Abstract
Availability of free iron is extremely limited in the mammalian host, and the acquisition of iron in the host is essential for successful infection by pathogenic bacteria. Expression of many genes involved in acquiring iron is regulated in response to the level of iron availability, and iron regulation is mediated by Fur. In this study, cellular levels of Vibrio vulnificus HupA, a heme receptor protein, and the hupA transcript were found to increase in cells grown at 40°C compared to cells grown at 30°C. The results suggested that change in growth temperature, in addition to iron availability, is an environmental cue controlling the expression of the hupA gene. The influence of global regulatory proteins on the expression of hupA was examined, and the cyclic AMP receptor protein (CRP) was found to activate the expression of hupA at the transcriptional level. CRP exerts its effects by directly binding to DNA upstream of the hupA promoter PhupA, and a CRP binding site, centered at 174 bp upstream of the transcription start site, was identified by a DNase I protection assay. Finally, a hupA mutant showed reduced virulence in mice and in tissue cultures, in which growth of the hupA mutant was impaired, indicating that HupA of V. vulnificus is essential for survival and multiplication during infection.
Iron is essential for the host and the pathogen, and both require the metal as a cofactor or as a prosthetic group for biologically important proteins involved in many basic cellular functions (39, 41). Iron is rarely found as free iron in the mammalian host and is rather sequestered as bound to high-affinity iron-binding proteins such as transferrin, lactoferrin, and ferritin or as complexed to the heme of hemoproteins (41, 43). Due to the limited availability of iron to pathogens and the inevitable competition with the host, pathogens have evolved sophisticated mechanisms for the acquisition of iron from the host tissues, and the mechanisms are closely linked to their virulence. Like the pathogenesis of many other bacteria, the pathogenesis of Vibrio vulnificus, a causative agent of food-borne diseases such as gastroenteritis and life-threatening septicemia, depends primarily on the organism's ability to uptake and utilize iron (2, 24, 40, 42, 44).
Specialized iron-acquiring systems consisting of various components have been identified from V. vulnificus. Okujo et al. (33) identified the structure of the phenolate siderophore of the pathogen, named vulnibactin, that solubilizes and chelates iron with high affinity. A mutant in which the gene venB was specifically inactivated was screened from a random TnphoA library of V. vulnificus, and the venB mutant was not able to produce the catechol siderophores or to acquire iron from transferrin (24). The uptake of the iron-chelating siderophores or transferrin in V. vulnificus depends on vuuA, a gene encoding a siderophore receptor, VuuA, an outer membrane protein (42). The expression of these genes is regulated at the transcriptional level by Fur, an iron-binding regulatory protein, in response to the level of iron, indicating that the availability of iron is one of the signals used for controlling expression of the genes (22, 42). However, until now, the question of whether other environmental cues, such as temperature change, could stimulate the expression of iron-acquiring systems has not yet been addressed. Furthermore, relatively little is known about regulatory proteins other than Fur that affect the expression of these iron-regulated genes.
Previously, a gene encoding an outer-membrane protein with 712 amino acids, HupA, which is required for heme utilization as an iron source, was identified from V. vulnificus (22). Expression of HupA, a heme receptor protein, was modulated in response to the level of iron availability, and the iron modulation is mediated by Fur and HupR, a LysR homologue (23). Here, our effort to further understand the regulatory mechanisms of the hupA expression was initiated by elucidating that the cellular level of HupA increased in V. vulnificus grown at 40°C rather than at 30°C, suggesting that change in growth temperature is an environmental cue controlling the expression of hupA. Furthermore, the present study examined the influence of global regulatory proteins on the expression of hupA, and the cyclic AMP receptor protein (CRP) appeared to activate the expression of hupA by directly binding to the hupA promoter. Finally, the virulence of the hupA mutant was compared to that of the parental wild type, and the possible roles of HupA in the pathogenesis of V. vulnificus were explored.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
The strains and plasmids used in this study are listed in Table 1. Escherichia coli strains used for plasmid DNA replication or the conjugational transfer of plasmids were grown in Luria-Bertani (LB) broth or in LB broth containing 1.5% (wt/vol) agar. V. vulnificus strains were grown in iron-replete medium, an LB medium supplemented with 2.0% (wt/vol) NaCl (LBS). LBS with the addition of 2,2′-dipyridyl to a final concentration of 0.1 mM and 0.2 mM was used as a medium low in iron and an iron-depleted medium, respectively. All the medium components were purchased from Difco (Detroit, MI), and the chemicals were purchased from Sigma (St. Louis, MO).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source/reference |
|---|---|---|
| Bacterial strains | ||
| V. vulnificus | ||
| ATCC 29307 | Clinical isolate; virulent | 8 |
| HLM101 | M06/24-O, Δfur | 16 |
| KC74 | ATCC 29307 with crp::nptI; Kmr | 11 |
| SM02 | ATCC 29307 with hupA::nptI; Kmr | This study |
| HS02 | ATCC 29307 with lrp::nptI; Kmr | 10 |
| HS03 | ATCC 29307 with smcR::nptI; Kmr | 12 |
| KP101 | ATCC 29307, ΔrpoS | 12 |
| KC94 | ATCC 29307 with toxRS::nptI; Kmr | Laboratory collection |
| E. coli | ||
| SM10λpir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu λ pir; conjugal donor | 29 |
| Plasmids | ||
| pDM4 | R6K γ ori sacB; suicide vector; oriT of RP4; Cmr | 30 |
| pGEM-T Easy | PCR product cloning vector; Apr | Promega |
| pRSET A | Expression vector; Apr | Invitrogen |
| pHK0201 | pRSET A carrying the crp coding region; Apr | 4 |
| pJH0311 | Broad-host-range vector; Apr Cmr | 7 |
| pOH0801 | pGEM-T Easy carrying a 0.35-kb fragment of the putative promoter region of hupA; Apr | This study |
| pSM501 | 1.3-kb fragment carrying part of the hupA coding region in pGEM-T Easy; Apr | This study |
| pSM502 | pSM501 with hupA::nptI; Apr Kmr | This study |
| pSM503 | pDM4 with hupA::nptI; Cmr Kmr | This study |
| pSM506 | pJH0311 carrying the hupA coding region; Apr Cmr | This study |
| pSM0610 | pGEM-T Easy carrying a 0.23-kb fragment of the putative promoter region of hupA; Apr | This study |
| pUC4K | pUC4 with nptI; Apr Kmr | Pharmacia |
Apr, ampicillin resistant; Kmr, kanamycin resistant; Cmr, chloramphenicol resistant.
