Abstract
Protective antigen (PA)-based anthrax vaccines acting on toxins are less effective than live attenuated vaccines, suggesting that additional antigens may contribute to protective immunity. Several reports indicate that capsule or spore-associated antigens may enhance the protection afforded by PA. Addition of formaldehyde-inactivated spores (FIS) to PA (PA-FIS) elicits total protection against cutaneous anthrax. Nevertheless, vaccines that are effective against cutaneous anthrax may not be so against inhalational anthrax. The aim of this work was to optimize immunization with PA-FIS and to assess vaccine efficacy against inhalational anthrax. We assessed the immune response to recombinant anthrax PA from Bacillus anthracis (rPA)-FIS administered by various immunization protocols and the protection provided to mice and guinea pigs infected through the respiratory route with spores of a virulent strain of B. anthracis. Combined subcutaneous plus intranasal immunization of mice yielded a mucosal immunoglobulin G response to rPA that was more than 20 times higher than that in lung mucosal secretions after subcutaneous vaccination. The titers of toxin-neutralizing antibody and antispore antibody were also significantly higher: nine and eight times higher, respectively. The optimized immunization elicited total protection of mice intranasally infected with the virulent B. anthracis strain 17JB. Guinea pigs were fully protected, both against an intranasal challenge with 100 50% lethal doses (LD50) and against an aerosol with 75 LD50 of spores of the highly virulent strain 9602. Conversely, immunization with PA alone did not elicit protection. These results demonstrate that the association of PA and spores is very much more effective than PA alone against experimental inhalational anthrax.
Bacillus anthracis is a gram-positive, aerobic, facultatively anaerobic, spore-forming, rod-shaped bacterium and is the etiologic agent of anthrax. B. anthracis resides in the soil as a dormant spore that is highly resistant to adverse conditions and can remain viable for years. The spore typically enters herbivores through ingestion; although anthrax is predominantly a disease of herbivores, humans can be infected through incidental exposure during handling of animals or animal products. In humans, the disease may take three forms—cutaneous, gastrointestinal, or pulmonary—depending on the site of entry. The most common human form is cutaneous anthrax, typically caused by spores infecting open wounds or skin abrasions. The mortality of cutaneous anthrax is near 20% if untreated (21). Gastrointestinal anthrax may in some cases extend to neuromeningitidis and generally leads to fatal systemic disease if untreated (5, 21). Naturally acquired pulmonary anthrax is very unusual. However, the mortality of pulmonary anthrax is almost 100% if not treated very early (80). Inhalational anthrax manifests as the rapid development of nonspecific, flulike symptoms that, if untreated, progress quickly to shock, respiratory distress, and death (21, 80).
Inhaled spores are deposited in alveolar spaces where they are ingested by macrophages (39, 66) and by dendritic cells (DCs) (9, 15). Then, the intracellular spores germinate into nascent bacilli that escape from the macrophage, multiply extracellularly in the lymphatic system and spread into the bloodstream, where rapid multiplication continues (38, 39); alternatively, phagocytized spores are transported by migrating macrophages to the mediastinal and peribronchial lymph nodes, where they germinate into bacilli (66). DCs may be central to this step of the infection (15). Anthrax disease appears to result from a two-step process involving overwhelming bacterial replication and subsequent toxin production. Nevertheless, the fate of spores within macrophages, the resistance of macrophages to anthrax toxins and the role of macrophages in B. anthracis dissemination all remain controversial (19, 20, 38, 39, 83). An alternative mechanism has been recently described, suggesting that inhaled spores establish an initial infection in nasally associated lymphoid tissues where they germinate. The bacteria then disseminate first to the draining lymph nodes, then to the spleen and lungs, and finally to the blood (37).
B. anthracis has two major virulence determinants. One is a tripartite protein complex toxin composed of lethal factor (LF), edema factor (EF), and protective antigen (PA) all encoded by plasmid pXO1. The other is antiphagocytic poly-γ-d-glutamic acid (γPDGA) capsule encoded by plasmid pXO2. EF and LF combine with PA to form the edema toxin (ET) and lethal toxin (LT), respectively, which both impair host immune defenses and probably act synergistically in vivo to cause edema formation and death (58, 75). The PA-LF/PA-EF complex is internalized by receptor-mediated endocytosis and, after acidification of the endosome, the toxin is translocated into the host cell cytosol, where it exerts cytotoxic effects (89). LT is a zinc metalloprotease that inactivates mitogen-activated protein kinase kinases, leading to toxic effects on susceptible macrophages (3, 18, 24, 54) and impairment of the bactericidal activity of alveolar macrophages, thus facilitating B. anthracis survival (35, 65). ET is a calmodulin-dependent adenylate cyclase that catalyzes the production of cyclic AMP from host ATP, perturbing water homeostasis, which in turn causes massive edema (55). ET is also cytotoxic in a cell-dependent manner and may contribute to the disease through directly killing cells, leading to tissue necrosis (79) and multiorgan failure, resulting in host death (28). LT and ET cooperatively inhibit activation of both DCs (14, 76) and T cells (57), thereby suppressing both the innate immune response and the priming of adaptive immune responses. Therefore, preventing either the entry of the toxin complex into the host cell or its translocation into the cytosol would make a major contribution to protection.
The PDGA capsule is a poorly immunogenic polypeptide but seems to be vital for the dissemination of B. anthracis in the bodies of infected animals (12). The in vivo synthesis of capsule determines the outcome of infection (22, 49), and capsule degradation enhances both in vitro macrophage phagocytosis and neutrophil killing of encapsulated B. anthracis (68).
The potential use of B. anthracis spores as a weapon of biological warfare or as inhaled weapons of bioterrorism has increased the need for a safe and effective vaccine to protect humans against inhalational anthrax (6, 31).
The current United Kingdom licensed anthrax vaccine, anthrax vaccine precipitate, is an alum-precipitated filtrate of B. anthracis 34F2 Sterne strain culture consisting mainly of PA (77). The U.S. licensed anthrax vaccine absorbed (AVA/Biothrax) also consists mainly of PA, in this case extracted from cultures of the unencapsulated, toxin-producing strain of B. anthracis V770-NP1-R adsorbed onto aluminum hydroxide (33). Both vaccines contain small amounts of EF and LF and probably other components that presumably contribute to vaccine efficacy (33, 77, 88).
These vaccines have the major disadvantage of inducing only a limited duration of protection and require frequent booster injections if sufficient immunity is to be maintained (32). Furthermore, such PA-based vaccines, acting on toxins, are less effective than live attenuated vaccines such as the Sterne strain, suggesting that additional antigens may contribute in a significant manner to protective immunity (4, 16, 42, 51, 59, 85).
Various animal models have been used for testing the protective activities of vaccines against anthrax infection, including mice (10, 30, 86), rats (46), guinea pigs (10, 26, 46, 70), hamsters (27), rabbits (26, 50, 60, 61), and nonhuman primates (26, 40, 44, 60). These studies emphasize the large differences of protection between species. For instance, PA-based vaccines confer better protection to guinea pigs, rabbits, and nonhuman primates than to mice, probably because the γPDGA capsule is the primary virulence factor in mice (87). Indeed, many reports suggest that capsule antigen(s) (13, 47, 64, 67, 81) and spore antigen(s) (10, 16, 23) might confer additional protection. An immunodominant glycoprotein antigen of the spore surface (BclA) has been identified among the various surface proteins of the exosporium and may contribute to protective immunity (72, 74). Sera from animals immunized with living spores of the toxinogenic unencapsulated STI-1 strain of B. anthracis have been reported to express both antitoxin and antispore activities, the latter involving inhibition of spore germination, which was attributed by some authors to both anti-PA and anti-LF antibodies (73). Furthermore, PA-based vaccines induce antispore activity characterized by stimulation of phagocytosis of opsonized spores by murine macrophages in vitro and by inhibition of spore germination. As a consequence, anti-PA antibody-specific immunity may contribute to impeding the early stages of infection with B. anthracis spores (84).
Brossier et al. demonstrated that the addition of formaldehyde-inactivated spores (FIS) of B. anthracis to PA antigen (PA-FIS) elicits total protection of mice and guinea pigs against subcutaneous (s.c.) challenge with a virulent B. anthracis strain (10). However, vaccines that are effective for the s.c. route of infection may not be so against the pulmonary route (30).
Several studies have demonstrated that either live spore-based vaccines or PA-based vaccines may confer variable protection against different B. anthracis strains and isolates in both mice and guinea pigs (26, 43, 51, 82, 85). Therefore, we used two different B. anthracis challenge strains in our study, namely, strains 9602 and 17JB from the Institut Pasteur collection. Although both strains are encapsulated and toxinogenic (cap+ tox+), harboring both pXO1 and pXO2 plasmids, they differ in virulence, as shown by the 50% lethal doses (LD50) (s.c. route), estimated to be about 50 and 500 spores per mouse, respectively (10). Strain 9602 is as virulent as the Ames strain (10, 43); strain 17JB (the atypical Pasteur vaccine strain 2-17JB (78), harboring both pXO1 and pXO2 (cap+ tox+), is very similar to the so-called “Carbosap” strain used in Italy for immunization against ovine and bovine anthrax (25). It has residual pathogenicity characteristics that cause death in mice and guinea pigs but expresses no virulence in rabbits (25). Adone et al. demonstrated that the attenuation of the Carbosap vaccine strain is not due to the lack of virulence genes (cya, lef, and pagA), of regulatory genes (atxA and pagR), or of the gerX operon involved in germination within macrophages, or to divergence of the sequences of these genes from those of a wild-type virulent B. anthracis strain (1). Indeed, sophisticated advanced molecular analysis has been unable to identify the genetic differences accounting for differences in virulence between Carbosap and virulent strains (48).
