Abstract
Staphylococcal alpha-toxin is an important virulence factor for Staphylococcus aureus to cause severe infections. In this study, we explored whether the toxoid of alpha-toxin may be utilized to block the toxicity of wild-type alpha-toxin. We created a series of H35A mutated alpha-toxin expression strains and revealed that the H35A mutation eliminates the activity of alpha-toxin using a human lung epithelial cell line (A549). More importantly, we found that either the pretreatment or simultaneous treatment of the epithelial cells with alpha-toxin-H35A completely disrupted the cytotoxicity of alpha-toxin. Specifically, we demonstrated that alpha-toxin-H35A can effectively interfere with the pore formation and the internalization of alpha-toxin using cytotoxicity and immunofluorescence assays. In addition, we found that the removal of either the 30-amino-acid (aa) or 99-aa C-terminal region of alpha-toxin-H35A reactivated its cytotoxicity, indicating that interactions between the alanine residue at position 35 and these C-terminal regions may be associated with interrupting the toxic activity of alpha-toxin-H35A. Taken together, these results suggest that the alpha-toxin-H35A protein may be developed as a potential alternative therapeutic agent for treating early stages of S. aureus infections.
Alpha-toxin is a well-characterized virulence factor of Staphylococcus aureus that is able to cause a variety of infections including pneumonia, endocarditis, and toxic shock syndrome (24). The exported alpha-toxin is encoded by the gene hla, which is highly expressed in a stationary growth phase in vitro and at later stages of infection in animals (7, 9). The expression of alpha-toxin is coordinately modulated by different global regulatory systems including agr, sae, arl, sar, rot, and mgr (6, 20, 23, 25, 30).
Although multiple virulence factors are required for S. aureus to induce apoptosis in endothelial cells (10), alpha-toxin plays an important role among these factors (27). Specifically, alpha-toxin can interact with surface receptors of the host cells, form small heptameric pores, selectively release ions, and/or trigger cell signal transduction pathways, thus inducing apoptosis and/or death in various cell types (3, 8, 11, 14, 27, 33, 34). Alpha-toxin is required for the modulation of S. aureus-induced cytotoxicity in Jurkat T lymphocytes, human peripheral blood lymphocytes, and monocytes (8). Recently, we have demonstrated that alpha-toxin can interact with α5β1-integrin to interfere with S. aureus adhering to and internalizing into human lung epithelial cells (A549) (21). The interaction of alpha-toxin with α5β1-integrin contributes to the cytotoxicity of alpha-toxin that is required for S. aureus to induce the apoptosis and death of epithelial cells (22).
Moreover, the role played by alpha-toxin varies according to the stage of infection and the quantities produced. It has been demonstrated that the overproduction of alpha-toxin significantly reduces virulence in a model of experimental endocarditis (4). However, alpha-toxin is a critical virulence factor in experimental brain abscesses (18), intraperitoneal infection (13, 17), and pneumonia (36). It was reported previously that H35L mutagenesis leads to the elimination of pathogenicity of alpha-toxin in an animal model (28). We also confirmed that the H35L mutation in alpha-toxin abrogates cytotoxicity in epithelial A549 cells (22). The H35L mutated alpha-toxin (alpha-toxin-H35L) may be a potential vaccine candidate since alpha-toxin-H35L could provide both active and passive immunity against S. aureus-induced infection (28, 29), especially for cases of necrotizing pneumonia caused by community-acquired methicillin-resistant S. aureus (CA-MRSA) (36, 37). Therefore, alpha-toxin may be an attractive target for developing an alternative approach to interfere with CA-MRSA-induced infection.
In this study, we questioned whether a mutated toxin is able to interrupt the virulence of wild-type alpha-toxin. In order to address this question, we created a series of H35A mutated alpha-toxin expression strains and explored whether we can utilize the attenuated alpha-toxin-H35A to inhibit the cytotoxicity of alpha-toxin by using a human lung epithelial cell line (A549). In addition, we determined the mechanisms of alpha-toxin-H35A in the interference of pathogenicity.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
The bacterial strains and plasmids used in this study are listed in Table 1. S. aureus cells were cultured in Trypticase soy broth (TSB) at 37°C with shaking. Escherichia coli strains were grown in Luria-Bertani (LB) medium. Transformants containing recombinant plasmids were selected on LB agar containing ampicillin (100 μg/ml), chloramphenicol (50 μg/ml), or erythromycin (300 μg/ml) for E. coli and TSA containing chloramphenicol (10 μg/ml) or erythromycin (5 μg/ml) for S. aureus.
TABLE 1.
Strains and plasmids used for this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| S. aureus strains | ||
| RN4220 | Laboratory S. aureus strain; rsbU | 19 |
| Sa371ko | WCUH29 saeS::Tet Tcr | 20 |
| Sa371ko/hlaOE | hla overexpression strain | 21 |
| SaXL0107 | Sa371ko carrying pXL0107 | 21 |
| SaXL0207 | Sa371ko carrying pXL0207 | This study |
| SaXL0407 | Sa371ko carrying pXL0407 | This study |
| SaXL0507 | Sa371ko carrying pXL0507 | This study |
| SaXL0607 | Sa371ko carrying pXL0607 | This study |
| SaXL0707 | Sa371ko carrying pXL0707 | This study |
| SaXL0807 | Sa371ko carrying pXL0807 | This study |
| SaXL0907 | Sa371ko carrying pXL0907 | This study |
| SaXL1007 | Sa371ko carrying pXL1007 | This study |
| Plasmids | ||
| pYH4 | Tc-inducible shuttle vector; Ermr | 38 |
| pYH4/hlaOE | hla overexpression plasmid | 21 |
| pXL0107 | pYH4/hla-H35LOE | 21 |
| pXL0207 | pYH4/hla-H35AOE | This study |
| pXL0407 | pYH4/hla80-H35AOE | This study |
| pXL0507 | pYH4/hla94-H35AOE | This study |
| pXL0607 | pYH4/hla124-H35AOE | This study |
| pXL0707 | pYH4/hla194-H35AOE | This study |
| pXL0807 | pYH4/hla263-H35AOE | This study |
| pXL0907 | pYH4/hla-mycOE | This study |
| pXL1007 | pYH4/hla-H35A-mycOE | This study |
Cell culture.