Proteomic analysis and identification of hupA.
The V. vulnificus cells grown at 30°C and 40°C, respectively, were harvested, disrupted by sonication (ultrasonic processor; Sonics & Materials, Inc., Newtown, CT), and spun down by centrifugation. Proteins in the supernatant were dialyzed (Slide-A-Lyzer Dialysis Cassette; Pierce, Rockford, IL), resolved by 2D gel electrophoresis, and silver stained as described elsewhere (31). The protein spots that were more abundant in V. vulnificus cells grown at 40°C were excised, digested with trypsin (Promega, Madison, WI), and used for mass spectrometry (MS) analysis with a matrix-assisted laser desorption ionization-time of flight MS (Voyager-DE STR Biospectrometry Workstation, Hamburg, Germany). One protein among the spots was identified as the V. vulnificus HupA on the basis of the V. vulnificus YJ016 genome databases, which were retrieved from GenBank (BA000038). Therefore, a part of the gene hupA, representing the region of hupA encoding the 110th to 545th amino acids, was amplified from the genomic DNA of V. vulnificus ATCC 29307 by PCR using a pair of oligonucleotide primers, HupA051 and HupA052 (Table 2). The amplified 1.3-kb hupA was ligated into pGEM-T Easy (Promega) to result in pSM501 and was used for construction of the V. vulnificus hupA mutant (Table 1).
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Oligonucleotide sequence (5′ → 3′)a | Use(s) |
|---|---|---|
| HupA051 | TGATGGCGGTCCCTACTCTTTTA | Mutant construction |
| HupA052 | TTCCGTCAAACCACCAACTCTT | Mutant construction |
| HupA053 | CGAGCTCACTATGGATTTAAGGTAGTA | Complementation of hupA |
| HupA054 | GGGGTACCGTTAGTTAGAACTCATATT | Complementation of hupA |
| HupA0603 | TTGTACTGGGCTAGCGAGAGCGAATAG | Primer extension |
| HupA501-1 | CAAGCTTTTGAATTTGATAACTC | Gel mobility shift assay, DNase I footprinting |
| HupA502 | CCTTAAATCCATAGTAGGCG | Gel mobility shift assay, DNase I footprinting |
| RtxA081 | GTGATGACGCAAGTGGGTAAAGG | Real-time PCR analysis |
| RtxA082 | TAAGGCTACGGCTGTGGTATTCG | Real-time PCR analysis |
Regions of oligonucleotide not complementary to the corresponding genes are underlined.
RNA purification and transcript analysis.
Total cellular RNAs from the V. vulnificus strains were isolated using a TRIzol reagent kit in accordance with the manufacturer's protocol (Invitrogen, Carlsbad, CA). For Northern blot analysis, 20 μg of total RNA was separated by agarose gel electrophoresis, transferred to a nylon membrane, and hybridized as previously described (36, 38). Northern slot analysis was carried out by the same procedure used for Northern blot analysis, except that separation of the total RNA was omitted. The PCR product amplified using a pair of oligonucleotide primers, HupA051 and HupA052 (Table 2), was digested with HindIII, and a 0.4-kb DNA fragment representing the region of hupA encoding the 438th to 545th amino acids was isolated. The DNA probe HUPAP was prepared by labeling the 0.4-kb DNA fragment with [α-32P]dCTP using the Prime-a-Gene labeling system (Promega) as previously described (37) and was used for hybridizations (Fig. 1).
FIG. 1.
Dependency of the V. vulnificus hupA expression on temperatures and iron levels. (A and B) Wild-type V. vulnificus was grown in iron-replete medium (LBS) at different temperatures as indicated, and then samples removed at an optical density at 600 nm of 0.6 were analyzed for protein profiles (A) and the hupA transcript (B). (A) A protein spot whose abundance increased in the V. vulnificus cells grown at 40°C is indicated with arrows and was subjected to matrix-assisted laser desorption ionization-time of flight MS analysis. (B) Total RNAs isolated as described in Materials and Methods were separated (bottom) and then hybridized to HUPAP, a 32P DNA probe (top). The relative levels of the hupA transcripts are presented relative to the level of the hupA in cells grown at 40°C. The two bands represent rRNAs (bottom), and the molecular size markers (Invitrogen) and the hupA transcripts are shown in kilobases. (C) Total RNAs were isolated from V. vulnificus grown either in iron-replete medium (+) or in medium low in iron (−) transferred to nylon membrane (Roche, IN). The relative levels of the hupA transcripts are presented relative to the level of hupA in cells grown with medium low in iron at 40°C. The hupA transcripts in the RNAs were determined by Northern slot blot analyses using the HUPAP as a 32P DNA probe.
