalo, F. Walker, and C. Carbon. 1997. Use of a new mouse model of Acinetobacter baumannii pneumonia to evaluate the postantibiotic effect of imipenem. Antimicrob. Agents Chemother. 41345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karim, M., W. Khan, B. Farooqi, and I. Malik. 1991. Bacterial isolates in neutropenic febrile patients. J. Pak. Med. Assoc. 4135-37. [PubMed] [Google Scholar]
- 10.Knapp, S., C. W. Wieland, S. Florquin, R. Pantophlet, L. Dijkshoorn, N. Tshimbalanga, S. Akira, and T. van der Poll. 2006. Differential roles of CD14 and Toll-like receptors 4 and 2 in murine Acinetobacter pneumonia. Am. J. Respir. Crit. Care Med. 173122-129. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi, S. D., J. M. Voyich, C. Burlak, and F. R. DeLeo. 2005. Neutrophils in the innate immune response. Arch. Immunol. Ther. Exp. (Warsaw) 53505-517. [PubMed] [Google Scholar]
- 12.Kuo, L. C., C. C. Lai, C. H. Liao, C. K. Hsu, Y. L. Chang, C. Y. Chang, and P. R. Hsueh. 2007. Multidrug-resistant Acinetobacter baumannii bacteraemia: clinical features, antimicrobial therapy and outcome. Clin. Microbiol. Infect. 13196-198. [DOI] [PubMed] [Google Scholar]
- 13.Liese, J. G., V. Jendrossek, A. Jansson, T. Petropoulou, S. Kloos, M. Gahr, and B. H. Belohradsky. 1996. Chronic granulomatous disease in adults. Lancet 347220-223. [DOI] [PubMed] [Google Scholar]
- 14.Mastroeni, P., A. Vazquez-Torres, F. C. Fang, Y. Xu, S. Khan, C. E. Hormaeche, and G. Dougan. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J. Exp. Med. 192237-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller, R. A., and B. E. Britigan. 1997. Role of oxidants in microbial pathophysiology. Clin. Microbiol. Rev. 101-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan, C. 2006. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6173-182. [DOI] [PubMed] [Google Scholar]
- 17.Nauseef, W. M. 2007. How human neutrophils kill and degrade microbes: an integrated view. Immunol. Rev. 21988-102. [DOI] [PubMed] [Google Scholar]
- 18.Pacher, P., J. S. Beckman, and L. Liaudet. 2007. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 87315-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renckens, R., J. J. Roelofs, S. Knapp, A. F. de Vos, S. Florquin, and T. van der Poll. 2006. The acute-phase response and serum amyloid A inhibit the inflammatory response to Acinetobacter baumannii pneumonia. J. Infect. Dis. 193187-195. [DOI] [PubMed] [Google Scholar]
- 20.Snelgrove, R. J., L. Edwards, A. J. Rae, and T. Hussell. 2006. An absence of reactive oxygen species improves the resolution of lung influenza infection. Eur. J. Immunol. 361364-1373. [DOI] [PubMed] [Google Scholar]
- 21.Swain, S. D., T. W. Wright, P. M. Degel, F. Gigliotti, and A. G. Harmsen. 2004. Neither neutrophils nor reactive oxygen species contribute to tissue damage during Pneumocystis pneumonia in mice. Infect. Immun. 725722-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Faassen, H., R. KuoLee, G. Harris, X. Zhao, J. W. Conlan, and W. Chen. 2007. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect. Immun. 755597-5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vazquez-Torres, A., J. Jones-Carson, P. Mastroeni, H. Ischiropoulos, and F. C. Fang. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 192227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]