A549 human lung epithelial cells (ATCC CCL 185) were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (Invitrogen, CA). Cultures of A549 cells were maintained in medium containing penicillin (5 μg/ml) and streptomycin (100 μg/ml) (Invitrogen, CA). Assays were performed using RPMI 1640 medium with different concentrations of purified toxins.
Site-directed mutagenesis and construction of truncated alpha-toxin-H35A.
The alpha-toxin-H35L mutant is nontoxic in vivo (28). We created H35A site-directed mutagenesis in an alpha-toxin overexpression plasmid DNA, pYH4/hlaOE (21), using a QuikChange multisite-directed mutagenesis kit (Stratagene, TX) according to the manufacturer's instructions except that primer extension was allowed to continue for 2 min. The primers used for site-directed mutagenesis are listed in Table 2. The resulting plasmid, pXL0207, was electroporated into RN4220 and then into Sa371ko. The formed strain was designated SaXL0207. The site-specific mutations were confirmed by DNA sequencing. To create truncated alpha-toxin-H35A, the truncated hla DNA fragments were obtained by PCR using the primers listed in Table 2 and the H35A mutated plasmid pXL0207 as a template. The PCR products were purified and digested with AscI and ligated into the PmeI and AscI sites of pYH4 as described previously (21). The resulted recombinant plasmid DNAs were electroporated into RN4220 and then into Sa371ko, respectively, and confirmed by DNA sequencing. The resulting S. aureus strains were designated SaXL0407, SaXL0507, SaXL0607, SaXL0707, and SaXL0807. The truncated alpha-toxin-H35A proteins were prepared from the supernatants of the cultures of the above-described S. aureus strains grown overnight in TSB as described previously (21).
TABLE 2.
Primers used for this study
| Primer | Sequence |
|---|---|
| H35AFor | 5′-GAAAATGGCATGGCGAAAAAAGTATTTTATAG-3′ |
| H35ARev | 5′-CTATAAAATACTTTTTTCGCCATGCCATTTTC-3′ |
| HlaFor | 5′-AAACTATGAAAACACGTATAGTCAGC-3′ |
| HlaRev | 5′-TTGGCGCGCCTTAATTTGTCATTTCTTCTTTTTC-3′ |
| Peptide-hlaRev318AscI | 5′-TTGGCGCGCCATTAGCGCCTTCTTCGCTATA-3′ |
| Peptide-hlaRev360AscI | 5′-TTGGCGCGCCTTCATTATCAGGTAGTTGCAAC-3′ |
| Peptide-hlaRev450AscI | 5′-TTGGCGCGCCCACGTTACCGTTGAATCCATAAG-3′ |
| Peptide-hlaRev660AscI | 5′-TTGGCGCGCCTTATTGATTGCCATATACCGGGTTC-3′ |
| Peptide-hlaRev867AscI | 5′-TTGGCGCGCCTTATGTTGAAGTCCAATGCAATTGG-3′ |
| Hla-c-mycRev | 5′-TTGGCGCGCCTTATTGATGAATATCTTCTTCACTAATTAATTTGTCATTTCTTCTTTTTC-3′ |
Construction of myc-tagged hla overexpression strains in S. aureus.
To identify potential mechanisms of alpha-toxin-H35A attenuation, we created c-myc-tagged hla overexpression strains using a tetracycline-inducible shuttle vector, pYH4 (38). Both alpha-toxin and alpha-toxin-H35A coding regions were obtained by PCR using high-fidelity Pfx DNA polymerase (Invitrogen). Forward primer HlaFor and reverse primer Hla-c-mycRev, containing an AscI-recognized sequence and a c-myc sequence, were used (Table 2). The PCR products were digested and ligated into the PmeI and AscI sites of pYH4. The resulting recombinant plasmid DNAs, pXL0907 (pYH4/hla-myc) and pXL1007 (pYH4/hla-H35A-myc), were confirmed by sequencing to ensure proper spacing with the ribosome binding site and to make certain that no mutations were introduced by PCR. pXL0907 and pXL1007 plasmid DNAs were electroporated into S. aureus laboratory strain RN4220 and then into Sa371ko (20), resulting in SaXL0907 and SaXL1007, respectively. The bacteria were incubated in TSB containing an appropriate antibiotic in the presence of anhydrotetracycline (500 ng/ml). The exported toxins were prepared from the supernatants of cultures of SaXL0907, SaXL1007, and Sa371ko/hlaOE (21) grown overnight using Microcon Ultracel-5K centrifugal filter devices (Millipore) according to the manufacturer's instructions. The concentrations of the prepared alpha-toxin and exported proteins were determined by using a BCA protein assay kit (Pierce). The hemolytic activity was examined using sheep blood agar plates. An obvious alpha-hemolytic ring was observed on the blood agar plate containing the prepared alpha-toxin, whereas no hemolytic activity was observed on the blood agar plate containing the same amount of exported proteins from control strain Sa371ko.
Cytotoxicity assays.
A cytotoxicity assay was performed by measuring lactate dehydrogenase (LDH) release as described previously (20). All cells were grown in 96-well plates to 70% confluence. To induce lysis, monolayer cells were exposed to alpha-toxin or alpha-toxin-H35A and incubated for 16 h at 37°C with 5% CO2. At the end of the experiment, cell viability was determined by measuring LDH release using the CellTiter 96 aqueous nonradioactive cell proliferation assay (Promega, MI) according to the manufacturer's instructions. Each experiment was repeated three times, and all of the percentages of cell death related to the control (no death) were calculated and statistically analyzed by a Student's t test using Microsoft Excel 2003 software. P values of <0.05 were considered to be significant.