For quantitative real-time PCR, the RNA was prepared from bacterial cells exposed to INT-407 cells as described previously (15). Briefly, the hupA mutant SM02 and the wild type were incubated with INT-407 cells at a multiplicity of infection (MOI) of 30 for 1 h. The mixture of the INT-407 and V. vulnificus cells was centrifuged at 250 × g for 10 min to precipitate INT-407 cells, and the bacterial cells were then harvested from the supernatant by centrifugation at 2,430 × g for 20 min. Quantitative real-time PCRs were performed in a final volume of 20 μl of 2XiQ SYBR Green Supermix (Bio-Rad Laboratories) containing cDNA synthesized with the SuperScript first-strand synthesis system for reverse transcription-PCR (Invitrogen). Real-time PCRs were performed in triplicate using the iCycler iQ real-time detection system (Bio-Rad Laboratories) with a pair of primers, RtxA081 and RtxA082 (Table 2). Relative expression levels of rtxA were calculated by using a standard curve obtained from PCR on serially diluted genomic DNA as templates and the 16S rRNA expression level as the internal reference for normalization.
For the primer extension experiments, end-labeled 27-base primer HupA0603 (Table 2), complementary to the coding region of hupA, was added to the RNA and then extended with SuperScript II RNase H− reverse transcriptase (Invitrogen). The cDNA products were purified and resolved on a sequencing gel alongside sequencing ladders generated from pSM0610 that carries the 234-bp hupA upstream DNA (Table 1) with the same primer used for the primer extension. The Northern hybridization and primer extension products were visualized and quantified using a phosphorimage analyzer (BAS1500; Fuji Photo Film Co., Ltd., Tokyo, Japan) and the Image Gauge (version 3.12) program.
Gel-mobility shift assay and DNase I footprinting.
The 350-bp upstream region of hupA, extending from residues −323 to +27, was amplified by PCR using 32P-labeled HupA502 and unlabeled HupA501-1 as the primers (Table 2). The expression and purification of the His-tagged CRP were carried out using pHK0201, carrying the V. vulnificus crp gene, as described elsewhere (4). Binding of CRP to the labeled DNA and electrophoretic analysis of the DNA-CRP complexes have already been described (12).
The same labeled 350-bp hupA upstream DNA was used for the DNase I protection assays. The binding of CRP to the labeled DNA and DNase I digestion of the DNA-CRP complexes followed the procedures previously described by Choi et al. (4). After precipitation with ethanol, the digested DNA products were resolved on a sequencing gel alongside sequencing ladders of pOH0801 (Table 1), generated using HupA502 as the primer. The pOH0801 carries the 350-bp hupA upstream DNA. The gels were visualized as described above for the Northern analysis.
Generation of the V. vulnificus hupA mutant.
To inactivate hupA in pSM501 in vitro, a 1.2-kb nptI DNA conferring resistance to kanamycin (32) was inserted into a unique XbaI site present 300 bp apart from the translational initiation codon of hupA, and the resulting 2.5-kb hupA::nptI was ligated with SphI-SacI-digested pDM4 (30) to form pSM503 (Table 1). E. coli SM10 λ pir tra (containing pSM503) (29) was used as a conjugal donor to V. vulnificus ATCC 29307 to generate the hupA mutant by homologous recombination. Double crossovers, in which wild-type hupA on the chromosome was replaced with the hupA::nptI allele, were confirmed using previously described methods (31), and a hupA::nptI mutant chosen for further analysis was named SM02 (Table 1).
Utilization of heme as the sole iron source.
The ability of V. vulnificus strains to use heme as an iron source was assayed by measuring growth on the iron-depleted LBS broth that had been supplemented with 10 μM hemin. Hemin (Sigma) was solubilized in 10 mM NaOH and added to the iron-depleted LBS by using previously described procedures (22). Log-phase cultures were washed using phosphate-buffered saline (pH 7.4) and used as inoculum. Growth was monitored by measuring the A600 of the cultures.
Cytotoxicity assays.
ATCC 29307 and the hupA mutant SM02 were grown in an LBS broth overnight at 30°C. The following day, 0.1 ml of the cultures was inoculated into 100 ml of the LBS broth and shaken at 30°C. After 4 h of cultivation, both bacterial cultures were harvested by centrifugation and suspended in a cell culture medium, minimum essential medium containing 1% (vol/vol) fetal bovine serum (Gibco-BRL, Gaithersburg, MD) to the same level of concentrations. The preparation of the INT-407 human intestinal epithelial cells (ATCC CCL-6) and infection with the bacterial suspensions were performed on a 96-well tissue culture plate (Nunc, Roskilde, Denmark) as described previously (13, 34). The cytotoxicity was then determined by measuring the activity of lactate dehydrogenase (LDH) in the supernatant by using a cytotoxicity detection kit (Roche, Mannheim, Germany) and expressed using the total LDH activity of the cells completely lysed by 1% Triton X-100 as 100%.
Bacterial growth rates during infection.
The INT-407 cells were infected using the hupA mutant SM02 and the wild type at an MOI of 30, and growth rates of the bacterial strains during the infection were monitored. For this purpose, samples of the supernatant of the INT-407 cells were removed at regular intervals and bacterial cells in the supernatant were determined by counting CFU on LBS agar plates.
LD50 determination.
The 50% lethal doses (LD50s) of the wild-type and hupA mutant SM02 were compared using ICR mice (specific pathogen-free mice; Seoul National University), as described elsewhere (9, 19). A group (n = 6) of 6-week-old healthy female mice were injected intraperitoneally with 0.1 ml of serial dilutions of the bacterial suspensions. The infected mice were observed for 24 h, and the LD50s were calculated by the method of Reed and Muench (35). All manipulations of mice were approved by the Animal Care and Use Committee at Seoul National University.
Data analysis.
Averages and standard errors of the mean (SEM) were calculated from at least three independent determinations. The statistical significance of the difference among the V. vulnificus strains was evaluated using Student's unpaired t test (SAS software; SAS Institute Inc., Cary, NC). Significance was accepted at a P value of <0.05.