There are various possible causes of these differences in virulence and pathogenesis, including (i) involvement of unknown virulence factors and/or mechanisms involved in attenuation, (ii) differences in expression and activity of the known virulence factors and their regulators (48), and (iii) differences in pXO2 plasmid copy number (17). Nevertheless, like the Vollum strain, 17JB remains a relevant model for the study of vaccine efficacy: it is less pathogenic than wild-type strains such as 9602 or Ames but is nevertheless cap+ tox+.
In summary, despite obvious efficacy in nonhuman primates, the currently licensed anthrax vaccines have shortcomings, such as a limited duration of protection and the need for frequent booster injections. Moreover, trace amounts of LF, EF, and probably other components are likely to have contributed to the efficacy of the vaccine in the reported studies. For instance, AVA provides partial protection in a guinea pig model of inhalational anthrax, whereas a recombinant anthrax PA from B. anthracis (rPA)-based vaccine elicits no protection (53). Furthermore, PA-based vaccines may confer variable protection against different B. anthracis strains and isolates, and large differences in the level of protection afforded are observed between animal species. These limitations have stimulated interest in the development of improved anthrax vaccines. The data discussed above suggest that other antigens in addition to PA are required for full protection.
The aim of the present study was to optimize the PA-FIS vaccine immunization protocol so as to elicit protection against inhalational anthrax in an experimental model of lung infection. We assessed the systemic and mucosal immune response to PA-FIS in mice and guinea pigs, immunized either through the s.c. or the intranasal (i.n.) route or both. Second, we assessed the protection afforded in an experimental model of inhalational anthrax of mice and guinea pigs infected by nasal instillation or an aerosol.
MATERIALS AND METHODS
Bacterial strains.
The spore stocks of the B. anthracis clinical isolate 9602 (5) and isolate 17JB were from the Institut Pasteur de Paris collection.
Preparation of B. anthracis spores.
Spores of B. anthracis strains were prepared as previously described (39, 58). RPLC2 spores used in vaccine formulations were purified by differential centrifugation through a Radioselectan gradient as previously described (74), inactivated overnight in 4% paraformaldehyde, washed, and stored in deionized water. All spore stocks were stored at 4°C until use.
Animals.
Female Swiss mice were obtained from the Centre d'Elevage Janvier (Le Genest-St-Isle, France) and used (first immunization) when 8 weeks old (25 to 30 g). Female Hartley guinea pigs obtained from Charles River Laboratories (l'Arbresle, France) were used when 3 to 4 weeks old (250 to 300 g).
All animals were cared for according to the guidelines of the European convention for the protection of vertebrate animals used for experimental and other scientific purposes (ETS 123). Experiments were conducted in compliance with the principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council of the USA (1996 edition). All experimental protocols were approved by the CRSSA Animal Use Committee.
For protection studies all animals were kept in an animal biosafety level 3 containment area.
Immunization of mice and guinea pigs.
The vaccine consists of a mix of rPA (List Biological Laboratories, Inc., Campbell, CA) and FIS from the previously described RPLC2 derivative of the Sterne strain of B. anthracis (11). rPA was given at a dose of 10 μg to mice and 30 μg to guinea pigs. FIS was used at a dose of 108 spores per injection as previously described (10). Immunization was performed through the s.c. route with 0.3% aluminum hydroxide gel (Sigma-Aldrich, St. Louis, MO) as an adjuvant and/or by nasal instillation (i.n.) with 5 μg of cholera toxin (List Biological Laboratories) as an adjuvant. Mice immunized through the s.c. route were given 200 μl of the vaccine suspension. For nasal immunization, mice were given a total volume of 50 μl, with 25 μl placed into each nare. Guinea pigs were immunized with a volume of 1 ml through the s.c. route and 50 μl per nostril through the i.n. route. All animals were lightly anesthetized with O2-halothane before nasal immunization. Animals were given one or two booster injections at 15-day intervals as appropriate. Control animals were immunized with adjuvant only.
i.n. challenge of animals.
Mice were lightly anesthetized with O2-halothane, and 25 μl of the challenge spore suspension was deposited on each nare for inhalation into the lungs. Guinea pigs were challenged in the same way with a 50-μl inoculum per nostril. Mice and guinea pigs were observed until all died or for 3 weeks postinfection.
The spore load in the lung was determined at time zero (within 10 min of challenge). Mice and guinea pigs were euthanized by O2-CO2 inhalation. The lungs were dissected aseptically and homogenized in either 3 ml (mouse) or 6 ml (guinea pig) of sterile saline in a tissue homogenizer (Potter cell grinder) on ice. Aliquots (200 μl) of 10-fold serial dilutions of the homogenate were plated on brain heart infusion agar (Difco/Becton Dickinson France, Pont de Claix, France) and incubated at 37°C for 24 h. Colonies were counted, and the bacterial load is expressed as the total number of B. anthracis CFU per organ.
LD50 values were determined by the method of Reed and Muench (62).
Aerosol challenge of guinea pigs.
Animals were exposed for 30 min to a muzzle-only aerosol (Ysebaert, Frépillon, France) containing B. anthracis 9602 spores. An aqueous suspension containing 2.9 × 107 spores per ml was aerosolized. The actual inhaled dose was 2.7 × 105 spores, corresponding to approximately 75 LD50, as assessed by counting the bacteria in the lungs of two control animals.
Immunoassays for anti-PA and antispore antibody in sera and lung mucosal secretions.
Anti-PA and antispore total immunoglobulin G (IgG) were measured by enzyme-linked immunosorbent assay (ELISA) in the sera and bronchoalveolar lavage (BAL) fluids from uninfected animals, 3 weeks after the last booster immunization. Blood was drawn by cardiac puncture from animals deeply anesthetized with O2-halothane. After euthanasia, BAL was performed with 5 ml (guinea pigs) or 1 ml (mice) of sterile phosphate-buffered saline (PBS) containing protease inhibitor.
Anti-PA ELISA was performed as previously described (59). Briefly, 96-well microplates (Nunc Maxisorb immunoplate) were coated with rPA (200 ng/well) overnight at 4°C, washed three times with 0.1% Tween in PBS, and blocked by incubation for 60 min with 2% bovine serum albumin (BSA) in PBS at room temperature. Then, serial twofold dilutions of the samples in 2% BSA-PBS were added, followed by incubation for 120 min at room temperature. After three washes with 0.1% Tween in PBS, horseradish peroxidase-labeled goat anti-mouse antibodies (Amersham Biosciences) were added, and incubation was continued for 1 h at room temperature. After three washing steps, freshly prepared 0.2% orthophenylenediamine (Sigma) and 0.03% H2O2 in 0.1 M citrate buffer (pH 5.2) were added to each well for 10 min. The reaction was stopped with 50 μl of 2 N H2SO4, and plates were read at 492 nm (against 620 nm). Titers are expressed as the log2 of the reciprocal of the dilution giving an optical density value of 0.5.
Antispore ELISA was performed as previously described (10). Briefly, 96-well microplates (Nunc Maxisorb immunoplate) were coated with FIS of the Δsap Δeag 7702 Sterne strain (107/well) and left drying overnight at 37°C. After a 15-min fixation step in 4% paraformaldehyde in PBS at room temperature, wells were washed three times with 0.1% Tween in PBS and saturated with 2% BSA in PBS at room temperature. The protocol thereafter was as for anti-PA ELISA.
Toxin-neutralizing assay.
Neutralizing assays with macrophages were performed as previously described (10). Briefly, murine macrophages (RAW 264.7) were seeded in 96-well plates (Corning) (2 × 105 cells/well) and incubated overnight at 37°C under a 5% CO2 atmosphere. PA (100 ng/ml, the 100% effective concentration) was preincubated for 1 h at 37°C under a 5% CO2 atmosphere with serial twofold dilutions of either sera or BAL fluids from immune mice or guinea pigs. The complexes were then incubated with LF (200 ng/ml) and the macrophages for 4 h at 37°C under a 5% CO2 atmosphere. Cell viability was quantified by the MTT colorimetric assay as previously described and the plates were read at 540 nm. Titers are expressed as the log2 of the reciprocal of the dilution giving 50% of the maximum optical density (viable cells without PA and LF) minus the background values (dead cells after incubation with PA and LF at a 100% effective concentration).
Statistics.
Survival curves were created by using the Kaplan-Meier method under GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA). They were compared by using the log rank test. A P value of <0.05 was considered to indicate a statistically significant difference between two curves.
Antibody titers (log10 values) were compared according to the unpaired Student t test, with a two-tailed P value, using GraphPad InStat version 2.03 for Mac. A P value of ≤0.05 was considered to indicate a statistically significant difference between two means.
RESULTS
Protection against B. anthracis i.n. challenge in s.c.-immunized guinea pigs and mice.
Immunization of guinea pigs with PA-FIS through the s.c. route elicited total protection against i.n. challenge with 50 LD50 of the highly virulent strain 9602 of B. anthracis. All vaccinated animals infected i.n. with 4.7 × 106 spores survived. All 10 animals in the control group died within 3 days of exposure (Fig. 1A).