Calcium influx assay.
The concentration of cytosolic free calcium was determined by measuring the calcium indicator Fluo-4 as described previously (26), with modifications. Briefly, cells were grown in 96-well plates to 70% confluence. Cells were treated with culture medium containing 10 mM Fluo-4 and 0.1% pluronic acid F-127 and incubated for up to 30 min at room temperature. Treated cells were washed twice subsequently with phosphate-buffered saline (PBS) buffer, diluted to 6 × 106 cells/ml, and added to each well of a 96-well black-wall, clear-bottom microtiter plate. The monolayer cells were exposed to alpha-toxin, alpha-toxin-H35A, or alpha-toxin mixed with alpha-toxin-H35A and incubated for 3 h at 37°C with 5% CO2. The abundant alpha-toxin was removed by washing cells with PBS. Next, 100 μl of 2 mM CaCl2 was added into each well and incubated for 30 min at 37°C. Pore formation was determined by measuring calcium influx or the change in fluorescence using a SpectraMax M2 microplate reader (excitation wavelength of 488 nm and emission wavelength of 530 nm) (Molecular Devices). The percent relative fluorescence was calculated as [(RLU for treated cells − RLU for control)/RLU for control] × 100, where RLU is relative light units.
Immunofluorescence assay.
A549 cells were grown on 10-mm coverslips to 90% confluence in 24-well tissue culture plates. The monolayer cells were exposed to alpha-toxin-myc and/or alpha-toxin-H35A-myc for 1 h, fixed with 100% ethyl alcohol, and permeated with 0.1% (vol/vol) Triton X-100 in PBS (pH 7.4). Coverslips were incubated with a c-myc monoclonal antibody (9E-10) (University of Iowa) at 37°C for 30 min, washed with PBS, and then incubated with the secondary antibody [CyTM3-conjugated Affinipure goat anti-mouse immunoglobulin G(H+L)] at 37°C for 30 min and washed with PBS. The stained cells were observed using an Olympus IX70 inverted microscope.
RESULTS
The H35A mutation abolishes the cytotoxicity of alpha-toxin.
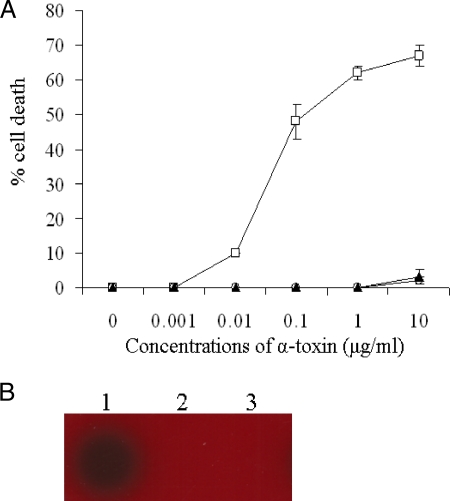
It was previously reported that the H35L mutation in alpha-toxin leads to an attenuation of pathogenicity in vivo (28) and cytotoxicity (22). To determine the impact of the H35A mutation on cytotoxicity, we performed cytotoxicity assays using human lung epithelial cells (A549). The LDH release from the epithelial cells was measured 16 h after exposure to alpha-toxin, alpha-toxin-H35A, or alpha-toxin-H35L, which was used as a negative control. Consistent with previous findings (22), wild-type alpha-toxin caused significant death of the epithelial cells in a dose-dependent manner (Fig. 1A). The lung epithelial cells (A549) were sensitive to the wild-type alpha-toxin treatment, with as little as 0.1 μg/ml of alpha-toxin leading to approximately 50% cell death (Fig. 1A). In contrast, no apparent cell death was detected after exposure to 10 μg/ml mutated alpha-toxin-H35A or alpha-toxin-H35L (Fig. 1A). Moreover, we conducted erythrocytolysis assays to further confirm the attenuation of mutated alpha-toxin using sheep blood agar plates. Wild-type alpha-toxin dramatically induced the lysis of erythrocytes, whereas similarly to alpha-toxin-H35L, alpha-toxin-H35A did not cause the lysis of erythrocytes (Fig. 1B). These results indicate that the H35A mutation eradicates the cytotoxicity of staphylococcal alpha-toxin.
FIG. 1.
Effect of mutations of alpha-toxin on cytotoxicity. (A) Influence of mutations of alpha-toxin on death of epithelial cells. Monolayers of A549 cells (2 × 105 cells/well) were exposed by adding different concentrations of alpha-toxin (open squares), alpha-toxin-H35A (open circles), or alpha-toxin-H35L (solid triangles). Cell viability was determined by measuring LDH release 16 h after treatment and is expressed as an average of data from at least three experiments ± standard deviations. (B) Impact of mutations of alpha-toxin on its capacity to lyse sheep erythrocytes. The same amount of mutated toxins was added onto the blood agar plate, the plate was incubated overnight at 37°C, and hemolytic activity was observed. Lane 1, alpha-toxin (used as a positive control); lane 2, alpha-toxin-H35A; lane 3, alpha-toxin-H35L (used as a negative control) (22).
Alpha-toxin-H35A impedes virulence of staphylococcal alpha-toxin.