RESULTS
Transcription of hupA is controlled by temperature and iron.
When protein profiles of the V. vulnificus grown in iron-replete medium at 30°C and 40°C, respectively, were compared, HupA was found to be more abundant in the culture grown at 40°C (Fig. 1A). This variation of HupA occurs possibly either at the transcriptional level or at the posttranscriptional level of hupA expression. To distinguish between these two possibilities, changes in the level of the hupA mRNA were monitored in the same amount of total RNA isolated from wild-type cells grown in LBS at different temperatures. The relative levels of the hupA mRNA increased as the growth temperatures increased from 30°C to 37°C or 40°C, suggesting that the effect of the upshift in growth temperature on the level of HupA was correlated with the increase in the mRNA level of the gene hupA (Fig. 1B).
The effect of growth temperature on the induction of hupA expression in V. vulnificus grown with different levels of iron was examined using Northern slot analyses (Fig. 1C). At both 30°C and 40°C, expression of hupA, determined based on the intensity of the bands of the hupA transcript, increased in cells grown with a low level of iron compared to expression in cells grown with a high level of iron. This is consistent with the previous observation that the expression of hupA is regulated by the level of iron in the growth medium (22). However, it is noteworthy that the relative level of hupA expression in cells grown at 40°C is greater than that in cells grown 30°C regardless of the iron level in the medium (Fig. 1C). When taken together, these results indicate that expression of V. vulnificus hupA is induced by high growth temperatures at the transcriptional level, and the induction occurs regardless of the iron level in the medium. Thus, hereafter, the cultures grown with low levels of iron at 40°C were used to further characterize the regulation of hupA expression.
Effect of a mutation in the gene crp on the hupA transcription.
The levels of the hupA transcript in the wild type and mutants which lack transcription factors SmcR, CRP, ToxRS, Lrp, and RpoS (Table 1) were compared in order to extend our understanding of the regulation of hupA expression. Expression of hupA did not differ in the wild type or in rpoS, smcR, toxRS, or lrp mutants (data not shown). The level of the hupA transcript was reduced only in the crp mutant KC74 (Table 1) among the mutants tested (Fig. 2A). When the hupA transcripts in RNA isolated from the wild type and KC74 grown at 40°C were determined, it was apparent that the downregulation of hupA transcription due to the disruption of crp occurs regardless of the level of iron (Fig. 2A). These results suggested that CRP acts as a positive regulator for hupA expression.
FIG. 2.
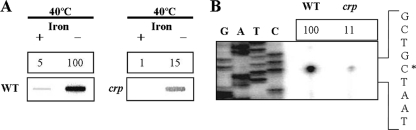
Effect of CRP on the cellular level of the hupA transcript and PhupA activity. Cultures of the wild type and crp mutant KC74 were grown at 40°C with either iron-replete medium or medium low in iron, and total RNAs were isolated as described in Materials and Methods. (A) The hupA transcripts in the RNAs were determined by Northern slot blot analyses using the HUPAP as a 32P DNA probe. +, iron-replete medium; −, medium low in iron. The relative levels of the hupA transcripts are presented relative to the level of the hupA in the wild type grown with medium low in iron. (B) PhupA activities were determined by primer extension of the RNA derived from each strain grown with medium low in iron. Lanes G, A, T, and C represent the nucleotide sequencing ladders of pSM0610. The relative levels of the PhupA activity are presented relative to the level of the PhupA activity in the wild type. The asterisk indicates the transcription start site. WT, wild type; crp, crp mutant.
To confirm the effect of CRP on the expression of hupA, the activity of the hupA promoter was compared for the wild type and the crp mutant KC74 grown with medium with low levels of iron at 40°C by primer extension analyses (Fig. 2B). A single reverse transcript was produced from the primer extension of RNA isolated from the wild type and KC74, and the 5′ end of the hupA transcript is located 35 bp upstream of the translational initiation codon of HupA and subsequently designed +1. The putative promoter upstream of the transcription start site was named PhupA. Based on the intensity of the reverse transcripts, PhupA activity was significantly decreased in KC74. Overall, these results led us to conclude that the expression of hupA in V. vulnificus is under the positive control of CRP and that CRP affects the level of hupA transcription by activating the hupA promoter PhupA, regardless of the iron level in the medium.
CRP activates hupA expression by directly binding to PhupA.
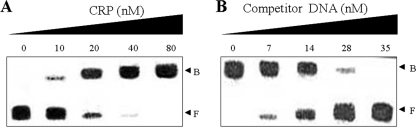
The 350-bp DNA fragment encompassing the putative hupA regulatory region was incubated with increasing amounts of CRP and then subjected to electrophoresis. As shown in Fig. 3A, the addition of CRP at a concentration of 10 nM resulted in a shift of the 350-bp DNA fragment to a single band with slower mobility. The binding of CRP was also specific because assays were performed in the presence of 200 ng of poly(dI-dC) as a nonspecific competitor. In a second gel-mobility shift assay, the same, but unlabeled, 350-bp DNA fragment was used as a self-competitor to confirm the specific binding of CRP to the PhupA (Fig. 3B). The unlabeled 350-bp DNA competed for the binding of CRP in a dose-dependent manner (Fig. 3B), confirming that CRP binds specifically to the hupA regulatory region.
FIG. 3.
Gel mobility shift assay results for binding of CRP to the hupA regulatory region. A 350-bp DNA fragment of the upstream region of PhupA was radioactively labeled and then used as a probe DNA. (A) The radiolabeled fragments (7 nM) were mixed with increasing amounts of CRP as indicated: 0, 10, 20, 40, and 80 nM of CRP in lanes 1 to 5, respectively. (B) For competition analysis, the same, but unlabeled, DNA fragment was used as a competitor. Various amounts of the competitor DNA were added to a reaction mixture containing 7 nM labeled DNA prior to the addition of CRP. Lanes 1 to 5, probe DNA incubated with 80 nM of CRP and 0, 7, 14, 28, and 35 nM of the competitor DNA, respectively, as indicated. B, bound DNA; F, free DNA.