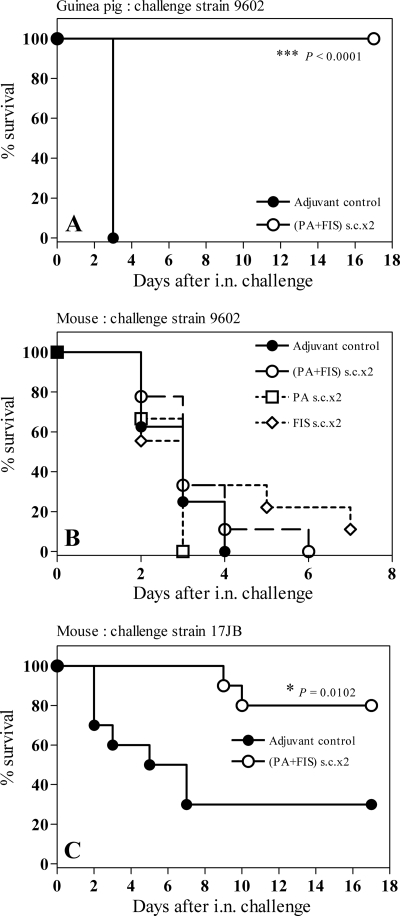
FIG. 1.
Efficacy of s.c. vaccination with PA-FIS in the guinea pig and mouse models of respiratory anthrax. (A) Guinea pigs were s.c. immunized twice with PA-FIS (s.c.×2; see Materials and Methods). Three weeks after the booster injection, all animals (six per group) were i.n. infected with 50 LD50 of B. anthracis 9602 spores; 2.6 × 106 spores were recovered from the lungs at time zero. The guinea pigs were observed for 17 days after exposure. The number of survivors in the PA-FIS group was significantly greater than that in the control group (***, P < 0.0001). (B and C) Mice were s.c. immunized twice with either PA-FIS, PA alone, or FIS alone (s.c.×2). Three weeks after the booster injection, all animals (10 per group) were either i.n. infected with 9 LD50 of B. anthracis 9602 spores and observed for 6 days after exposure (B) or i.n. infected with 8 LD50 of B. anthracis 17JB spores and observed for 17 days after exposure (C). The number of mice in the PA-FIS group surviving the 17JB challenge was significantly greater than that in the adjuvant control group (•) (*, P = 0.0102) (C).
On the contrary, s.c. immunization of mice with PA alone, FIS alone, or PA-FIS failed to elicit protection against i.n. challenge with 9 LD50 of strain 9602 of B. anthracis. All animals from the PA, FIS, and the PA-FIS groups that received 1.7 × 107 spores i.n. died within 3 to 7 days after challenge. The median survival was 3 days in the PA and PA-FIS groups (Fig. 1B).
However, s.c. immunization of mice with PA-FIS elicited partial protection against i.n. challenge with 8 LD50 of the B. anthracis strain 17JB (intermediate virulence, pXO2+, pXO1+). Eight of ten vaccinated mice infected i.n. with 2 × 107 spores survived, whereas seven of ten control animals died within 7 days of challenge. The median survival (the time by which half the subjects have died) was 6 days for control mice and too long to be determined for vaccinated mice (Fig. 1C).
These results show that s.c. immunization with PA-FIS elicits total protection of guinea pigs but not mice against lethal i.n. challenge with the highly virulent 9602 strain.
Increased survival after i.n. challenge with B. anthracis spores in mice immunized i.n. with PA-FIS.
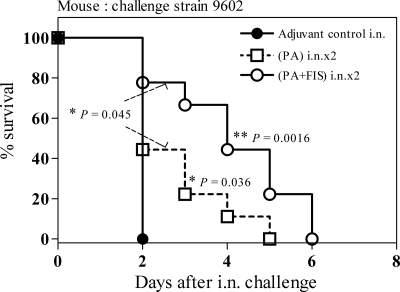
With the aim of improving the protective immune response of mice against pulmonary anthrax, we immunized them through the i.n. route. When mice were i.n. immunized (two i.n. immunizations administered 15 days apart [i.n.×2]) and then challenged i.n. with spores of the 9602 strain, PA-FIS resulted in longer survival than PA alone (*, P = 0.045) and than that of mice in the i.n. control group (*, P = 0.0016). All animals of the PA-FIS group died within 6 days of i.n. challenge with 1.75 × 107 spores, whereas mice of the control and PA groups died within 2 and 5 days, respectively. The median survival was 2 days in the control group and 4 days in the PA-FIS group (Fig. 2).
FIG. 2.
Efficacy of i.n. vaccination with PA-FIS in the mouse model of respiratory anthrax. Mice were immunized with either PA-FIS or PA alone through the i.n. route (i.n.×2). Three weeks after the booster injection all animals (eight per control group, nine per assay group) were infected i.n. with 9 LD50 of B. anthracis 9602 spores. The mice were observed for 6 days after exposure. The number of mice in the PA-FIS group surviving the challenge was significantly greater than that in either the control group (**, P = 0.0016) or the PA group (*, P = 0.045). The number of mice in the PA group surviving the challenge was significantly greater than that in the control group (*, P = 0.036).
Increasing the number of i.n. booster injections to two (PA-FIS; i.n.×3) did not increase protection (data not shown).
These results show that, contrary to s.c. immunization, i.n. immunization with PA-FIS delays the mortality of mice i.n. challenged with the highly virulent 9602 strain, compared to nonvaccinated control animals.
Comparison of antibody production in lung mucosal secretions and sera between s.c. and i.n. immunization in mice and guinea pigs.
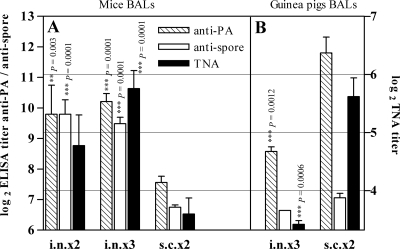
To assess the beneficial effects of nasal immunization, we studied the immune response in lung mucosal secretions from both mice and guinea pigs. Anti-PA and anti-spore antibody titers elicited by PA-FIS were measured in the BAL fluids from immunized animals. After immunization of mice given PA-FIS either i.n.×2 or i.n.×3, the mean titer of total IgG anti-PA in BAL fluid was 1,450; this was seven times greater than the titer after s.c.×2 immunization (titer = 210). Similarly, the total IgG antispore titer was significantly higher after i.n.×3 (titer = 795) or i.n.×2 immunization (titer = 1,034) than after s.c.×2 immunization (titer = 110). The toxin neutralizing antibody (TNA) titer was significantly higher after i.n.×3 immunization (titer = 71) than after s.c.×2 immunization (titer = 18) (Fig. 3A).
FIG. 3.
Comparison of antibody production in lung mucosal secretions between s.c. and i.n. immunization in mice and guinea pigs. Animals were immunized with PA-FIS either s.c. (s.c.×2) or i.n. (i.n.×2 or i.n.×3). Three weeks after the booster injection, BAL was performed on at least two animals from each experimental group. The titers of anti-PA and antispore total IgG were determined in duplicate by ELISA (left y axis). Anti-PA neutralizing antibody titers were determined by the TNA assay (TNA, right y axis) as described in Materials and Methods. The data reported represent means of two independent experiments, except for the i.n.×3 guinea pig group (one experiment involving four animals). Bars represent means ± the standard deviations of the log2 values of the titers measured. For each antibody, an unpaired Student t test was used to compare titers obtained after either i.n.×3 or i.n.×2 immunization to those after s.c.×2 immunization. A P value of ≤0.05 was considered to indicate a statistically significant difference between the means. According to the P value, the difference was significant (*), very significant (**), or extremely significant (***).
Conversely, both anti-PA and TNA titers were significantly higher in the BAL fluids from guinea pigs immunized s.c. than i.n. Total IgG anti-PA antibody titers were 12 times lower in the BAL fluids from i.n.×3-immunized guinea pigs (titer = 388) than s.c.×2-immunized animals (titer = 4,567); similarly, the TNA titer was five times lower (i.n. titer = 11 versus s.c. titer = 56; Fig. 3B). Furthermore, anti-PA (**, P = 0.0028), antispore (***, P = 0.0001), and TNA (**, P = 0.0015) titers were also significantly higher in the sera from s.c.×2-immunized guinea pigs than in the sera from i.n.×3-immunized animals (see Table 2).
TABLE 2.
Total IgG anti-PA and antispore antibody responses to either PA-FIS or PA alone in the sera from immunized mice and guinea pigsa
| Animal model | Antigen(s) | Immunization protocol | No. of animals | Anti-PA antibody titer
|
Antispore antibody titer
|
||||
|---|---|---|---|---|---|---|---|---|---|
| ELISA
|
TNAb
|
||||||||
| Median ± SD | P | Median ± SD | P | Median ± SD | P | ||||
| Mouse | PA+FIS | (s.c.+i.n.)×3 | 24 | 17.85 ± 1.07 | 14.75 ± 1.04 | 14.03 ± 1.23 | |||
| PA | (s.c.+i.n.)×3 | 4 | 18.08 ± 0.44 | NS | 15.08 ± 0.27 | NS | |||
| PA+FIS | i.n.×2 | 4 | 16.40 ± 1.10 | 0.0186* | 12.79 ± 0.54 | 0.0012† | 13.23 ± 0.78 | NS | |
| PA+FIS | i.n.×3 | 8 | 16.86 ± 1.92 | NS | 13.66 ± 0.99 | 0.0148* | 13.40 ± 1.43 | NS | |
| PA+FIS | s.c.×2 | 12 | 17.95 ± 1.10 | NS | 11.64 ± 1.75 | 0.0001‡ | 13.10 ± 1.45 | NS | |
| Guinea pig | PA+FIS | (s.c.+i.n.)×3 | 7 | 18.76 ± 0.10 | 14.31 ± 0.45 | 16.14 ± 0.49 | |||
| PA | (s.c.+i.n.)×3 | 2 | 17.43 ± 1.49 | 0.0224* | 13.24 ± 0.19 | 0.0160* | |||
| PA+FIS | i.n.×3 | 4 | 15.83 ± 0.86 | 0.0001‡ | 10.61 ± 0.74 | 0.0001‡ | 11.02 ± 0.59 | 0.0001‡ | |
| PA+FIS | s.c.×2 | 6 | 18.65 ± 1.12 | NS | 14.16 ± 1.35 | NS | 14.31 ± 0.36 | 0.0001‡ | |
In each column of values (ELISA anti-PA, anti-PA-TNA, and ELISA antispore), an unpaired Student t test (5%, two-tailed P value) was used to compare the medians obtained with each of the protocols (rows) to the median of PA-FIS [(s.c.+i.n.)×3] in the same column. A P value of ≤0.05 was considered to indicate a statistically significant difference between the means. According to the P value, the difference was significant (*), very significant (†), extremely significant (‡), or not significant (NS). Median ± SD values are log2 ± the standard deviation. Antibody titers were measured in the sera from immunized animals as described in Materials and Methods.