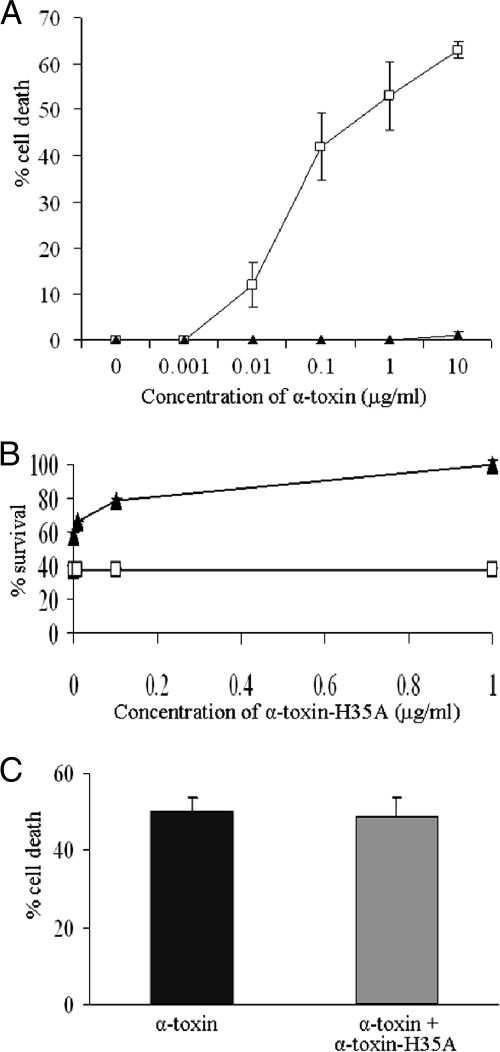
The above-described studies demonstrate that alpha-toxin-H35A lacks cytotoxicity. This led us to hypothesize that the H35A mutated toxin may be utilized as a potential therapeutic agent to interfere with the process of disease that is caused by staphylococcal alpha-toxin. To test this possibility, we examined the impact of alpha-toxin-H35A on the cytotoxicity of alpha-toxin by measuring LDH release. Epithelial cells were treated with alpha-toxin-H35A 30 min prior to exposure to wild-type alpha-toxin. The result showed that pretreatment with 1 μg/ml of mutated alpha-toxin-H35A eliminated the toxicity of 10 μg/ml of alpha-toxin (Fig. 2A). To further determine the efficacy of alpha-toxin-H35A, we simultaneously treated epithelial cells with a certain amount of alpha-toxin and different concentrations of alpha-toxin-H35A for 16 h. Treatment with alpha-toxin (10 μg/ml) alone caused more than 60% cell death, whereas the addition of an extra 0.01 μg/ml of alpha-toxin-H35A obviously increased the level of surviving epithelial cells to above 60% (Fig. 2B). Moreover, the addition of an extra 1 μg/ml of alpha-toxin-H35A effectively blocked the cytotoxicity of alpha-toxin (Fig. 2B). The above-described results indicate that the H35A mutated toxin may be useful for interrupting the activity of alpha-toxin. To address this possibility, we treated epithelial cells with 10 μg/ml of alpha-toxin 30 min before adding an extra 1 μg/ml of alpha-toxin-H35A. The viability of epithelial cells was determined by measuring LDH release. Unfortunately, the addition of the extra H35A mutated toxin had no impact on the death of cells that had been exposed to alpha-toxin (Fig. 2C).
FIG. 2.
Effect of mutated H35A toxin on the capacity for alpha-toxin-induced cell death. (A) Alpha-toxin-H35A inhibits alpha-toxin-induced cell death. Monolayers of A549 cells (2 × 105 cells/well) were pretreated for 30 min with 1 μg/ml of alpha-toxin-H35A (solid triangles) or control (open squares) without any treatment before exposure to different concentrations of alpha-toxin. Cell viability was determined by measuring LDH release 16 h after the final treatment and is expressed as an average of data from at least three experiments ± standard deviations. (B) Efficacy of alpha-toxin-H35A in inhibition of alpha-toxin-induced cell death. Monolayers of A549 cells (2 × 105 cells/well) were exposed to different mixtures of toxins containing 10 μg/ml of alpha-toxin in the presence of different amounts of alpha-toxin-H35A (solid triangles) or without alpha-toxin-H35A (open squares). Cell viability was determined by measuring LDH release 16 h after the final treatment and is expressed as an average of data from at least three experiments ± standard deviations. (C) Impact of alpha-toxin-H35A on the progress of alpha-toxin-induced cell death. Monolayers of A549 cells (2 × 105 cells/well) were exposed to alpha-toxin (10 μg/ml) for 30 min before extra alpha-toxin-H35A (1 μg/ml) was added (gray bar). Cell viability was determined by measuring LDH release 16 h after the final treatment and is expressed as an average of data from at least three experiments ± standard deviations.
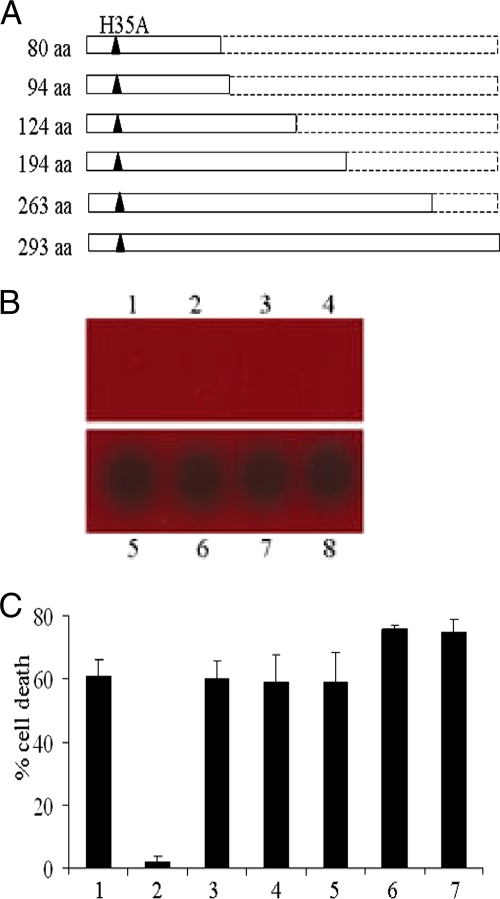
Different truncations of alpha-toxin-H35A restore cytotoxicity.