Identification of the CRP binding site using in vitro DNase I protection analysis.
To determine the precise location of the CRP binding site in the hupA regulatory region, a DNase I footprinting experiment was performed using the same 350-bp DNA fragment used for the gel shift assays. DNase I footprinting revealed a clear protection pattern in the upstream region of hupA between −164 and −187 (Fig. 4A). Several nucleotides showed enhanced cleavage, which is frequently observed in the DNase I protection analysis of CRP binding sites (4, 12, 20). The protected region overlapped with a consensus sequence for CRP binding, extending from −166 to −182 (Fig. 4B). This CRP binding site is centered −174 bp upstream from the transcription start site of hupA. These observations confirmed that CRP activates PhupA directly by binding to a specific CRP binding site in the upstream region of hupA.
FIG. 4.
DNase I protection analysis for identification of CRP binding site and sequence analysis of the hupA upstream region. (A) The 32P-labeled 350-bp hupA regulatory region was incubated with increasing amounts of CRP and then digested with DNase I. Lane 1, no CRP added; lanes 2 to 6, CRP at 50, 100, 150, 200, and 250 nM, respectively. Lanes G, A, T, and C represent the nucleotide sequencing ladders of pOH0801. The hypersensitivity and protection in the presence of CRP are indicated by thick lines and open boxes, respectively. (B) The transcription start site is indicated by a bent arrow (PhupA). The sequences proposed for the binding sites of CRP are shown in a shaded box. The conserved nucleotide sequences for the binding of CRP and Fur are indicated above the V. vulnificus DNA sequence in uppercase letters. The positions of the putative −10 and −35 regions are underlined with dotted lines for the promoter PhupA. The ATG translation initiation codon and putative ribosome-binding site (SD) are indicated in bold. ORF, open reading frame.
Effect of hupA mutation on utilization of heme as the sole iron source.
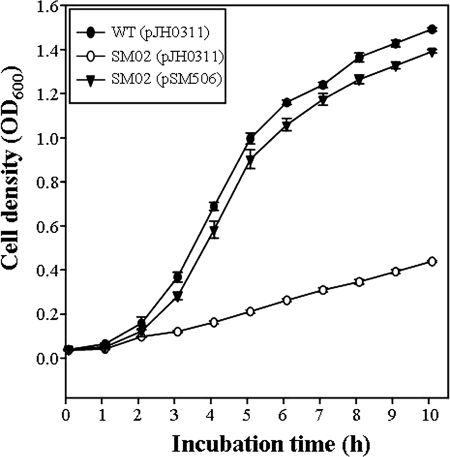
The insertional disruption of the gene hupA in SM02 was confirmed by PCR (data not shown). The hupA mutant SM02 was tested for the ability to use heme as the sole iron source by monitoring its growth in medium depleted of free iron but containing hemin (Fig. 5). The wild type was not able to grow on the iron-depleted LBS in the absence of hemin (negative control) (data not shown). Supplementation of the iron-depleted LBS with 10 μM hemin as the sole iron source supported growth of the wild type. In contrast, SM02 that is deficient of functional hupA was not able to grow to a substantial level, indicating that the gene product of hupA is responsible for the growth of V. vulnificus when heme is the sole iron source (Fig. 5). To complement the hupA mutation, pSM506 (Table 1) was constructed by subcloning the entire hupA amplified by PCR using primers HupA053 and HupA054 (Table 2) into the broad-host-range vector pJH0311 (7). The lack of growth of SM02 with hemin was restored by the reintroduction of hupA on pSM506. Therefore, it was confirmed that the attenuated growth of SM02 resulted from the inactivation of functional hupA rather than from any polar effects on genes downstream of hupA.
FIG. 5.
Cultures of the wild-type (WT), hupA mutant SM02, and complemented strains were grown on iron-depleted medium with 10 μM hemin at 40°C. OD600, optical density at 600 nm.
Comparison of cytotoxicity and growth rate of the V. vulnificus strains.
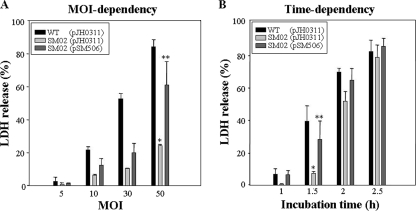
To examine the effects of the hupA mutation on the ability of V. vulnificus to damage epithelial cells, the LDH activities from monolayers of INT-407 cells infected with 100 μl of a suspension of the wild type and hupA mutant SM02 strains at different MOIs and incubated for 1.5 h were determined (Fig. 6A). The hupA mutant SM02 exhibited significantly less LDH-releasing activity when the MOI was up to 50 (Fig. 6A). The level of LDH activity released from the INT-407 cells infected with SM02 was almost fourfold less than that from cells infected with the wild type. The INT-407 cells were also infected at an MOI of 30, and the LDH activity from the cells was compared at different incubation times as indicated (Fig. 6B). When the cells were incubated for as long as 2 h, the cells infected with SM02 exhibited lower levels of LDH activity than the cells infected with the wild type. Again, the lower LDH activities were restored to levels comparable to those obtained from the cells infected with the wild type when the cells were infected with the complemented strain, SM02(pSM506) (Fig. 6A and B). These results suggest that HupA is important for V. vulnificus to infect and injure host cells.
FIG. 6.