TNA, lethal TNA titer.
In short, delayed mortality of i.n.-immunized mice (Fig. 2) correlated with greater specific antibody production in lung mucosal secretions than in s.c.-immunized mice (Fig. 3A). In contrast to mice, guinea pigs are well protected by s.c. immunization (Fig. 1), which elicits a high bronchial immune response that is not improved by i.n. immunization (Fig. 3B).
Effects of double immunization (s.c. plus i.n.) on mucosal and systemic responses to PA-FIS in mice and guinea pigs.
The findings described above suggest that it may be beneficial to combine s.c. and i.n. routes of immunization, eliciting both systemic and mucosal responses for a species-independent optimized protective immunization protocol. Combining s.c. and i.n. routes of immunization, in a (s.c.+i.n.)×3 schedule, resulted in significantly higher titers of both the anti-PA and antispore antibodies in mouse BAL fluids than after s.c.×2 immunization (Table 1). No such difference was observed in mouse sera for either anti-PA or antispore titers (Table 2).
TABLE 1.
Total IgG anti-PA and antispore antibody responses to either PA-FIS or PA alone in the lung mucosal secretions from immunized mice and guinea pigsa
| Animal model | Antigen(s) | Immunization protocol | No. of animals | Anti-PA antibody titer
|
Antispore antibody titer
|
||||
|---|---|---|---|---|---|---|---|---|---|
| ELISA
|
TNAb
|
||||||||
| Median ± SD | P | Median ± SD | P | Median ± SD | P | ||||
| Mouse | PA+FIS | (s.c.+i.n.)×3 | 19 | 11.70 ± 1.22 | 6.64 ± 1.52 | 9.24 ± 1.42 | |||
| PA+FIS | i.n.×2 | 4 | 9.79 ± 1.91 | 0.0173* | 4.78 ± 1.05 | 0.0304* | 9.79 ± 0.95 | NS | |
| PA+FIS | i.n.×3 | 10 | 10.21 ± 0.83 | 0.0019† | 5.76 ± 0.98 | NS | 9.49 ± 0.67 | NS | |
| PA+FIS | s.c.×2 | 12 | 7.56 ± 0.70 | 0.0001‡ | 3.60 ± 0.96 | 0.0001‡ | 6.75 ± 0.26 | 0.0001‡ | |
| Guinea pig | PA+FIS | (s.c.+i.n.)×3 | 6 | 12.24 ± 0.54 | 5.22 ± 0.97 | 7.35 ± 0.39 | |||
| PA | (s.c.+i.n.)×3 | 2 | 11.26 ± 0.41 | NS | 5.11 ± 1.71 | NS | |||
| PA+FIS | i.n.×3 | 4 | 8.57 ± 0.31 | 0.0001‡ | 3.42 ± 0.13 | 0.0067† | 6.71 ± 0.08 | 0.0136* | |
| PA+FIS | s.c.×2 | 6 | 11.80 ± 1.28 | NS | 5.62 ± 0.79 | NS | 7.06 ± 0.37 | NS | |
In each column of values (ELISA anti-PA, anti-PA TNA, and ELISA antispore), an unpaired Student t test (5%, two-tailed P value) was used to compare the median obtained with each of the protocols (rows) to the median of PA-FIS [(s.c.+i.n.)×3] in the same column. A P value of ≤0.05 was considered to indicate a statistically significant difference. According to the P value, the difference was either significant (*), very significant (†), extremely significant (‡), or not significant (NS). Median ± SD values are log2 ± the standard deviation. Antibody titers were measured in the BAL fluids from immunized animals as described in Materials and Methods.
TNA, lethal TNA titer.
Nevertheless, the TNA titer was significantly higher in both sera (Table 2) and BAL fluids (Table 1) in (s.c.+i.n.)×3-immunized mice than in s.c.×2-immunized mice.
There was no significant difference in the titers of anti-PA and antispore IgA antibodies in mouse BAL fluids after (s.c.+i.n.)×3 and i.n.×3 immunization (data not shown).
In contrast to mice, the optimized (s.c.+i.n.)×3 immunization protocol with PA-FIS did not elicit a significantly higher mucosal response than s.c.×2 immunization in guinea pigs. None of the anti-PA, antispore, and TNA titers were significantly different in the BAL fluid (Table 1). Nevertheless, and again unlike in mice, anti-PA, antispore, and TNA titers in guinea pig BAL fluids (Table 1) and sera (Table 2) were very significantly higher after (s.c.+i.n.)×3 immunization than after i.n.×3 immunization.
In brief, s.c.-i.n. immunization of mice with PA-FIS led to significantly greater anti-PA titers and lethal toxin neutralization activity in BAL fluid than either s.c. immunization or i.n. immunization alone. Antibody titers in s.c.-plus-i.n.-immunized guinea pigs were significantly greater than titers of i.n.-immunized but not s.c.-immunized guinea pigs, thus confirming the above observations.
The (s.c.+i.n.)×3 optimized immunization protocol with PA-FIS elicits protection against inhalational anthrax. (i) Protection of mice challenged i.n.
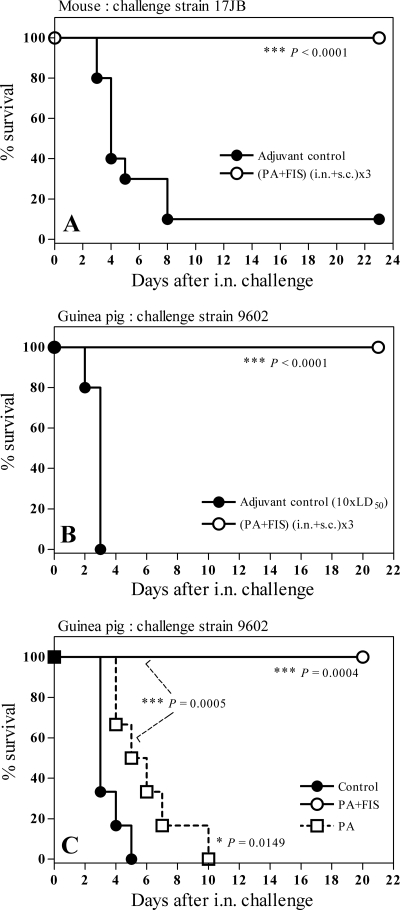
The optimized immunization protocol described above, i.e., (s.c.+i.n.)×3, significantly delayed the death of mice infected i.n. with 10 LD50 of strain 9602 with respect to control mice (*, P = 0.042; data not shown). Furthermore, the (s.c.+i.n.)×3 immunization elicited 100% protection in mice infected i.n. with 33 LD50 of spores from strain 17JB (Fig. 4A); nine of the ten control mice i.n. infected with 8.30 × 107 spores died within 8 days, whereas all ten mice in the PA-FIS group survived (Fig. 4A).
FIG. 4.
Protection of mice and guinea pigs after a double immunization with PA-FIS. (A) Mice were immunized both s.c. and i.n., (s.c.+i.n.)×3, by the optimized protocol. Three weeks after the second booster injection all animals (10 per group) were i.n. infected with 33 LD50 (8.3 × 107) of B. anthracis 17JB spores. Mice were observed for 23 days after exposure. The number of mice in the PA-FIS group surviving the challenge was statistically greater than that in the adjuvant control group (***, P < 0.0001). Guinea pigs were immunized with either PA-FIS or PA alone both s.c. and i.n.. i.e., (s.c.+i.n.)×3. Three (B) or two (C) weeks after the booster injection, all animals (six per group) were i.n. infected with either 100 LD50 (8 × 106) (B) or 5 LD50 (4 × 105) (C) of B. anthracis 9602 spores. Guinea pigs were observed for 21 days after exposure. In both cases, the number of guinea pigs in the PA-FIS group surviving the challenge was significantly higher than that in the adjuvant control group (***, P < 0.001 [B]; ***, P = 0.0004 [C]). In the experimental results shown in panel C, the number of animals in the PA-FIS group surviving the challenge was significantly higher than that in the PA group (***, P = 0.0005). Also, in panel C the number of survivors in the PA group was significantly higher than that in the adjuvant control group (*, P = 0.0149).
(ii) Protection of guinea pigs against i.n. challenge.
The optimized protocol completely protected guinea pigs against i.n. challenge with 100 LD50 of B. anthracis 9602 spores (Fig. 4B), whereas PA alone provided no protection against doses as low as 5 LD50 (Fig. 4C). All six control guinea pigs challenged i.n. with 8 × 106 spores of B. anthracis 9602 spores died within 3 days, whereas all guinea pigs immunized (s.c.+i.n.)×3 with PA-FIS survived (Fig. 4B). In another experiment, all six guinea pigs immunized (s.c.+i.n.)×3 with PA alone, and challenged i.n. with 4 × 105 spores of B. anthracis 9602 spores, died within 5 days, whereas all guinea pigs immunized (s.c.+i.n.)×3 with PA-FIS survived. The median survival was 3 days in the control group and 5.5 days in the PA group (Fig. 4C).