To further explore whether any short peptide of alpha-toxin-H35A is able to inhibit the toxicity of alpha-toxin, we constructed a series of truncated alpha-toxin-H35A expression strains as shown in Fig. 3A. To ensure the expression of these truncated toxins, we prepared exported proteins from cultures of the above-described hla mutant strains grown overnight and performed sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis (data not shown). The effects of deletion mutations of alpha-toxin-H35A on cytotoxicity were first determined by measuring their capacity to lyse sheep erythrocytes. Alpha-toxin and alpha-toxin-H35A were used as positive and negative controls, respectively. Both our prepared alpha-toxin and standard alpha-toxin effectively lysed the sheep erythrocytes (Fig. 3B, lanes 5 and 6); in contrast, similarly to alpha-toxin-H35A (293 amino acids [aa]), the 80-aa peptide-H35A, the 94-aa peptide-H35A, and the 124-aa peptide-H35A revealed an incapacity to lyse the erythrocytes (Fig. 3B, lanes 1 to 4). However, both the 194-aa peptide-H35A and the 263-aa peptide-H35A fully restored the capacity of lysing erythrocytes (Fig. 3B, lanes 7 and 8). We then examined the impact of these truncated H35A toxins on the inhibition of alpha-toxin's cytotoxicity by measuring LDH release using epithelial cells. Treatment with alpha-toxin alone led to more than 60% death of the epithelial cells (Fig. 3C, column 1). The addition of extra alpha-toxin-H35A completely impeded the toxicity of alpha-toxin (Fig. 3C, column 2). However, neither shorter peptide of truncated alpha-toxin-H35A in this study could block the cytotoxicity of alpha-toxin (Fig. 3C, columns 3 to 7). In contrast, both the 194-aa peptide-H35A and the 263-aa peptide-H35A exhibited a slightly additive toxic effect when combined with alpha-toxin (Fig. 3C, column 6 and 7), indicating that the two deletion mutations reactivate the cytotoxicity of alpha-toxin-H35A.
FIG. 3.
Effect of secondary mutations in alpha-toxin-H35A on cytotoxicity. (A) Diagrams represent different truncations of alpha-toxin-H35A. Open rectangles represent the length of truncated alpha-toxin-H35A with amino acids. Black triangles represent the H35A residue. (B) Impact of secondary mutations in alpha-toxin-H35A on its capacity to lyse sheep erythrocytes. The same amount of truncated toxins (1 μg/ml) was added to the blood agar plate, the plate was incubated overnight at 37°C, and hemolytic activity was observed. Lane 1, 80-aa peptide-H35A; lane 2, 94-aa peptide-H35A; lane 3, 124-aa peptide-H35A; lane 4, alpha-toxin-H35A (293 aa); lane 5, standard alpha-toxin; lane 6, prepared alpha-toxin; lane 7, 194-aa peptide-H35A; lane 8, 263-aa peptide-H35A. (C) Efficacy of truncated alpha-toxin-H35A in inhibition of alpha-toxin-induced cell death. Monolayers of A549 cells (2 × 105 cells/well) were exposed to different mixtures of toxins containing 10 μg/ml of alpha-toxin alone (column 1) or in the presence of different truncated alpha-toxin-H35A proteins. Column 2, alpha-toxin-H35A (293 aa); column 3, 80-aa peptide-H35A; column 4, 94-aa peptide-H35A; column 5, 124-aa peptide-H35A; column 6, 194-aa peptide-H35A; column 7, 263-aa peptide-H35A. Cell viability was determined by measuring LDH release 16 h after treatment and is expressed as an average of data from at least three experiments ± standard deviations.
The H35A mutation eliminates pore formation and internalization of alpha-toxin.
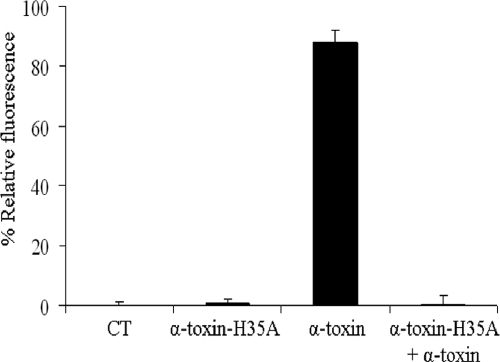
It has been well established that the ability of alpha-toxin to induce cell death is dependent on its pore formation in the plasma membrane of mammalian erythrocytes (5). To understand the reason why the H35A mutated toxin can effectively inhibit the toxicity of alpha-toxin, we examined the effect of the H35A mutation on pore formation by measuring calcium influx using the Fluo-4 calcium indicator. Intensive fluorescence signals were detected in the alpha-toxin-treated epithelial cells, indicating dramatic calcium influx and pore formation (Fig. 4). However, weak fluorescence signals were revealed in the negative control cells and the alpha-toxin-H35A-treated cells, indicating a base level of calcium influx (Fig. 4). Furthermore, the addition of alpha-toxin-H35A eradicated the ability of alpha-toxin-induced calcium influx, indicating that alpha-toxin-H35A is able to inhibit the pore formation of alpha-toxin (Fig. 4).
FIG. 4.
Effect of the H35A mutation in alpha-toxin on pore formation. Monolayers of A549 cells were treated with culture medium containing Fluo-4 and 0.1% pluronic acid F-127, diluted, and added to each well of a 96-well black-wall, clear-bottom microtiter plate. The monolayer cells were exposed to alpha-toxin, alpha-toxin-H35A, or alpha-toxin mixed with alpha-toxin-H35A and incubated for 3 h at 37°C with 5% CO2. The abundant toxins were removed, and 100 μl of 2 mM CaCl2 was added into each well and incubated for 30 min at 37°C. Pore formation was determined by measuring calcium influx or the change in fluorescence. CT, negative control without any treatment. Each experiment was repeated three times, and all of the percent relative fluorescence values, compared to the control, were calculated.