Effect of hupA mutation on virulence of V. vulnificus toward INT-407 cells. INT-407 cells were infected with the wild-type (WT), hupA mutant SM02, or complemented strains at various MOIs for 1.5 h (A) or at an MOI of 30 for various incubation times (B). The cell cytotoxicity was determined by an LDH release assay. Data are means ± SEM from three independent experiments. *, P < 0.01; **, P < 0.1, relative to groups infected with the wild-type V. vulnificus at each MOI or each incubation time.
Since it has been reported that RtxA toxin is causing lysis of epithelial cells (18, 25), the rtxA transcripts in the hupA mutant and parental wild type were compared using quantitative real-time PCR to see if the hupA mutation altered rtxA expression during infection (Fig. 7A). The cellular level of the rtxA transcript in the hupA mutant was not significantly different from that of the wild type, indicating that the decreased cytotoxicity of the hupA mutant is caused by factors other than less expression of RtxA.
FIG. 7.
Expression of rtxA and growth rates of the wild type (WT) and hupA mutant SM02 during infection. (A) The relative levels of rtxA expression in the wild type and SM02 were determined by quantitative real-time PCR analysis. Details for preparation of total cellular RNA and real-time PCR are given in Materials and Methods, and the expression levels of rtxA were normalized to the 16S rRNA expression level. (B) Growth of the strains during the infection of INT-407 cells was monitored. The strains were used to infect the INT-407 cells at an MOI of 30, and then bacterial cells in the supernatant were determined by counting CFU on LBS agar plates at time intervals as indicated. Data are means ± SEM from three independent experiments.
To examine whether reduced cytotoxicity of the hupA mutant resulted from defects in its growth, we compared the growth rate of the hupA mutant with that of the wild type. The growth rate of the hupA mutant in either LBS or minimal essential medium with 1% fetal bovine serum was not significantly different from that of the wild type (data not shown). During infection, however, the growth rate of the hupA mutant in the supernatant of the INT-407 cells was significantly lower than that of the wild type (Fig. 7B). These results suggest that the decreased virulence of the hupA mutant likely resulted from its growth defect when infected the INT-407 cells, indicating that HupA could play a role in the pathogenesis of V. vulnificus by assuring growth of the pathogen during infection.
HupA is important for virulence in mice.
A total of six non-iron-treated mice were used for each inoculation group, and the LD50s with V. vulnificus strains were compared. The LD50 for SM02 was 4.0 × 107 CFU and greater than that for the wild type, an LD50 of 2.7 × 106 CFU. Therefore, for the mouse model of intraperitoneal infection, in which the hupA mutant exhibited more than 1 log increase in the LD50 over the wild type, the hupA mutant appeared less virulent than its parental wild type. This result indicates that the HupA and utilization of heme as an iron source is apparently important for the pathogenesis of V. vulnificus. Thus, when taken together, the results of the present study make it reasonable to conclude that the hupA gene is essential for the virulence of V. vulnificus in mice as well as in tissue cultures.
DISCUSSION
Bacterial pathogenicity is a multifactorial and complex phenomenon that involves the products of many genes contributing not only to diseases but also to the survival and replication on or within a host (28). Many of these genes are not expressed or are less expressed during in vitro growth and are preferentially expressed on or within host tissues in response to environmental signals (21). Distinguishing between host and nonhost environments and the subsequent differential expression of virulence factors may allow a more efficient utilization of resources and be crucial in obtaining maximum effectiveness of pathogenesis. Changes in environment, such as temperature, types and levels of nutrients, pH, osmolarity, oxygen levels, and concentrations of various ions, are the most common environmental signals that pathogenic bacteria routinely encounter within the host (27). Among them, changes in temperature have been implicated as one of the most frequently used cues controlling the expression of numerous virulence factors in pathogenic bacteria (14).
The majority, approximately 95%, of iron is sequestered in the form of heme, primarily as hemoglobin within the mammalian host (43). Therefore, a successful infection of pathogenic bacteria depends on adequate heme acquisition for growth and virulence. A gene, hupA, encoding the heme receptor of V. vulnificus was identified, and the hupA mutant was not able to use heme as a source of iron (22). As a result of the present study, it is apparent that change in temperature is an environmental signal, in addition to iron availability, used by V. vulnificus to regulate the expression of hupA (Fig. 1). It is likely that multiple signals may provide the additional levels of control for the precise expression of the heme uptake system. As such, the pathogenic bacteria could use temperature fluctuations to sense the host milieu where the major types of iron resources are heme or hemoproteins.
The expression of many of the iron-acquiring systems is controlled at the transcriptional level by a global regulatory protein called Fur (1). The level of hupA transcript in the fur mutant HLM101 (17) (Table 1) was significantly higher than that of its parental wild type (data not shown), indicating that the transcription of hupA is negatively regulated by Fur as observed previously (22). The present study has shown that the expression of V. vulnificus hupA is also dependent on CRP (Fig. 2, 3, and 4). CRP, which is a central regulator of energy (catabolic) metabolism, would make expression of the heme uptake protein metabolically coordinated, such that the acquired iron in cells should be used in the most efficient way as a cofactor or as a prosthetic group for essential enzymes involved in energy metabolism. Indeed, CRP regulation has also been observed in the synthesis of the V. vulnificus VuuA, a vulnibactin receptor protein (5). This metabolically coordinated acquisition and effective utilization of iron, one of the most limited resources, would facilitate establishing and maintaining infection and would be crucial for the overall success of the organism during pathogenesis.