(iii) Protection of guinea pigs against aerosol challenge.
Similar to the findings for i.n. challenge, the optimized protocol elicited protection of guinea pigs against aerosol challenge with B. anthracis 9602 spores. PA alone did not provide protection and caused only a short but significant delay of mortality. Five of six guinea pigs immunized (s.c.+i.n.)×3 with PA-FIS survived aerosol challenge with 75 LD50 of B. anthracis 9602 spores, whereas all six animals from the PA group died within 7 days of exposure. The median survival was 2 days in the control group and 5.5 days in the PA group (Fig. 5).
FIG. 5.
Efficacy of PA-FIS against aerosol challenge in the guinea pig model of inhalational anthrax. Guinea pigs were immunized with either PA-FIS or PA alone both s.c. and i.n., i.e., (s.c.+i.n.)×3. Three weeks after the second booster injection all animals (six per group) were exposed to a muzzle-only aerosol challenge with B. anthracis 9602 spores. An aqueous suspension containing 2.9 × 107 spores per ml was aerosolized. The actual inhaled dose was 2.7 × 105 spores, corresponding to approximately 75 LD50, as determined by bacterial counting in the lungs of two control animals at time zero. Guinea pigs were observed for 21 days after exposure. The number of guinea pigs in the PA-FIS group surviving the challenge was significantly higher than those in both the adjuvant control group (***, P = 0.0005) and the PA group (***, P = 0.0008). The number of survivors in the PA group was significantly higher than that in the adjuvant control group (**, P = 0.0013).
To sum up, although s.c. immunization is protective in guinea pigs, a double s.c.-i.n. immunization with PA-FIS is the best way of eliciting total protection in both mice and guinea pigs against experimental inhalational anthrax.
DISCUSSION
The potential use of B. anthracis spores for biowarfare has led to the need for a safe and efficient vaccine to protect humans against inhalational anthrax. Current licensed anthrax vaccines, based on PA, have serious drawbacks: they confer protection for only a limited duration and require frequent booster injections to maintain sufficient immunity. Furthermore, such PA-based vaccines, acting on toxins, are less effective than live attenuated vaccines, suggesting that additional antigens may make a significant contribution to protective immunity, especially by containing septicemia. Coimmunization with PA and FIS has previously been shown to confer protection against experimental cutaneous anthrax, whereas immunization with either PA alone or FIS alone was not protective in mice and only partially protective in guinea pigs (10). Here, we assessed the efficacy of the PA-FIS vaccine candidate against pulmonary anthrax in experimental rodent models of inhalational anthrax.
s.c. immunization with PA-FIS elicited 100% protection of guinea pigs against i.n. challenge with 50 LD50 of spores of the virulent strain 9602 of B. anthracis but failed to elicit any protection of mice against i.n. challenge with 9 LD50 of the same strain.
The difference in vaccine efficacy between mice and guinea pigs may be partly due to the γDPGA capsule being the primary virulence factor in mice (87) but less important in guinea pigs (and also rabbits and nonhuman primates).
Indeed, i.n. challenge with the nontoxinogenic 9602P strain, a derivative of 9602 carrying a pagA gene deletion, the virulence of which is due solely to its multiplication (10), was as lethal as 9602 i.n. challenge in naive mice (Y. P. Gauthier, unpublished results). PA-FIS immunization similarly failed to protect mice against i.n. challenge with 9602P, indicating that septicemia was not prevented (Gauthier, unpublished). Other studies have demonstrated the dominance of the capsule component as a virulence factor in mice (22): most mouse strains are very sensitive to infection by even low doses of toxin-producing encapsulated stains of B. anthracis (30, 86). However, susceptibility to the toxin component varies greatly between mouse strains, from resistant (e.g., CBA/J, BALB/cJ, and C57L/J mice) to susceptible (e.g., A/J and DBA/2J mice) to infection with the unencapsulated toxin-producing Sterne strain (86). These observations demonstrate the diversity of the protective activity of PA-based vaccines in mice, varying from moderate in CBA/J mice to very weak in A/J mice (45, 85). Thus, vaccine efficacy may vary greatly depending on the mouse strain, the B. anthracis challenge strain and the route of infection (30). More generally, there is no direct correlation between anti-PA titers and protection in mice or hamsters (27, 30, 45, 85), whereas both the anti-PA IgG titer and toxin neutralization correlate with protection in rabbits (50, 61). Brossier et al. demonstrated that s.c. immunization with PA alone is unable to protect either Swiss mice or guinea pigs against s.c. lethal challenge with the encapsulated nontoxinogenic strain 9602P, whereas immunization with FIS alone elicits partial protection of mice (50% survival) and total protection (100% survival) of guinea pigs against this strain (10). These findings and our results highlight the large differences between mice and guinea pigs with regard to their ability to raise protective immunity against B. anthracis. Nevertheless, we observed that s.c. immunization with PA-FIS partially protected mice against i.n. challenge with B. anthracis strain 17JB, in contrast to its inefficacy against challenge with strain 9602. Brossier et al. showed that s.c. immunization with PA-FIS elicits total protection of Swiss mice against s.c. challenge with 17JB, whereas mice immunized with either PA or FIS alone were only partially protected (10).
In an effort to improve the protective immune response against pulmonary anthrax, we immunized mice and guinea pigs with PA-FIS via the i.n. route to stimulate a mucosal immune response. Administration of vaccines via the nasal route can, unlike parenteral immunization, efficiently induce both mucosal and systemic antibody responses against infectious diseases. This is a consequence of the large amounts of associated lymphoid tissue and antigen-presenting cells in the nasal mucosa, which is also the site of pathogen entry (56).
Mice immunized with PA-FIS through the i.n. route (i.n.×2), but not those immunized s.c., survived significantly longer after i.n. challenge with strain 9602 than did both mice immunized with PA alone and control mice. This suggests potentially synergistic protective activity of antispore and anti-PA immune responses against anthrax spore infection at mucosal surfaces.
We measured anti-PA and antispore antibody titers elicited by PA-FIS in lung mucosal secretions (BAL fluids) from both mice and guinea pigs. As expected, total anti-PA and antispore IgG values for mouse BAL fluid were significantly higher in i.n.-immunized mice than in s.c.-immunized animals. Also, TNA titers were significantly greater in i.n.×3-immunized animals than in s.c.×2-immunized mice.
Surprisingly, in contrast to mice, both anti-PA and TNA titers were significantly higher in BAL from s.c.-immunized guinea pigs than in BAL from i.n.-immunized animals.
Nasal immunization of mice with either rPA or purified PA coadministered with either CT (8) or other adjuvants (7, 29, 34, 71) yields high levels of both plasma and mucosal anti-PA, as well as neutralizing anti-PA. Anti-PA and antispore responses in lung mucosal secretions of mice and guinea pigs immunized either i.n. or s.c. have not previously been compared. Oral vaccination (mucosal immunization) of guinea pigs with a nontoxinogenic nonencapsulated B. anthracis live spore vaccine expressing rPA has been reported to elicit very high specific humoral and mucosal responses and generate long-lasting protective immunity (2). Nevertheless, the proportion of guinea pigs that develop measurable anti-PA antibodies and protective immunity is lower after per os immunization than after s.c. immunization. Furthermore, although s.c. vaccination with effective spore doses elicits protection of all immunized guinea pigs against a high lethal challenge, oral vaccination is consistently only partially protective (2, 16). A clinical study showed that i.n. administration of live attenuated measles vaccine to humans elicited immune responses that were very much lower than those generated by s.c. administration (69); this observation is in agreement with our observations.
To sum up, i.n. immunization of mice elicited both a better mucosal immune response to PA-FIS and better protection against inhalational anthrax than could be obtained with s.c. immunization. In contrast, s.c. immunization was more effective than nasal immunization in guinea pigs as assessed by either the specific antibody response or protection. These observations show that the induction of an efficient mucosal response is dependent on the animal species tested and suggest that it may be beneficial to combine the two routes of immunization for a species-independent optimized protective immunization protocol.
Combining both the s.c. and the i.n. routes of immunization, the optimized (s.c.+i.n.)×3 PA-FIS immunization schedule elicited a significantly higher anti-PA and antispore mucosal response in mice than either s.c.×2 or i.n.×3 immunization. No such difference was observed in mouse sera. However, this optimized immunization did not elicit a better mucosal response than s.c.×2 immunization in guinea pigs. Unlike the findings for mice, anti-PA, antispore, and TNA titers were significantly higher in the BAL fluids and sera from (s.c.+i.n.)×3-immunized guinea pigs than in i.n.×3-immunized animals. These various observations demonstrate the differences between mice and guinea pigs regarding the mucosal immune response to PA-FIS after mucosal and s.c. immunization.
Nevertheless, despite the differences between mice and guinea pigs regarding the comparative efficacy of i.n. and s.c. immunization, the optimized (s.c.+i.n.)×3 immunization with PA-FIS elicited 100% protection in both species against experimental inhalational anthrax with B. anthracis spores of strains 17JB and 9602, respectively. Flick-Smith et al. reported that, despite differences in rPA-specific antibody titers, A/J mice vaccinated with rPA by a combined intramuscular (i.m.-i.n.) schedule were more consistently protected against both injected and aerosol lethal challenges with B. anthracis STI spores than after either i.m. or i.n. immunization alone (29). These results and data emphasize the importance of stimulating both the mucosal and systemic immune systems to elicit full protection against inhalational anthrax, because the respiratory tract epithelium is the initial site of infection after inhalation of spores.