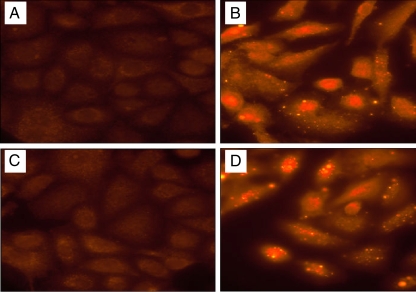
To further elucidate the mechanism of alpha-toxin-H35A in the interference of cytotoxicity, we created c-myc-tagged alpha-toxin and c-myc-tagged alpha-toxin-H35A and determined whether alpha-toxin-H35A affects the internalization of alpha-toxin by using fluorescence microscopy. Either alpha-toxin or alpha-toxin-H35A alone was able to enter the epithelial cells; in contrast, the addition of alpha-toxin-H35A dramatically blocked the capacity of alpha-toxin to internalize into the epithelial cells (Fig. 5). To rule out the possibility that c-myc-tagged alpha-toxin may lose its toxicity, we performed cytotoxicity assays by measuring LDH release. Consistent with unlabeled alpha-toxin, c-myc-tagged alpha-toxin was able to cause cell death, whereas c-myc-tagged alpha-toxin-H35A had no toxicity and enabled an inhibition of alpha-toxin-induced cell death (data not shown).
FIG. 5.
Effect of H35A mutated alpha-toxin on internalization of alpha-toxin. Monolayers of A549 cells grown on 10-mm coverslips were exposed to alpha-toxin-myc and/or alpha-toxin-H35A-myc, fixed, and permeated with 0.1% Triton X-100 in PBS. Cell-bound and internalized c-myc-tagged toxins were probed with anti-c-myc monoclonal antibody and then labeled with CyTM3-conjugated anti-mouse immunoglobulin G. The stained cells were observed using an Olympus IX70 inverted microscope. (A) Control cells without any treatment. (B) Cells were treated with alpha-toxin-myc (1 μg/ml). (C) Cells were treated with a mixture of alpha-toxin-myc (1 μg/ml) and alpha-toxin-H35A (0.1 μg/ml). (D) Cells were treated with alpha-toxin-H35A-myc (0.1 μg/ml).
DISCUSSION
Alpha-toxin is an important virulence factor and a potential vaccine candidate (29), especially for S. aureus-induced necrotizing pneumonia (36, 37). In this study, we have created H35A and a series of truncated alpha-toxin-H35A mutated toxins and demonstrated that the H35A mutation eliminates the cytotoxicity of alpha-toxin. Furthermore, we explored whether the H35A mutated toxin is able to interrupt the cytotoxicity of alpha-toxin using a human lung epithelial cell line (A549). Our data demonstrate that mutated alpha-toxin-H35A can effectively block the pore formation and the internalization of alpha-toxin, which in turn eliminates the cytotoxicity of alpha-toxin if exposed to epithelial cells simultaneously. This suggests that at an early stage of infection caused by S. aureus, especially CA-MRSA, alpha-toxin-H35A may be applied as a therapeutic agent to augment the efficacy of antibiotic treatment. It is necessary to investigate whether alpha-toxin-H35A can inhibit the cytotoxicity of alpha-toxin during infection. However, we found that alpha-toxin-H35A had no impact on the death of the epithelial cells that had been exposed to alpha-toxin. This suggests that at a later stage of infection, i.e., after a dramatic amount of alpha-toxin has been released from S. aureus, the addition of alpha-toxin-H35A may not benefit the efficiency of antibiotic treatment.
Pore-forming toxins are often associated with the pathogenesis of various pathogens and constitute almost one-third of the bacterial toxins characterized to date (1, 2, 32). The continuous emergence of multiple-antibiotic-resistant bacterial pathogens, particularly methicillin-resistant S. aureus and vancomycin-intermediate-resistant S. aureus, has caused serious concerns in public health and highlighted the urgent need for developing novel and/or alternative therapeutic agents. Therefore, it would be valuable to identify a molecule to inhibit pore formation for any given pore-forming toxin. It has been revealed that several amino acid derivatives of β-cyclodextrin inhibit the toxic activity of alpha-toxin and lethal toxin (LeTx) via blocking ion conductance (15, 16). In this study, we also examined whether alpha-toxin-H35A is able to block the cytotoxicity of anthrax LeTx and found that the pretreatment of mouse macrophages (J744A.1) with alpha-toxin-H35A had no influence on the capacity of LeTx-induced cell death (data not shown). This suggests that the inhibitory activity of alpha-toxin-H35A may be distinct from those of the amino acid derivatives of β-cyclodextrin. We are in the process of determining whether alpha-toxin-H35A possesses the ability to inhibit the activity of pore-forming toxins from other pathogens. An investigation of the efficacy of alpha-toxin-H35A treatment for S. aureus-caused infection in vivo is beyond scope of this study.
Our results are consistent with previous findings that His35 is critical for the pore formation of staphylococcal alpha-toxin (33), and mutations of His35 exhibit either abolished or reduced hemolytic activity (28, 35). Similarly to the H35L mutation, the H35A mutation in alpha-toxin disrupts pore formation and contributes to the interference of the toxic activity of alpha-toxin, including hemolytic activity and inducing the death of the epithelial cells. Surprisingly, we found that simultaneous treatment with the H35A mutated toxin could fully protect A549 cells from a 10-fold concentration of alpha-toxin (Fig. 2B), suggesting that the H35A mutation may dramatically increase the affinity of binding of alpha-toxin to unknown receptors on A549 cells. A similar protective effect by the H35A mutated toxin was observed by using a monkey kidney cell line (MARK145) (data not shown). These findings indicate that the single H35A amino acid mutation not only affects the biological activity of alpha-toxin but also changes the conformation of the molecule. In addition, we found that although both alpha-toxin and the H35A mutated toxin could enter A549 cells, the simultaneous treatment with the H35A mutated toxin blocked the internalization of alpha-toxin. This may be attributable to the high affinity of H35A mutated toxin for A549 cells, which in turn allows the receptors of alpha-toxin to be preoccupied by the H35A mutated toxin. The other possibility is that the H35A mutated toxin interferes with the oligomerization of alpha-toxin to form the heptamer and pore, which may be critical for the internalization of alpha-toxin.