A binding site for the Fur protein was found at an appropriate distance from the transcription start site of PhupA (Fig. 4B). The assigned sequence for the Fur binding, GATAATGATAATCATTATC, scored a 79% homology to the consensus Fur binding sequence (6). A CRP binding sequence centered 174 bp upstream of the transcription start site of PhupA was also determined in the present study (Fig. 4B). The 174-bp-upstream location of the transcription start site is unusually distant for direct activation by CRP (3). Generally, activators binding this far upstream of the promoter are not able to activate RNA polymerase (RNAP) directly and rather cooperate and interact with additional transcriptional regulator(s) on the promoter DNA. As such, the additional regulatory proteins convey the activator's signal to RNAP and/or induce structural changes of the DNA (forming a DNA loop) to bring the activators to RNAP (3, 26). This implies that another factor(s), if yet unidentified, may be involved in the activation of PhupA to deliver the remote effects of CRP to RNAP.
In summary, it is apparent that transcription of the V. vulnificus gene hupA encoding a heme receptor protein is controlled by growth temperature. In addition, CRP positively regulates PhupA activity and exerts its effect by directly binding to a specific CRP binding site centered 174-bp upstream of the transcription start site. Finally, the hupA mutant showed reduced virulence in a mouse model and in tissue cultures in which growth of the hupA mutant was impaired, indicating that HupA of V. vulnificus is essential for pathogenesis by assuring survival and multiplication during infection.
Acknowledgments
We thank K.-H. Lee for providing the V. vulnificus Fur mutant HM101.
This study was supported by grants to S.H.C. from the Basic Science Research Project, Korea Science and Engineering Foundation (R-01-2007-000-20590-0), and the National Research Laboratory Grant (R0A-2007-000-20039-0), Republic of Korea.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 12 January 2009.
REFERENCES
- 1.Bagg, A., and J. B. Neilands. 1987. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry 265471-5477. [DOI] [PubMed] [Google Scholar]
- 2.Biosca, E. G., B. Fouz, E. Alcaide, and C. Amaro. 1996. Siderophore-mediated iron acquisition mechanisms in Vibrio vulnificus biotype 2. Appl. Environ. Microbiol. 62928-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browning, D. F., and S. J. Busby. 2004. The regulation of bacterial transcription initiation. Nat. Rev. Microbiol. 257-65. [DOI] [PubMed] [Google Scholar]
- 4.Choi, H. K., N. Y. Park, D. I. Kim, H. J. Chung, S. R. Ryu, and S. H. Choi. 2002. Promoter analysis and regulatory characteristics of vvhBA encoding cytolytic hemolysin of Vibrio vulnificus. J. Biol. Chem. 27747292-47299. [DOI] [PubMed] [Google Scholar]
- 5.Choi, M. H., H. Y. Sun, R. Y. Park, C. M. Kim, Y. H. Bai, Y. R. Kim, J. H. Rhee, and S. H. Shin. 2006. Effect of the crp mutation on the utilization of transferrin-bound iron by Vibrio vulnificus. FEMS Microbiol. Lett. 257285-292. [DOI] [PubMed] [Google Scholar]
- 6.de Lorenzo, V., S. Wee, M. Herrero, and J. B. Neilands. 1987. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J. Bacteriol. 1692624-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goo, S. Y., H. J. Lee, W. H. Kim, K. L. Han, D. K. Park, H. J. Lee, S. M. Kim, K. S. Kim, K. H. Lee, and S. J. Park. 2006. Identification of OmpU of Vibrio vulnificus as a fibronectin-binding protein and its role in bacterial pathogenesis. Infect. Immun. 745586-5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollis, D. G., R. E. Weaver, C. N. Baker, and C. Thornsberry. 1976. Halophilic Vibrio species isolated from blood cultures. J. Clin. Microbiol. 3425-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong, H. G., and S. H. Choi. 2008. Evidence that AphB essential for the virulence of Vibrio vulnificus is a global regulator. J. Bacteriol. 1903768-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong, H. S., J. E. Rhee, J. H. Lee, H. K. Choi, D. I. Kim, M. H. Lee, S. J. Park, and S. H. Choi. 2003. Identification of Vibrio vulnificus lrp and its influence on survival under various stresses. J. Microbiol. Biotechnol. 13159-163. [Google Scholar]
- 11.Jeong, H. S., K. C. Jeong, H. K. Choi, K. J. Park, K. H. Lee, J. H. Rhee, and S. H. Choi. 2001. Differential expression of Vibrio vulnificus elastase gene in a growth phase-dependent manner by two different types of promoters. J. Biol. Chem. 27613875-13880. [DOI] [PubMed] [Google Scholar]
- 12.Jeong, H. S., M. H. Lee, K. H. Lee, S. J. Park, and S. H. Choi. 2003. SmcR and cyclic AMP receptor protein coactivate Vibrio vulnificus vvpE encoding elastase through the RpoS-dependent promoter in a synergistic manner. J. Biol. Chem. 27845072-45081. [DOI] [PubMed] [Google Scholar]
- 13.Jeong, K. C., H. S. Jeong, S. E. Lee, S. S. Chung, J. H. Rhee, A. M. Starks, G. M. Escudero, P. A. Gulig, and S. H. Choi. 2000. Construction and phenotypic evaluation of a Vibrio vulnificus vvpE mutant for elastolytic protease. Infect. Immun. 685096-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konkel, M. E., and K. Tilly. 2000. Temperature-regulated expression of bacterial virulence genes. Microbes Infect. 2157-166. [DOI] [PubMed] [Google Scholar]