Despite the combined s.c.-i.n. schedule, alhydrogel-adjuvanted PA alone failed to elicit protection of guinea pigs against i.n. or aerosol challenge with B. anthracis 9602.
PA-based vaccines are more effective in guinea pigs than in mice (7, 10, 45). However, vaccination of guinea pigs with PA alone generally confers only limited protection against aerosol or i.n. lethal challenge with B. anthracis spores (7, 41). Furthermore, guinea pigs are commonly described as being difficult to protect consistently with alum-adjuvanted PA (26, 41, 45, 53). Nevertheless, in our study guinea pigs immunized with the alum-containing PA-FIS vaccine were fully protected against aerosol and i.n. lethal challenge with 75 and 100 LD50, respectively, of the highly virulent B. anthracis strain 9602. Protection was elicited by combined s.c. plus i.n. immunization, as well as by s.c. immunization alone. It has been reported that immunization with FIS alone elicits total protection of guinea pigs against s.c. infection with the PA-deficient strain 9602P (10), indicating that the sensitivity of guinea pigs to anthrax disease is not solely due to toxemia and that antibodies to PA are not sufficient to confer protection in this species. This suggests that antibodies specific to other B. anthracis antigens (spore or others), stimulation of cell-mediated immunity, or both are needed for protection.
Marcus et al. demonstrated that primary immunization with threshold levels of PA could induce a long-term T-cell immunological memory response in guinea pigs without inducing detectable anti-PA antibodies. However, protection is attained only after boosting, concomitant with the detection of neutralizing antibodies in the circulation (52). Other authors have confirmed that, despite the absence of correlation between protective immunity and anti-PA titers determined by ELISA in the guinea pig model, neutralizing antibodies to PA are a major component of the protective immunity of guinea pig against anthrax (63). Nevertheless, vaccination of guinea pigs with PA combined with Ribi adjuvant (MPL-TDM-CWS) is more effective against both aerosol (53) and parenteral (i.m.) (45) challenge with the Ames strain than immunization with either PA adsorbed onto Al(OH)3 (45, 53) or the licensed human anthrax vaccine (45), thereby suggesting the possible role of cell-mediated immunity in protection against anthrax (53). Similar observations have been reported for mice (85).
In conclusion, as demonstrated for mice (36, 45, 53, 85), although the production of antibody is necessary, a cell-mediated immune response may be required for protection of guinea pigs against anthrax infection. The notion that spore antigen(s) exerts a protective role through eliciting a cell-mediated immune response in guinea pig is strengthened by recent reports that mouse immunization with FIS induces a protective cellular immune response by gamma interferon-producing CD4 T lymphocytes, whereas humoral immunity is not protective (36).
In summary, our study clearly shows that the addition of killed spores to PA antigen greatly enhances the protective efficacy of the PA-based vaccine and elicits total protection against inhalational anthrax. Our findings emphasize the importance of stimulating both the mucosal and systemic immune systems to elicit full protection against inhalational anthrax: the best protection of mice against inhalational anthrax was elicited with a combined s.c.-i.n. immunization schedule and increased protection of mice correlated with higher antispore, anti-PA, and TNA titers in lung mucosal secretions than those elicited by s.c. immunization alone. Nevertheless, no such clear correlation was observed in guinea pigs, and s.c. immunization alone is strongly protective in this model. These findings and other reported observations demonstrate that there is no absolute correlation between specific antibody titers and protection and implicate cell-mediated immunity in protection against inhalational anthrax.
Acknowledgments
This study was supported by a grant from the French Ministry of Defense (Livre Rouge/EMA).
We thank Françoise Desor for expert production and purification of spores and Emmanuelle Cadio for performing ELISAs and neutralization assays. We are indebted to David Bois and Didier Riou for taking care of the animals and for assistance with animal experiments.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 29 December 2008.
This work is dedicated to the memory of Martine Levy.
REFERENCES
- 1.Adone, R., P. Pasquali, G. La Rosa, C. Marianelli, M. Muscillo, A. Fasanella, M. Francia, and F. Ciuchini. 2002. Sequence analysis of the genes encoding for the major virulence factors of Bacillus anthracis vaccine strain “Carbosap.” J. Appl. Microbiol. 93117-121. [DOI] [PubMed] [Google Scholar]
- 2.Aloni-Grinstein, R., O. Gat, Z. Altboum, B. Velan, S. Cohen, and A. Shafferman. 2005. Oral spore vaccine based on live attenuated nontoxinogenic Bacillus anthracis expressing recombinant mutant protective antigen. Infect. Immun. 734043-4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banks, D. J., M. Barnajian, F. J. Maldonado-Arocho, A. M. Sanchez, and K. A. Bradley. 2005. Anthrax toxin receptor 2 mediates Bacillus anthracis killing of macrophages following spore challenge. Cell. Microbiol. 71173-1185. [DOI] [PubMed] [Google Scholar]
- 4.Barnard, J. P., and A. M. Friedlander. 1999. Vaccination against anthrax with attenuated recombinant strains of Bacillus anthracis that produce protective antigen. Infect. Immun. 67562-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthier, M., J. L. Fauchere, J. Perrin, B. Grignon, and D. Oriot. 1996. Fulminant meningitis due to Bacillus anthracis in 11-year-old girl during Ramadan. Lancet 347828. [DOI] [PubMed] [Google Scholar]
- 6.Bhalla, D. K., and D. B. Warheit. 2004. Biological agents with potential for misuse: a historical perspective and defensive measures. Toxicol. Appl. Pharmacol. 19971-84. [DOI] [PubMed] [Google Scholar]
- 7.Bielinska, A. U., K. W. Janczak, J. J. Landers, P. Makidon, L. E. Sower, J. W. Peterson, and J. R. Baker, Jr. 2007. Mucosal immunization with a novel nanoemulsion-based recombinant anthrax protective antigen vaccine protects against Bacillus anthracis spore challenge. Infect. Immun. 754020-4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyaka, P. N., A. Tafaro, R. Fischer, S. H. Leppla, K. Fujihashi, and J. R. McGhee. 2003. Effective mucosal immunity to anthrax: neutralizing antibodies and Th cell responses following nasal immunization with protective antigen. J. Immunol. 1705636-5643. [DOI] [PubMed] [Google Scholar]
- 9.Brittingham, K. C., G. Ruthel, R. G. Panchal, C. L. Fuller, W. J. Ribot, T. A. Hoover, H. A. Young, A. O. Anderson, and S. Bavari. 2005. Dendritic cells endocytose Bacillus anthracis spores: implications for anthrax pathogenesis. J. Immunol. 1745545-5552. [DOI] [PubMed] [Google Scholar]
- 10.Brossier, F., M. Levy, and M. Mock. 2002. Anthrax spores make an essential contribution to vaccine efficacy. Infect. Immun. 70661-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brossier, F., M. Weber-Levy, M. Mock, and J. C. Sirard. 2000. Role of toxin functional domains in anthrax pathogenesis. Infect. Immun. 681781-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Candela, T., and A. Fouet. 2005. Bacillus anthracis CapD, belonging to the gamma-glutamyltranspeptidase family, is required for the covalent anchoring of capsule to peptidoglycan. Mol. Microbiol. 57717-726. [DOI] [PubMed] [Google Scholar]
- 13.Chabot, D. J., A. Scorpio, S. A. Tobery, S. F. Little, S. L. Norris, and A. M. Friedlander. 2004. Anthrax capsule vaccine protects against experimental infection. Vaccine 2343-47. [DOI] [PubMed] [Google Scholar]
- 14.Cleret, A., A. Quesnel-Hellmann, J. Mathieu, D. Vidal, and J. N. Tournier. 2006. Resident CD11c+ lung cells are impaired by anthrax toxins after spore infection. J. Infect. Dis. 19486-94. [DOI] [PubMed] [Google Scholar]
- 15.Cleret, A., A. Quesnel-Hellmann, A. Vallon-Eberhard, B. Verrier, S. Jung, D. Vidal, J. Mathieu, and J. N. Tournier. 2007. Lung dendritic cells rapidly mediate anthrax spore entry through the pulmonary route. J. Immunol. 1787994-8001. [DOI] [PubMed] [Google Scholar]