Despite the importance of His35 for pore formation, secondary mutations of alpha-toxin-H35A enable the restoration of cytotoxicity. These mutations include the removal of either 30 aa or 99 aa residues from the C terminus of alpha-toxin-H35A. This phenomenon has been revealed in several secondary-site mutations in H35N, which lead to the reactivation of H35N (31). Previous studies suggested that conformational rearrangements at D108 and K154 are important for alpha-toxin assembly (16) and that the N terminus, H35, the triangle region, S217, and the prestem residues are involved in pore transition (12). Our findings suggest that the C-terminal 195- to 293-aa region and the C-terminal 264- to 293-aa region interact with the N terminus and/or the alanine residue at position 35, which may be associated with interrupting pore formation with unknown mechanisms.
The above-described data suggest that alpha-toxin-H35A may be a potent, supplemental, therapeutic agent for treating S. aureus-associated infections at the early stage. However, we cannot rule out the possibility that treatment with alpha-toxin-H35A would induce the host to generate antibodies against alpha-toxin, which in turn may promote the colonization of S. aureus. We have revealed that the neutralization of alpha-toxin with anti-alpha-toxin antiserum dramatically increases the adhesion and invasion of S. aureus by epithelial cells (21). Therefore, it is necessary to investigate whether the H35A mutated toxin can inhibit the cytotoxicity and colonization of S. aureus during infection. In addition, using c-myc-tagged toxin, we found that alpha-toxin-H35A is able to enter epithelial cells. It remains to be determined whether the internalized alpha-toxin-H35A has any potential impact on signal pathways of the host cells while alpha-toxin-H35A has a lack of toxic activity after 16 h of exposure to epithelial cells.
Acknowledgments
We thank Katherine Doll and the reviewers for critical reading of the manuscript.
This work is supported by grant AI057451 from the NIAID and by AHC Faculty Research development grant no. 03-02 at the University of Minnesota.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 22 December 2008.
REFERENCES
- 1.Alouf, J. E., and M. R. Popoff. 2006. The comprehensive sourcebook of bacterial protein toxins, p. xxiii. Elsevier, Boston, MA.
- 2.Aroian, R., and F. G. van der Goot. 2007. Pore-forming toxins and cellular non-immune defenses (CNIDs). Curr. Opin. Microbiol. 1057-61. [DOI] [PubMed] [Google Scholar]
- 3.Bantel, H., B. Sinha, W. Domschke, G. Peters, K. Schulze-Osthoff, and R. U. Jänicke. 2001. α-Toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling. J. Cell Biol. 155637-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayer, A., M. Ramos, B. Menzies, M. Yeaman, A. Shen, and A. L. Cheung. 1997. Hyperproduction of alpha-toxin by Staphylococcus aureus results in paradoxically reduced virulence in experimental endocarditis: a host defense role for platelet microbicidal proteins. Infect. Immun. 654652-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhakdi, S., R. Jursch, M. Bröker, H. Ronneberger, and K. D. Hungerer. 1994. Histidine residues near the N terminus of staphylococcal alpha-toxin as reporters of regions that are critical for oligomerization and pore formation. Infect. Immun. 622249-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung, A. L., A. S. Bayer, G. Zhang, H. Gresham, and Y. Q. Xiong. 2004. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 401-9. [DOI] [PubMed] [Google Scholar]
- 7.Da Silva, M., J. Zahm, D. Gras, O. Bjolet, M. Abely, J. Hinnrasky, M. Milliot, M. de Assis, C. Hologne, N. Bonnet, M. Merten, M. Plotkowski, and E. Puchelle. 2004. Dynamic interaction between airway epithelial cells and Staphylococcus aureus. Am. J. Physiol. Lung Cell. Mol. Physiol. 287L453-L551. [DOI] [PubMed] [Google Scholar]
- 8.Essmann, F., H. Bantel, G. Totzke, I. H. Engels, B. Sinha, K. Schulze-Osthoff, and R. U. Janicke. 2003. Staphylococcus aureus alpha-toxin-induced cell death: predominant necrosis despite apoptotic caspase activation. Cell Death Differ. 101260-1272. [DOI] [PubMed] [Google Scholar]
- 9.Goerke, C., U. Fluckiger, A. Steinhuber, W. Zimmerli, and C. Wolz. 2001. Impact of the regulatory loci agr, sarA and sae of Staphylococcus aureus on the induction of α-toxin during device-related infection resolved by direct quantitative transcript analysis. Mol. Microbiol. 401439-1447. [DOI] [PubMed] [Google Scholar]
- 10.Haslinger-Loffler, B., B. C. Kahl, M. Grundmeier, K. Strangfeld, B. Wagner, U. Fischer, A. L. Cheung, G. Paters, K. Schulze-Osthoff, and B. Sinha. 2005. Multiple virulence factors are required for Staphylococcus aureus-induced apoptosis in endothelial cells. Cell. Microbiol. 71087-1097. [DOI] [PubMed] [Google Scholar]
- 11.Haslinger, B., K. Strangfeld, G. Peters, K. Schulze-Osthoff, and B. Sinha. 2003. Staphylococcus aureus α-toxin induces apoptosis in peripheral blood mononuclear cells: role of endogenous tumor necrosis factor-α and the mitochondrial death pathway. Cell. Microbiol. 5729-741. [DOI] [PubMed] [Google Scholar]
- 12.Jayasinghe, L., G. Miles, and H. Bayley. 2006. Role of the amino latch of staphylococcal alpha-hemolysin in pore formation: a co-operative interaction between the N terminus and position 217. J. Biol. Chem. 2812195-2204. [DOI] [PubMed] [Google Scholar]