- 15.Lee, B. C., J. H. Lee, M. W. Kim, B. S. Kim, M. H. Oh, K. S. Kim, T. S. Kim, and S. H. Choi. 2008. The importance of Vibrio vulnificus rtxE for virulence and its expression induced by exposure to host cell. Infect. Immun. 761509-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, H. J., K. J. Park, A. Y. Lee, S. G. Park, B. C. Park, K. H. Lee, and S. J. Park. 2003. Regulation of fur expression by RpoS and Fur in Vibrio vulnificus. J. Bacteriol. 1855891-5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, H. J., S. H. Bang, K. H. Lee, and S. J. Park. 2007. Positive regulation of fur gene expression via direct interaction of Fur in a pathogenic bacterium, Vibrio vulnificus. J. Bacteriol. 1892629-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, J. H., M. W. Kim, B. S. Kim, S. M. Kim, B. C. Lee, T. S. Kim, and S. H. Choi. 2007. Identification and characterization of the Vibrio vulnificus rtxA essential for cytotoxicity in vitro and virulence in mice. J. Microbiol. 45146-152. [PubMed] [Google Scholar]
- 19.Lee, J. H., N. Y. Park, S. J. Park, and S. H. Choi. 2003. Identification and characterization of the Vibrio vulnificus phosphomannomutase gene. J. Microbiol. Biotechnol. 13149-154. [Google Scholar]
- 20.Lee, J. H., and S. H. Choi. 2006. Coactivation of Vibrio vulnificus putAP operon by cAMP receptor protein and PutR through cooperative binding to overlapping sites. Mol. Microbiol. 60513-524. [DOI] [PubMed] [Google Scholar]
- 21.Lee, S. H., and A. Camilli. 2000. Novel approaches to monitor bacterial gene expression in infected tissue and host. Curr. Opin. Microbiol. 397-101. [DOI] [PubMed] [Google Scholar]
- 22.Litwin, C. M., and B. L. Byrne. 1998. Cloning and characterization of an outer membrane protein of Vibrio vulnificus required for heme utilization: regulation of expression and determination of the gene sequence. Infect. Immun. 663134-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litwin, C. M., and J. Quackenbush. 2001. Characterization of a Vibrio vulnificus LysR homologue, HupR, which regulates expression of the haem uptake outer membrane protein, HupA. Microb. Pathog. 31295-307. [DOI] [PubMed] [Google Scholar]
- 24.Litwin, C. M., T. W. Rayback, and J. Skinner. 1996. Role of catechol siderophore synthesis in Vibrio vulnificus virulence. Infect. Immun. 642834-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, M., A. F. Alice, H. Naka, and J. H. Crosa. 2007. The HlyU protein is a positive regulator of rtxA1, a gene responsible for cytotoxicity and virulence in the human pathogen Vibrio vulnificus. Infect. Immun. 753282-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLeod, S. M., and R. C. Johnson. 2001. Control of transcription by nucleoid proteins. Curr. Opin. Microbiol. 4152-159. [DOI] [PubMed] [Google Scholar]
- 27.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 1741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller, J. F., J. J. Mekalanos, and S. Falkow. 1989. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science 243916-922. [DOI] [PubMed] [Google Scholar]
- 29.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 1702575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milton, D. L., R. O'Toole, P. Horstedt, and H. Wolf-Watz. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 1781310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh, M. H., H. G. Jeong, and S. H. Choi. 2008. Proteomic identification and characterization of Vibrio vulnificus proteins induced upon exposure to INT-407 intestinal epithelial cells. J. Microbiol. Biotechnol. 18968-974. [PubMed] [Google Scholar]
- 32.Oka, A., H. Sugisaki, and M. Takanami. 1981. Nucleotide sequence of the kanamycin resistance transposon Tn903. J. Mol. Biol. 147217-226. [DOI] [PubMed] [Google Scholar]
- 33.Okujo, N., M. Saito, S. Yamamoto, T. Yoshida, S. Miyoshi, and S. Shinoda. 1994. Structure of vulnibactin, a new polyamine-containing siderophore from Vibrio vulnificus. Biometals 7109-116. [DOI] [PubMed] [Google Scholar]
- 34.Park, N. Y., J. H. Lee, M. W. Kim, H. G. Jeong, B. C. Lee, T. S. Kim, and S. H. Choi. 2006. Identification of the Vibrio vulnificus wbpP gene and evaluation of its role in virulence. Infect. Immun. 74721-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27439-497. [Google Scholar]
- 36.Rhee, J. E., H. G. Jeong, J. H. Lee, and S. H. Choi. 2006. AphB influences acid tolerance of Vibrio vulnificus by activating expression of the positive regulator CadC. J. Bacteriol. 1886490-6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhee, J. E., K. S. Kim, and S. H. Choi. 2005. CadC activates pH-dependent expression of the Vibro vulnificus cadBA operon at a distance through direct binding to an upstream region. J. Bacteriol. 1877870-7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 39.Schaible, U. E., and S. H. E. Kaufmann. 2004. Iron and microbial infection. Nat. Rev. Microbiol. 2946-953. [DOI] [PubMed] [Google Scholar]
- 40.Stelma, G. N., Jr., A. L. Reyes, J. T. Peeler, C. H. Johnson, and P. L. Spaulding. 1992. Virulence characteristics of clinical and environmental isolates of Vibrio vulnificus. Appl. Environ. Microbiol. 582776-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wandersman, C., and P. Delepelaire. 2004. Bacterial iron sources: from siderophores to hemophores. Annu. Rev. Microbiol. 58611-647. [DOI] [PubMed] [Google Scholar]
- 42.Webster, A. C., and C. M. Litwin. 2000. Cloning and characterization of vuuA, a gene encoding the Vibrio vulnificus ferric vulnibactin receptor. Infect. Immun. 68526-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilks, A., and K. A. Burkhard. 2007. Heme and virulence: how bacterial pathogens regulate, transport and utilize heme. Nat. Prod. Rep. 24511-522. [DOI] [PubMed] [Google Scholar]
- 44.Wright, A. C., L. M. Simpson, and J. D. Oliver. 1981. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect. Immun. 34503-507. [DOI] [PMC free article] [PubMed] [Google Scholar]