- 16.Cohen, S., I. Mendelson, Z. Altboum, D. Kobiler, E. Elhanany, T. Bino, M. Leitner, I. Inbar, H. Rosenberg, Y. Gozes, R. Barak, M. Fisher, C. Kronman, B. Velan, and A. Shafferman. 2000. Attenuated nontoxinogenic and nonencapsulated recombinant Bacillus anthracis spore vaccines protect against anthrax. Infect. Immun. 684549-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coker, P. R., K. L. Smith, P. F. Fellows, G. Rybachuck, K. G. Kousoulas, and M. E. Hugh-Jones. 2003. Bacillus anthracis virulence in guinea pigs vaccinated with anthrax vaccine adsorbed is linked to plasmid quantities and clonality. J. Clin. Microbiol. 411212-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Comer, J. E., C. L. Galindo, A. K. Chopra, and J. W. Peterson. 2005. GeneChip analyses of global transcriptional responses of murine macrophages to the lethal toxin of Bacillus anthracis. Infect. Immun. 731879-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cote, C. K., N. Van Rooijen, and S. L. Welkos. 2006. Roles of macrophages and neutrophils in the early host response to Bacillus anthracis spores in a mouse model of infection. Infect. Immun. 74469-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon, T. C., A. A. Fadl, T. M. Koehler, J. A. Swanson, and P. C. Hanna. 2000. Early Bacillus anthracis-macrophage interactions: intracellular survival and escape. Cell. Microbiol. 2453-463. [DOI] [PubMed] [Google Scholar]
- 21.Dixon, T. C., M. Meselson, J. Guillemin, and P. C. Hanna. 1999. Anthrax. N. Engl. J. Med. 341815-826. [DOI] [PubMed] [Google Scholar]
- 22.Drysdale, M., S. Heninger, J. Hutt, Y. Chen, C. R. Lyons, and T. M. Koehler. 2005. Capsule synthesis by Bacillus anthracis is required for dissemination in murine inhalation anthrax. EMBO J. 24221-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enkhtuya, J., K. Kawamoto, Y. Kobayashi, I. Uchida, N. Rana, and S. Makino. 2006. Significant passive protective effect against anthrax by antibody to Bacillus anthracis inactivated spores that lack two virulence plasmids. Microbiology 1523103-3110. [DOI] [PubMed] [Google Scholar]
- 24.Erwin, J. L., L. M. DaSilva, S. Bavari, S. F. Little, A. M. Friedlander, and T. C. Chanh. 2001. Macrophage-derived cell lines do not express proinflammatory cytokines after exposure to Bacillus anthracis lethal toxin. Infect. Immun. 691175-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fasanella, A., S. Losito, T. Trotta, R. Adone, S. Massa, F. Ciuchini, and D. Chiocco. 2001. Detection of anthrax vaccine virulence factors by polymerase chain reaction. Vaccine 194214-4218. [DOI] [PubMed] [Google Scholar]
- 26.Fellows, P. F., M. K. Linscott, B. E. Ivins, M. L. Pitt, C. A. Rossi, P. H. Gibbs, and A. M. Friedlander. 2001. Efficacy of a human anthrax vaccine in guinea pigs, rabbits, and rhesus macaques against challenge by Bacillus anthracis isolates of diverse geographical origin. Vaccine 193241-3247. [DOI] [PubMed] [Google Scholar]
- 27.Fellows, P. F., M. K. Linscott, S. F. Little, P. Gibbs, and B. E. Ivins. 2002. Anthrax vaccine efficacy in golden Syrian hamsters. Vaccine 201421-1424. [DOI] [PubMed] [Google Scholar]
- 28.Firoved, A. M., G. F. Miller, M. Moayeri, R. Kakkar, Y. Shen, J. F. Wiggins, E. M. McNally, W. J. Tang, and S. H. Leppla. 2005. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am. J. Pathol. 1671309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flick-Smith, H. C., J. E. Eyles, R. Hebdon, E. L. Waters, R. J. Beedham, T. J. Stagg, J. Miller, H. O. Alpar, L. W. Baillie, and E. D. Williamson. 2002. Mucosal or parenteral administration of microsphere-associated Bacillus anthracis protective antigen protects against anthrax infection in mice. Infect. Immun. 702022-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flick-Smith, H. C., E. L. Waters, N. J. Walker, J. Miller, A. J. Stagg, M. Green, and E. D. Williamson. 2005. Mouse model characterisation for anthrax vaccine development: comparison of one inbred and one outbred mouse strain. Microb. Pathog. 3833-40. [DOI] [PubMed] [Google Scholar]
- 31.Friedlander, A. M. 1999. Clinical aspects, diagnosis, and treatment of anthrax. J. Appl. Microbiol. 87303. [DOI] [PubMed] [Google Scholar]
- 32.Friedlander, A. M., P. R. Pittman, and G. W. Parker. 1999. Anthrax vaccine: evidence for safety and efficacy against inhalational anthrax. JAMA 2822104-2106. [DOI] [PubMed] [Google Scholar]
- 33.Friedlander, A. M., S. L. Welkos, and B. E. Ivins. 2002. Anthrax vaccines. Curr. Top. Microbiol. Immunol. 27133-60. [DOI] [PubMed] [Google Scholar]
- 34.Gaur, R., P. K. Gupta, A. C. Banerjea, and Y. Singh. 2002. Effect of nasal immunization with protective antigen of Bacillus anthracis on protective immune response against anthrax toxin. Vaccine 202836-2839. [DOI] [PubMed] [Google Scholar]
- 35.Gimenez, A. P., Y. Z. Wu, M. Paya, C. Delclaux, L. Touqui, and P. L. Goossens. 2004. High bactericidal efficiency of type IIa phospholipase A2 against Bacillus anthracis and inhibition of its secretion by the lethal toxin. J. Immunol. 173521-530. [DOI] [PubMed] [Google Scholar]
- 36.Glomski, I. J., J. P. Corre, M. Mock, and P. L. Goossens. 2007. Cutting edge: IFN-gamma-producing CD4 T lymphocytes mediate spore-induced immunity to capsulated Bacillus anthracis. J. Immunol. 1782646-2650. [DOI] [PubMed] [Google Scholar]
- 37.Glomski, I. J., A. Piris-Gimenez, M. Huerre, M. Mock, and P. L. Goossens. 2007. Primary involvement of pharynx and Peyer's patch in inhalational and intestinal anthrax. PLoS Pathog. 3e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guidi-Rontani, C., M. Levy, H. Ohayon, and M. Mock. 2001. Fate of germinated Bacillus anthracis spores in primary murine macrophages. Mol. Microbiol. 42931-938. [DOI] [PubMed] [Google Scholar]
- 39.Guidi-Rontani, C., M. Weber-Levy, E. Labruyere, and M. Mock. 1999. Germination of Bacillus anthracis spores within alveolar macrophages. Mol. Microbiol. 319-17. [DOI] [PubMed] [Google Scholar]
- 40.Henderson, D. W., S. Peacock, and F. C. Belton. 1956. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. J. Hyg. 5428-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivins, B., P. Fellows, L. Pitt, J. Estep, J. Farchaus, A. Friedlander, and P. Gibbs. 1995. Experimental anthrax vaccines: efficacy of adjuvants combined with protective antigen against an aerosol Bacillus anthracis spore challenge in guinea pigs. Vaccine 131779-1784. [DOI] [PubMed] [Google Scholar]
- 42.Ivins, B. E., J. W. Ezzell, Jr., J. Jemski, K. W. Hedlund, J. D. Ristroph, and S. H. Leppla. 1986. Immunization studies with attenuated strains of Bacillus anthracis. Infect. Immun. 52454-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ivins, B. E., P. F. Fellows, and G. O. Nelson. 1994. Efficacy of a standard human anthrax vaccine against Bacillus anthracis spore challenge in guinea pigs. Vaccine 12872-874. [DOI] [PubMed] [Google Scholar]
- 44.Ivins, B. E., M. L. Pitt, P. F. Fellows, J. W. Farchaus, G. E. Benner, D. M. Waag, S. F. Little, G. W. Anderson, Jr., P. H. Gibbs, and A. M. Friedlander. 1998. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine 161141-1148. [DOI] [PubMed] [Google Scholar]
- 45.Ivins, B. E., S. L. Welkos, S. F. Little, M. H. Crumrine, and G. O. Nelson. 1992. Immunization against anthrax with Bacillus anthracis protective antigen combined with adjuvants. Infect. Immun. 60662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones, W. I., Jr., F. Klein, J. S. Walker, B. G. Mahlandt, J. P. Dobbs, and R. E. Lincoln. 1967. In vivo growth and distribution of anthrax bacilli in resistant, susceptible, and immunized hosts. J. Bacteriol. 94600-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kozel, T. R., P. Thorkildson, S. Brandt, W. H. Welch, J. A. Lovchik, D. P. AuCoin, J. Vilai, and C. R. Lyons. 2007. Protective and immunochemical activities of monoclonal antibodies reactive with the Bacillus anthracis polypeptide capsule. Infect. Immun. 75152-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.La Rosa, G., M. Muscillo, M. Sali, E. De Carolis, C. Marianelli, F. Ciuchini, A. Fasanella, and R. Adone. 2006. Molecular study of genes involved in virulence regulatory pathways in Bacillus anthracis vaccine strain “Carbosap.” New Microbiol. 29307-310. [PubMed] [Google Scholar]
- 49.Little, S. F., and B. E. Ivins. 1999. Molecular pathogenesis of Bacillus anthracis infection. Microbes Infect. 1131-139. [DOI] [PubMed] [Google Scholar]
- 50.Little, S. F., B. E. Ivins, P. F. Fellows, M. L. Pitt, S. L. Norris, and G. P. Andrews. 2004. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine 22422-430. [DOI] [PubMed] [Google Scholar]
- 51.Little, S. F., and G. B. Knudson. 1986. Comparative efficacy of Bacillus anthracis live spore vaccine and protective antigen vaccine against anthrax in the guinea pig. Infect. Immun. 52509-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marcus, H., R. Danieli, E. Epstein, B. Velan, A. Shafferman, and S. Reuveny. 2004. Contribution of immunological memory to protective immunity conferred by a Bacillus anthracis protective antigen-based vaccine. Infect. Immun. 723471-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McBride, B. W., A. Mogg, J. L. Telfer, M. S. Lever, J. Miller, P. C. Turnbull, and L. Baillie. 1998. Protective efficacy of a recombinant protective antigen against Bacillus anthracis challenge and assessment of immunological markers. Vaccine 16810-817. [DOI] [PubMed] [Google Scholar]