- 13.Ji, Y., A. Marra, M. Rosenberg, and G. Woodnutt. 1999. Regulated antisense RNA eliminates alpha-toxin virulence in Staphylococcus aureus infection. J. Bacteriol. 1816585-6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonas, D., I. Walev, T. Berger, M. Liebetrau, M. Palmer, and S. Bhakdi. 1994. Novel path to apoptosis: small transmembrane pores created by staphylococcal α-toxin in T lymphocytes evoke internucleosomal DNA degradation. Infect. Immun. 621304-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karginov, V. A., E. M. Nestorovich, F. Schmidtmann, T. M. Robinson, A. Yohannes, N. E. Fahmi, S. M. Bezrukov, and S. M. Hecht. 2007. Inhibition of S. aureus alpha-hemolysin and B. anthracis lethal toxin by beta-cyclodextrin derivatives. Bioorg. Med. Chem. 155424-5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawate, T., and E. Gouaux. 2003. Arresting and releasing staphylococcal α-hemolysin at intermediate stages of pore formation by engineered disulfide bonds. Protein Sci. 12997-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kernodle, D. S., R. K. Voladri, B. E. Menzies, C. C. Hager, and K. M. Edwards. 1997. Expression of an antisense hla fragment in Staphylococcus aureus reduces alpha-toxin production in vitro and attenuates lethal activity in murine model. Infect. Immun. 65179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kielian, T., A. Cheung, and W. F. Hickey. 2001. Diminished virulence of an alpha-toxin mutant of Staphylococcus aureus in experimental brain abscesses. Infect. Immun. 696902-6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreiswirth, B., S. Lofdahl, M. Betley, M. O'Reilly, P. Schlievert, M. Bergdoll, and R. Novick. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305709-712. [DOI] [PubMed] [Google Scholar]
- 20.Liang, X., C. Yu, J. Sun, H. Liu, C. Landwehr, D. Holmes, and Y. Ji. 2006. Inactivation of a two-component signal transduction system, SaeRS, eliminates adherence and attenuates virulence of Staphylococcus aureus. Infect. Immun. 744655-4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang, X., and Y. Ji. 2006. Alpha-toxin interferes with integrin-mediated adhesion and internalization of Staphylococcus aureus by epithelial cells. Cell. Microbiol. 81656-1668. [DOI] [PubMed] [Google Scholar]
- 22.Liang, X., and Y. Ji. 2007. Involvement of alpha5beta1-integrin and TNF-alpha in Staphylococcus aureus alpha-toxin-induced death of epithelial cells. Cell. Microbiol. 91809-1821. [DOI] [PubMed] [Google Scholar]
- 23.Liang, X., L. Zheng, C. Landwehr, D. Lunsford, D. Holmes, and Y. Ji. 2005. Global regulation of gene expression by ArlRS, a two-component signal transduction regulatory system of Staphylococcus aureus. J. Bacteriol. 1875486-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339520-532. [DOI] [PubMed] [Google Scholar]
- 25.Luong, T. T., S. W. Newell, and C. Y. Lee. 2003. Mgr, a novel global regulator in Staphylococcus aureus. J. Bacteriol. 1853703-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marie, I., and J. Beny. 2002. Calcium imaging of murine thoracic aorta endothelium by confocal microscopy reveals inhomogeneous distribution of endothelial cells responding to vasodilator agents. J. Vasc. Res. 39260-267. [DOI] [PubMed] [Google Scholar]
- 27.Menzies, B. E., and I. Kourteva. 2000. Staphylococcus aureus α-toxin induces apoptosis in endothelial cells. FEMS Immunol. Med. Microbiol. 2939-45. [DOI] [PubMed] [Google Scholar]
- 28.Menzies, B. E., and D. S. Kernodle. 1994. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect. Immun. 621843-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menzies, B. E., and D. S. Kernodle. 1996. Passive immunization with antiserum to a nontoxic alpha-toxin mutant from Staphylococcus aureus is protective in a murine model. Infect. Immun. 641839-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novick, R. P. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 481429-1449. [DOI] [PubMed] [Google Scholar]
- 31.Panchal, R. G., and H. Bayley. 1995. Interactions between residues in staphylococcal alpha-hemolysin revealed by reversion mutagenesis. J. Biol. Chem. 27023072-23076. [DOI] [PubMed] [Google Scholar]
- 32.Rosado, C. J., S. Kondos, T. E. Bull, M. J. Kuiper, R. H. Law, A. M. Buckle, I. Voskoboinik, P. I. Bird, J. A. Trapani, J. C. Whisstock, and M. A. Dunstone. 2008. The MACPF/CDC family of pore-forming toxins. Cell. Microbiol. 101765-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song, L., M. R. Hobaugh, C. Shustak, S. Cheley, H. Bayley, and J. E. Gouaux. 1996. Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science 2741859-1866. [DOI] [PubMed] [Google Scholar]
- 34.Valeva, A., M. Palmer, and S. Bhakdi. 1997. Staphylococcal α-toxin: formation of the heptameric pore is partially cooperative and proceeds through multiple intermediate stages. Biochemistry 3613298-13304. [DOI] [PubMed] [Google Scholar]
- 35.Walker, B., and H. Bayley. 1995. Restoration of pore-forming activity in staphylococcal alpha-hemolysin by targeted covalent modification. Protein Eng. 8491-495. [DOI] [PubMed] [Google Scholar]
- 36.Wardenburg, J. B., R. J. Patel, and O. Schneewind. 2007. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect. Immun. 751040-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wardenburg, J. B., and O. Schneewind. 2008. Vaccine protection against Staphylococcus aureus pneumonia. J. Exp. Med. 205287-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng, L., J. Yang, C. Landwehr, F. Fan, and Y. Ji. 2005. Identification of an essential glycoprotease in Staphylococcus aureus. FEMS Microbiol. Lett. 245279-285. [DOI] [PubMed] [Google Scholar]