- 54.Moayeri, M., and S. H. Leppla. 2004. The roles of anthrax toxin in pathogenesis. Curr. Opin. Microbiol. 719-24. [DOI] [PubMed] [Google Scholar]
- 55.Mourez, M. 2004. Anthrax toxins. Rev. Physiol. Biochem. Pharmacol. 152135-164. [DOI] [PubMed] [Google Scholar]
- 56.Ogra, P. L., H. Faden, and R. C. Welliver. 2001. Vaccination strategies for mucosal immune responses. Clin. Microbiol. Rev. 14430-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paccani, S. R., F. Tonello, R. Ghittoni, M. Natale, L. Muraro, M. M. D'Elios, W. J. Tang, C. Montecucco, and C. T. Baldari. 2005. Anthrax toxins suppress T lymphocyte activation by disrupting antigen receptor signaling. J. Exp. Med. 201325-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pezard, C., P. Berche, and M. Mock. 1991. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect. Immun. 593472-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pezard, C., M. Weber, J. C. Sirard, P. Berche, and M. Mock. 1995. Protective immunity induced by Bacillus anthracis toxin-deficient strains. Infect. Immun. 631369-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phipps, A. J., C. Premanandan, R. E. Barnewall, and M. D. Lairmore. 2004. Rabbit and nonhuman primate models of toxin-targeting human anthrax vaccines. Microbiol. Mol. Biol. Rev. 68617-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pitt, M. L., S. F. Little, B. E. Ivins, P. Fellows, J. Barth, J. Hewetson, P. Gibbs, M. Dertzbaugh, and A. M. Friedlander. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 194768-4773. [DOI] [PubMed] [Google Scholar]
- 62.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27493-497. [Google Scholar]
- 63.Reuveny, S., M. D. White, Y. Y. Adar, Y. Kafri, Z. Altboum, Y. Gozes, D. Kobiler, A. Shafferman, and B. Velan. 2001. Search for correlates of protective immunity conferred by anthrax vaccine. Infect. Immun. 692888-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rhie, G. E., M. H. Roehrl, M. Mourez, R. J. Collier, J. J. Mekalanos, and J. Y. Wang. 2003. A dually active anthrax vaccine that confers protection against both bacilli and toxins. Proc. Natl. Acad. Sci. USA 10010925-10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ribot, W. J., R. G. Panchal, K. C. Brittingham, G. Ruthel, T. A. Kenny, D. Lane, B. Curry, T. A. Hoover, A. M. Friedlander, and S. Bavari. 2006. Anthrax lethal toxin impairs innate immune functions of alveolar macrophages and facilitates Bacillus anthracis survival. Infect. Immun. 745029-5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ross, J. M. 1957. The pathogenesis of anthrax following the administration of spores by the respiratory route. J. Pathol. Bacteriol. 73485-494. [Google Scholar]
- 67.Schneerson, R., J. Kubler-Kielb, T. Y. Liu, Z. D. Dai, S. H. Leppla, A. Yergey, P. Backlund, J. Shiloach, F. Majadly, and J. B. Robbins. 2003. Poly(gamma-d-glutamic acid) protein conjugates induce IgG antibodies in mice to the capsule of Bacillus anthracis: a potential addition to the anthrax vaccine. Proc. Natl. Acad. Sci. USA 1008945-8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scorpio, A., D. J. Chabot, W. A. Day, D. K. O'Brien, N. J. Vietri, Y. Itoh, M. Mohamadzadeh, and A. M. Friedlander. 2007. Poly-gamma-glutamate capsule-degrading enzyme treatment enhances phagocytosis and killing of encapsulated Bacillus anthracis. Antimicrob. Agents Chemother. 51215-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simon, J. K., M. F. Pasetti, J. F. Viret, R. Mischler, A. Munoz, R. Lagos, M. M. Levine, and J. D. Campbell. 2007. A clinical study to assess the safety and immunogenicity of attenuated measles vaccine administered intranasally to healthy adults. Hum. Vaccin. 354-58. [DOI] [PubMed] [Google Scholar]
- 70.Singh, Y., B. E. Ivins, and S. H. Leppla. 1998. Study of immunization against anthrax with the purified recombinant protective antigen of Bacillus anthracis. Infect. Immun. 663447-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sloat, B. R., and Z. Cui. 2006. Nasal immunization with a dual antigen anthrax vaccine induced strong mucosal and systemic immune responses against toxins and bacilli. Vaccine 246405-6413. [DOI] [PubMed] [Google Scholar]
- 72.Steichen, C., P. Chen, J. F. Kearney, and C. L. Turnbough, Jr. 2003. Identification of the immunodominant protein and other proteins of the Bacillus anthracis exosporium. J. Bacteriol. 1851903-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stepanov, A. V., L. I. Marinin, A. P. Pomerantsev, and N. A. Staritsin. 1996. Development of novel vaccines against anthrax in man. J. Biotechnol. 44155-160. [DOI] [PubMed] [Google Scholar]
- 74.Sylvestre, P., E. Couture-Tosi, and M. Mock. 2002. A collagen-like surface glycoprotein is a structural component of the Bacillus anthracis exosporium. Mol. Microbiol. 45169-178. [DOI] [PubMed] [Google Scholar]
- 75.Tournier, J. N., A. Quesnel-Hellmann, A. Cleret, and D. R. Vidal. 2007. Contribution of toxins to the pathogenesis of inhalational anthrax. Cell. Microbiol. 9555-565. [DOI] [PubMed] [Google Scholar]
- 76.Tournier, J. N., A. Quesnel-Hellmann, J. Mathieu, C. Montecucco, W. J. Tang, M. Mock, D. R. Vidal, and P. L. Goossens. 2005. Anthrax edema toxin cooperates with lethal toxin to impair cytokine secretion during infection of dendritic cells. J. Immunol. 1744934-4941. [DOI] [PubMed] [Google Scholar]
- 77.Turnbull, P. C. 1991. Anthrax vaccines: past, present and future. Vaccine 9533-539. [DOI] [PubMed] [Google Scholar]
- 78.Uchida, I., K. Hashimoto, and N. Terakado. 1986. Virulence and immunogenicity in experimental animals of Bacillus anthracis strains harbouring or lacking 110 MDa and 60 MDa plasmids. J. Gen. Microbiol. 132557-559. [DOI] [PubMed] [Google Scholar]
- 79.Voth, D. E., E. E. Hamm, L. G. Nguyen, A. E. Tucker, I. I. Salles, W. Ortiz-Leduc, and J. D. Ballard. 2005. Bacillus anthracis oedema toxin as a cause of tissue necrosis and cell type-specific cytotoxicity. Cell. Microbiol. 71139-1149. [DOI] [PubMed] [Google Scholar]
- 80.Walsh, J. J., N. Pesik, C. P. Quinn, V. Urdaneta, C. A. Dykewicz, A. E. Boyer, J. Guarner, P. Wilkins, K. J. Norville, J. R. Barr, S. R. Zaki, J. B. Patel, S. P. Reagan, J. L. Pirkle, T. A. Treadwell, N. R. Messonnier, L. D. Rotz, R. F. Meyer, and D. S. Stephens. 2007. A case of naturally acquired inhalation anthrax: clinical care and analyses of anti-protective antigen immunoglobulin G and lethal factor. Clin. Infect. Dis. 44968-971. [DOI] [PubMed] [Google Scholar]
- 81.Wang, T. T., P. F. Fellows, T. J. Leighton, and A. H. Lucas. 2004. Induction of opsonic antibodies to the gamma-d-glutamic acid capsule of Bacillus anthracis by immunization with a synthetic peptide-carrier protein conjugate. FEMS Immunol. Med. Microbiol. 40231-237. [DOI] [PubMed] [Google Scholar]
- 82.Welkos, S., D. Becker, A. Friedlander, and R. Trotter. 1990. Pathogenesis and host resistance to Bacillus anthracis: a mouse model, p. 49-52. In P. C. B. Turnbull (ed.), Proceedings of the International Workshop on Anthrax. Salisbury Medical Bulletin, vol. 68, special supplement. Salisbury Medical Society, Winchester, England. [Google Scholar]
- 83.Welkos, S., A. Friedlander, S. Weeks, S. Little, and I. Mendelson. 2002. In vitro characterisation of the phagocytosis and fate of anthrax spores in macrophages and the effects of anti-PA antibody. J. Med. Microbiol. 51821-831. [DOI] [PubMed] [Google Scholar]
- 84.Welkos, S., S. Little, A. Friedlander, D. Fritz, and P. Fellows. 2001. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology 1471677-1685. [DOI] [PubMed] [Google Scholar]
- 85.Welkos, S. L., and A. M. Friedlander. 1988. Comparative safety and efficacy against Bacillus anthracis of protective antigen and live vaccines in mice. Microb. Pathog. 5127-139. [DOI] [PubMed] [Google Scholar]
- 86.Welkos, S. L., T. J. Keener, and P. H. Gibbs. 1986. Differences in susceptibility of inbred mice to Bacillus anthracis. Infect. Immun. 51795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Welkos, S. L., N. J. Vietri, and P. H. Gibbs. 1993. Non-toxigenic derivatives of the Ames strain of Bacillus anthracis are fully virulent for mice: role of plasmid pX02 and chromosome in strain-dependent virulence. Microb. Pathog. 14381-388. [DOI] [PubMed] [Google Scholar]
- 88.Whiting, G. C., S. Rijpkema, T. Adams, and M. J. Corbel. 2004. Characterisation of adsorbed anthrax vaccine by two-dimensional gel electrophoresis. Vaccine 224245-4251. [DOI] [PubMed] [Google Scholar]
- 89.Young, J. A., and R. J. Collier. 2007. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu. Rev. Biochem. 76243-265. [DOI] [PubMed] [Google Scholar]